Abstract
Caspase activity is critical for both T-cell survival and death. However, little is known regarding what determines caspase activity in cycling T cells. Interleukin (IL)-2 and IL-15 confer very different susceptibilities to T-cell death. We therefore considered that IL-2 and IL-15 differentially regulate caspase activity to influence T-cell survival. We observed that IL-2-cultured primary murine effector T cells manifested elevated levels of caspase-3 activity compared with IL-15-cultured T cells. T cell receptor (TCR) restimulation further increased caspase activity and induced considerable cell death in IL-2-cultured T cells, but provoked only a minimal increase of caspase activity and cell death in IL-15-cultured T cells. IL-2 sensitization to cell death was caspase-3 mediated. Interestingly, increased active caspase-3 levels with IL-2 were independent of active initiator caspase-8 and caspase-9 that were similar with IL-2 and IL-15. Rather, caspase-3 activity was inhibited by posttranslational S-nitrosylation in IL-15-cultured T cells, but not in the presence of IL-2. This paralleled increased reactive nitrogen and oxygen species with IL-15 and reduced glycolysis. Taken together, these data suggest that the metabolic state conferred by IL-15 inhibits T-cell apoptosis in part by maintaining low levels of active caspase-3 via S-nitrosylation.
Similar content being viewed by others
Main
Interleukin (IL)-2 and IL-15 modulate T-cell growth, effector function, and survival.1, 2 IL-2 is induced and secreted by antigen-stimulated T cells through T cell receptor (TCR) and CD28 signaling1 and functions in an autocrine manner. In contrast, IL-15 is produced by nonlymphoid tissues, such as fibroblasts, LPS-stimulated monocytes, and dendritic cells.2, 3 However, despite the ability of both IL-2 and IL-15 to confer proliferative capacity to T cells,1 they nonetheless provide contrasting outcomes to T cells.4 Antigen restimulation in the presence of IL-2 can mediate peripheral tolerance through elimination of mature clonally expanded T cells via activation-induced cell death (AICD).5 In contrast, IL-15 contributes to an enhanced memory response by inhibiting AICD and leading to selective survival of memory cells during the contraction phase.6, 7 However, the mechanism for this difference in susceptibility to AICD is not well understood.
Caspases are needed for both T-cell proliferation and cell death.8, 9, 10, 11 Following T-cell stimulation, caspases are activated and their activity is sustained during the proliferative phase of the T cell.8, 9, 10 It is not known what determines the level of caspase activity in cycling T cells, nor how this affects their survival. Because T-cell survival depends on the ability to control the levels of active caspases, we considered the possibility that the differences in AICD susceptibility between IL-2 and IL-15 may reflect differences in caspase activity. Apoptosis following AICD is associated with the sequential activation of several caspases, including upstream caspase-8 and caspase-9, and downstream caspase-3.11 More recently, it has been reported that caspase-3 activity is inhibited by certain posttranslational modifications, such as S-nitrosylation of its active site cysteine163.12 S-nitrosylation and denitrosylation of proteins at critical cysteine residues is emerging as a method of rapidly modifying the function of numerous proteins, in a manner similar to phosphorylation.13 In human B- and T-cell lines, S-nitrosylation may prevent premature autoactivation of caspase-3.14, 15 Nitric oxide (NO) promotes protein S-nitrosylation, and NO production in T cells is enhanced by induction of inducible nitric oxide synthase (iNOS) expression under IL-15 conditions as compared with IL-2.16 NO reacts with reactive oxygen species (ROS) to form peroxynitrite (ONOO−) that can directly nitrosylate caspase-3 or can indirectly transnitrosylate it through its product, S-nitrosoglutathione (GSNO).17, 18
The nitrosylation state of a protein likely reflects the metabolic state of a cell, given that reactive species are by-products of the redox state of the cell. Upon antigen stimulation, quiescent T cells become activated and undergo a metabolic switch to aerobic glycolysis, similar to many cancer cells, a phenomenon known as the Warburg effect.19 To meet the biosynthetic demands required for proliferation and the production of effector molecules, AKT signaling in IL-2-cultured effector T cells increases both glucose and amino acid uptake.20 In contrast, IL-15 mediates fatty acid utilization.21, 22
Given the different metabolic states conferred by IL-2 and IL-15, we considered that this might be reflected in the levels of caspase activity, and specifically S-nitrosylation of caspase-3, as a possible mechanism by which IL-15 promotes the survival of T cells. We observe that there is a very robust increase in caspase activity as well as susceptibility to CD3-restimulated AICD in IL-2-cultured primary murine T cells, whereas the IL-15-cultured T cells manifest almost no increase in caspase activity or cell death upon CD3 restimulation. Consistent with this, IL-2-cultivated T cells manifest considerably higher levels of active caspase-3 compared with IL-15-propagated T cells. This was independent of initiator caspases, caspase-8 and -9. In addition, IL-15 promotes S-nitrosylation-mediated inactivation of caspase-3. This is likely because of the increased levels of reactive species in IL-15-cultured T cells that are generated in a metabolic state of reduced glycolysis. Taken together, these data support the model that IL-15 inhibits T-cell apoptosis and prolongs T-cell survival in part by maintaining low levels of active caspase-3 via S-nitrosylation.
Results
IL-2 promotes greater caspase activity and activation-induced cell death compared with IL-15
Although reports have suggested that IL-2, but not IL-15, promotes AICD,7 the mechanism for this difference is not well understood. Because caspase activity is required for both cell growth and death of T cells,8, 9, 10 we considered the possibility that differences in IL-2- and IL-15-regulated cell death might reflect their influence on caspase activity. We thus initially examined the level of caspase activity in murine T cells stimulated for 2 days with anti-CD3/CD28 in the presence of IL-2 to model in vivo antigen stimulation, then washed on day 3 free of cytokine and recultured for further expansion in the presence of IL-2 or IL-15 for 2 additional days. On day 5, caspase activity and cell death were assessed before and following anti-CD3 restimulation to induce AICD.
Caspase activity was determined using a DEVD-rhodamine substrate that measures global caspase activity. IL-2-cultured T cells manifested a dose-dependent high level of ambient caspase activity compared with T cells cultured in a broad range of concentrations of IL-15 (Figures 1a and b). Furthermore, upon anti-CD3 restimulation, the levels of caspase activity in IL-2-cultivated T cells increased substantially, whereas there was a negligible increase in caspase activity in IL-15-cultured T cells (Figure 1c). Cells were simultaneously assessed for cell death by TUNEL assay that revealed high levels of apoptosis upon CD3 restimulation for both CD4+ and CD8+ T cells under IL-2 conditions, but not under IL-15 conditions (Figures 1d and e). Thus, the high level of caspase activity induced by IL-2 likely increases the susceptibility of IL-2-cultured T cells to AICD.
IL-2 promotes caspase activity and activation-induced cell death compared with IL-15. Lymph node T cells were stimulated with anti-CD3/anti-CD28 for 2 days and then washed on day 3 and recultