Abstract
Aims
To detect the presence of lens epithelial cells in the anterior chamber of the eye at the end of phacoemulsification.
Methods
A prospective observational study was carried out on 50 patients undergoing phacoemulsification. Fluid from the anterior chamber was collected from these patients at the end of phacoemulsification. Thirty samples were processed for detection of viability using calcein AM–propidium iodide. Remaining samples were processed for immunofluorescence detection of αA-crystallin and vimentin. The nonparametric Mann–Whitney U test was applied.
Results
The presence of lens epithelial cells was confirmed in 27 of the first 30 samples. The total number of cells observed in these 27 samples were 64.70 ± 58.49. Within these 27 samples, 35.5% were live cells and 64.5% were dead. The cells were present as single cell or in groups. Twenty three percentage samples were also positive for nucleated lens fibres. In the remaining 20 samples, 89% cells were confirmed to be lens epithelial cells.
ConclusionsWe show for the first time, the presence of cells in the fluid of the anterior chamber at the end of phacoemulsification. The cells were positive for αA-crystallin and vimentin, thereby suggesting that they were lens epithelial cells.
Similar content being viewed by others
Introduction
Posterior capsule opacification (PCO) is a frequently encountered complication of all cataract surgeries and has important medical, social, and economic implications.1, 2 It occurs due to proliferation and differentiation of residual lens epithelial cells (LECs) in the posterior lens capsule.2 Besides PCO, this phenomenon is also involved in the pathogenesis of anterior capsular opacification (ACO).3 In our previous randomized experimental study on human cadaver eyes, we found that corticocleaving hydrodissection combined with rotation removed significant quantities of LECs.4 Although a few LECs are believed to remain in the anterior chamber even after the completion of cataract surgery, we have not come across any reports documenting this.
A pilot study was conducted on 10 consecutive eyes undergoing phacoemulsification with fluid being collected at the end of the surgery. In seven of the 10 eyes, the presence of a few cells in the anterior chamber at the end of the cataract surgery was documented. On the basis of this observation, we hypothesize that some LECs, which get detached from the capsular bag may remain in the anterior chamber even after it is thoroughly cleaned-up. To substantiate further, we designed a prospective observational study to detect LECs in the anterior chamber at the end of phacoemulsification.
Materials and methods
Patients’ inclusion
This prospective observational study comprised 50 consecutive patients with uncomplicated age-related cataract who had undergone phacoemulsification at our centre from March to July 2006. Patients older than 45 years, pupils dilating more than 7 mm, and otherwise normal eyes were included in the study. Exclusion criteria were glaucoma, shallow anterior chamber, uveitis, high myopia (axial length >27.0 mm), pseudoexfoliation, traumatic cataracts, subluxated cataracts, previous ocular surgeries, and patients allergic to dilating drops.
Surgical intervention
An experienced surgeon (ARV) performed all the surgeries using a standardized technique and topical anaesthesia. Two 1.0 mm paracenteses were made in the clear cornea. A soft shell technique using a small amount of 3% sodium hyaluronate and 4% chondroitin sulphate (Viscoat, Alcon Laboratories, Fort Worth, Texas, USA) was injected and the anterior chamber was filled with a high molecular weight ophthalmic viscosurgical device (sodium hyaluronate 1.0% (Provisc, Alcon Laboratories). This pushed the Viscoat towards the cornea so that it coated the endothelium.5 A 2.2 mm temporal clear corneal tunnel was made. Subsequently, a 5.0 mm capsulorhexis was created. A thorough, copious, gentle hydrodissection preceded rotation of the nucleus. Phacoemulsification was performed with the Infiniti phacoemulsification (Alcon Laboratories) using standardized surgical techniques described elsewhere.6, 7 This was followed by bimanual irrigation aspiration to ensure thorough and complete cortex removal. An AcrySof IOL (SN60AT, Alcon Laboratories) was implanted in the capsular bag. The irrigation fluid used during phacoemulsification was BSS plus (Alcon Laboratories). Bimanual irrigation and aspiration were used to thoroughly remove the residual viscoelastic substance and the IOL surface was tapped to remove the viscoelastic substance trapped under it. No further attempt was made to aspirate residual viscoelastic substance from behind the IOL optic.
Study design
The study was performed on a fluid collected from the anterior chamber after cataract surgery. A 26-gauge cannula mounted on a tubercular syringe was used to aspirate fluid from the anterior chamber. The fluid was immediately transferred to a microcentrifuge tube. The study was undertaken in two phases. The first phase included detecting the presence of cells in the anterior chamber at the end of phacoemulsification and the second involved in proving that these cells were LECs. Of the 50 samples, the first 30 were subjected to 1 μ M calcein AM and 0.5 μm propidium iodide (PI) to detect the presence of live cells. The remaining 20 samples were processed for immunofluorescence detection of αA-crystallin (n=10) and vimentin (n=10) to prove that the observed cells were LECs.
Viability assay
Of the 50 samples collected, the first 30 were subjected to a viability assay within 5 min of collection.8, 9 Calcein AM (Fluka, Buchs, Switzerland) and PI (Molecular Probes, Eugene, OR, USA) stocks were added to the fluid to achieve a final concentration of 1 μ M calcein AM and 0.5 μ M PI, respectively. The fluid was allowed to stand for 15 min at 37°C. It was then transferred to a clean glass slide, covered with a 20 × 10 mm cover slip, and viewed under an epifluorescence microscope (Axioskope II, Carl Zeiss, Germany). The entire cover slip area was scanned under phase-contrast using a 10X microscope objective. The cellular structures detected under the phase-contrast were further viewed under fluorescence light. Calcein (green)-positive live cells, and PI (red)-positive dead cells were observed using fluorescein and rhodamine filter sets, respectively.
Immunofluorescence detection of αA-crystallin and vimentin
Of the 50 samples, the remaining 20 were analysed separately for the immunofluorescence detection of αA-crystallin and vimentin. The fluid collected at the end of surgery was placed in a microcentrifuge tube and centrifuged at 1000 r.p.m for 10 min. The supernatant was removed and pellets containing cells were resuspended either in 50 μl methanol precooled at −26°C for the detection of αA-crystallin or in 2% paraformaldehyde in phosphate-buffered saline (PBS) for the detection of vimentin. These fixed cells were transferred to a glass slide and allowed to dry. After they were dry, they were washed with PBS for 6 min and incubated in 1% BSA in PBS for 10 min. To detect the presence of αA-crystallin, methanol fixed cells were incubated with 1:200 diluted anti αA-crystallin antibody (Santa Cruz, CA, USA) in PBS for 1 h at 37°C, washed in PBST (PBS containing 0.1% Tween 20), and incubated with 1:200 antirabbit antibody tagged with alexa fluor 546 (Molecular Probes, Eugene, OR, USA) in PBS. To detect the presence of vimentin paraformaldehyde, fixed cells were incubated with 1:100 diluted mouse antivimentin antibody (Sigma, St Louis, MO, USA) in PBS, washed with PBST, and incubated with 1:200 diluted alexa fluor 488 tagged antimouse antibody (Molecular Probes, Eugene, OR, USA) in PBS. After this labelling, both the samples were washed with PBST and the nuclei were counterstained with 300 nm DAPI (Sigma, St Louis, MO, USA) in PBS for 3 min. These samples were mounted on a slide in 90% glycerol with PBS containing 2.5% DABCO (1,4-diazabicyclo[2,2,2] octane) and observed under an epifluorescence microscope (Axioskope II, Carl Zeiss, Germany). DAPI-stained nuclei were obtained using a UV filter set; alexa fluor 546-labelled αA-crystallin was observed using a rhodamine filter; and alexa flour 488-labelled vimentin was observed using fluorescein filter sets. Ten random regions from each sample were selected and the total number of positive cells for αA-crystallin or vimentin was counted.
Statement of ethics
We certify that all associated institutional and governmental regulations concerning the ethical use of human volunteers were followed in this research.
Results
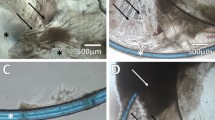
Of the first 30 samples subjected to assessment for viability, the presence of cells was confirmed in 27 (90%) samples. The cells were present individually or in groups and were either live or dead (Figure 1). The result of the viability assay is given in Table 1, whereas the percentage occurrence of live–dead and individual–group of cells is shown as a bar graph in Figure 2. The average number of cells was 64.7±58.49 (95% CI: 42.86–86.54). When these 27 samples were subjected to the viability assay, 22.97±18.70 (95% CI: 15.98–29.95) cells were found live (35.5%) and 41.73±43.19 (95% CI: 25.61–57.86) cells were dead (64.5%). Of the live cells observed, 7.37±6.02 cells were seen as single (95% CI: 5.12–9.62), whereas 15.60±16.48 (95% CI: 9.44–21.76) cells were observed in groups. Similarly, of the total dead cells observed, 16.87±21.97 cells (95% CI: 8.66–25.07) were seen as single, whereas 24.87±35.60 cells were found in groups. There were more dead cells documented than total live cells and this attained statistical significance (P<0.0006). Besides cells, seven (23.33%) samples showed the presence of nucleated fibre cells (Figure 2g and h). We could not detect viability in these fibre cells.
Cells and lens fibres obtained from the anterior chamber at the end of phacoemulsification. The panels on the left show the phase-contrast image whereas the panels on the right show a combined image for calcein-positive live cells (green) and propidium iodide-positive dead cells (red). The cells were present as individual live cells (a and b), individual dead cells (c and d), and a group of live and dead cells (e and f). Nucleated fibre cells were also detected in some samples (g and h) (bar=10 μm).
Percentage occurrence of live and dead cells in the fluid obtained from the anterior chamber at the end of phacoemulsification. Of the cells, 37.45% were present individually whereas 62.55% were present in groups. Of them, 35.5% cells were live (11.4% individual and 24.11% in group) whereas 64.5% cells were dead (26.07% individual and 38.44% in group).
The remaining 20 samples were observed for the detection of LECs by using αA-crystallin and vimentin. A total of 89.12±10.50 (95% CI: 84.20–94.0) cells were confirmed to be LECs in these 20 samples. In 10 samples, 87.75±13.46 (95% CI: 78.11–97.38) cells had αA-crystallin, whereas 90.49±6.87 (95% CI: 85.57–95.41) cells were confirmed with vimentin (Table 2). Both αA-crystallin and vimentin were located in the cytoplasm. αA-crystallin appeared diffused, whereas vimentin was in the form of a fibrous network. All the cells had a round to oval nuclei at the centre (Figure 3).
Immunofluorescence localization of αA-crystallin and vimentin in the cells obtained from the anterior chamber at the end of phacoemulsification. Panels on the left show the phase-contrast image. Cells were positive to αA-crystallin (top left, arrow) and vimentin (bottom left, arrow). Counterstaining of the nucleus was done by DAPI (arrow head) (bar=10 μm).
Discussion
The human lens epithelium possesses cells in the central, pre-equatorial, and equatorial zones. The cells of the central zone are mitotically quiescent but the cells of the pre-equatorial zone are mitotically active. The cells of the pre-equatorial zone proliferate and migrate to the equatorial zone where they undergo terminal differentiation to form fibre cells.10, 11, 12 Besides this normal differentiation, the LECs also have the ability to undergo epithelial mesenchymal transdifferentiation to form myofibroblast-like cells in response to any mechanical or physiological stress. This abnormal differentiation of LECs is involved in the pathogenesis of plaque in the intact lens and in the development of PCO and ACO after the removal of cataract.2, 4, 13, 14
We believe that the observed cells in this study could have been detached from any of the three zones of the lens epithelium. In our previous experimental study on human cadaver eyes, we observed that hydrodissection followed by rotation of the nucleus effectively scraped the LECs from the lens capsule.4 In the present study, it was interesting to observe the presence of live cells and nucleated fibres even after a thorough clean-up of the anterior chamber. We believe that hydrodissection combined with rotation of the nucleus causes friction between the cataract and LECs, leading to the removal of the LECs. Apart from hydrodissection and rotation, the phaco technique can also create turbulence in the anterior chamber during lens removal, and thereby detaching the LECs. The presence of αA-crystallin and vimentin confirmed that these cells were LECs. αA-crystallin is a water-soluble chaperon protein of the undifferentiated LECs.15, 16 Vimentin is an intermediate filament cytoskeleton protein of the LECs.17 Both αA-crystallin and vimentin were earlier used as marker proteins for the LECs.18
We speculate that live LECs remaining in the anterior chamber at the end of phacoemulsification could adhere to the posterior capsule and contribute to the development of PCO. This hypothesis has been illustrated in Figure 4. The LECs are shown to possess various adhesion molecules that help in their attachment to the lens capsule.19, 20, 21 Various substances like growth factors, fibronectin, and so on are released into the aqueous humour after the surgery due to the breakdown of blood aqueous humour, which facilitates the proliferation and differentiation of the adhered LECs.22, 23, 24 It would be worthwhile to further investigate the role of these live LECs in the incidence and severity of PCO. Our study may have clinical relevance in the context of both ACO and PCO.
The illustrative diagram showing the contribution of lens epithelial cells (LECs) remaining in the anterior chamber at the end of phacoemulsification to the development of posterior capsular opacification (PCO). These free LECs adhere to the posterior capsule (top). Proliferation and differentiation of the adhered LECs at the posterior capsule contribute to the development of PCO (bottom).
In the past, various speculations have been made about the presence of LECs in the anterior chamber following phacoemulsification. However, to our knowledge, no study has been conducted to confirm this hypothesis. This is the first study documenting the presence of LECs in the anterior chamber after phacoemulsification. In conclusion, our study confirmed the presence of LECs in the fluid of the anterior chamber at the end of phacoemulsification.
References
Schaumberg DA, Dana MR, Christen WG, Glynn RJ . A systematic overview of the incidence of posterior capsule opacification. Ophthalmol 1998; 105: 1213–1221.
Apple DJ, Solomon KD, Tetz MR . Posterior capsule opacification. Surv Ophthalmol 1992; 37: 73–116.
Werner L, Pandey SK, Apple DJ, Escobar-Gomez M, Mc Lendon L, Mackey TA . Anterior capsule opacification: correlation of pathologic findings with clinical sequelae. Ophthamology 2001; 108: 1657–1681.
Vasavada AR, Raj SM, Johar K, Mayank AN . Effect of hydrodissection alone and hydrodissection combined with rotation on lens epithelial cells. Surgical approach for the prevention of posterior capsule opacification. J Cataract Refract Surg 2006; 32: 145–150.
Arshinoff SA . Dispersive-cohesive viscoelastic soft shell technique. J Cataract Refract Surg 1999; 25: 167–173.
Vasavada AR, Singh R . Step-by-step, chop in situ and separation of very dense cataracts. J Cataract Refract Surg 1998; 24: 156–159.
Vasavada AR, Raj S . Step-down technique. J Cataract Refract Surg 2003; 29: 1077–1079.
Kodjikian L, Richter T, Halberstadt M . Toxic effects of indocyanine green, infracyanine green, and trypan blue on the human retinal pigmented epithelium. Graefes Arch Clin Exp Ophthalmol 2005; 243: 917–925.
Nanavaty MA, Johar K, Sivasankaran MA, Vasavada AR, Praveen MR, Zetterstrom C . Effect of trypan blue staining on the density and viability of lens epithelial cells in white cataract. J Cataract Refract Surg 2006; 32: 1483–1488.
Johar SR, Rawal UM, Jain NK, Vasavada AR . Sequential effects of ultraviolet radiation on the histomorphology, cell density and antioxidative status of the lens epithelium—an in vivo study. Photochem Photobiol 2003; 78: 306–311.
Kuwabara T . The maturation of the lens cell: a morphologic study. Exp Eye Res 1975; 20: 427–443.
Ramaekers CS, Bloemendal H . Cytoskeletal and contractile structures in lens cell differentiation. In: Bloemendal H (eds). Molecular and Cellular Biology of the Eye Lens, 1st edn. Wiley Interscience Publication: New York, 1991, pp 85–136.
Johar K, Vasavada AR, Tatsumi K, Dholakia S, Nihalani B, Lakshmana Rao SS . Anterior capsular plaque in congenital cataract: occurrence, morphology, immunofluorescence and ultrastructure. Invest Ophthalmol Vis Sci 2007; 48: 4209–4214.
Lovicu FJ, Steven P, Saika S, McAvoy JW . Aberrant lens fiber differentiation in anterior subcapsular cataract formation: a process dependent on reduced levels of Pax6. Invest Ophthalmol Vis Sci 2004; 45: 1946–1953.
Bloemendal H, de Jong W, Jaenicke R, Lubsen NH, Slingsby C, Tardieu A . Ageing and vision: structure, stability and function of lens crystallins. Prog Biophys Mol Biol 2004; 86: 407–485.
Horwitz J, Bova MP, Ding LL, Haley DA, Stewart PL . Lens alpha-crystallin: function and structure. Eye 1999; 13: 403–408.
Sandilands A, Prescott AR, Carter JM . Vimentin and CP49/filensin form distinct networks in the lens which are independently modulated during lens fibre cell differentiation. J Cell Sci 1995; 108: 1397–1406.
Sponer U, Pieh S, Soleiman A, Skorpik C . Upregulation of alphavbeta6 integrin, a potent TGF-beta1 activator, and posterior capsule opacification. J Cataract Refract Surg 2005; 31: 595–606.
Nishi O, Nishi K, Akaishi T, Shirasawa E . Detection of cell adhesion molecules in lens epithelial cells of human cataracts. Invest Ophthalmol Vis Sci 1997; 38: 579–585.
Volk T, Geiger B . A-CAM: a 135-kD receptor of intercellular adherens junctions. I. Immunoelectron microscopic localization and biochemical studies. J Cell Biol 1986; 103: 1441–1450.
Saika S, Kawashima Y, Miyamoto T, Okada Y, Tanaka S, Yamanaka O et al. Immunolocalization of hyaluronan and CD44 in quiescent and proliferating human lens epithelial cells. J Cataract Refract Surg 1998; 24: 1266–1270.
Kondo T, Nakatsu A, Masami P . A method of image analysis for primary angle closure glaucoma. Ophthalmologica 1995; 209: 113–116.
Kim KS, Lee BH, Kim IS . The measurement of fibronectin concentrations in human aqueous humor. Korean J Ophthalmol 1992; 6: 1–5.
de Jong-Hesse Y, Kampmeier J, Lang GK, Lang GE . Effect of extracellular matrix on proliferation and differentiation of porcine lens epithelial cells. Graefes Arch Clin Exp Ophthalmol 2005; 243: 695–700.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Vasavada, A., Johar, K., Praveen, M. et al. Confirmation of the presence of lens epithelial cells in the anterior chamber after phacoemulsification. Eye 23, 1170–1175 (2009). https://doi.org/10.1038/eye.2008.182
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/eye.2008.182
Keywords
This article is cited by
-
Cytocompatibility of self-assembled hydrogel from IKVAV-containing peptide amphiphile with neural stem cells
Journal of Wuhan University of Technology-Mater. Sci. Ed. (2009)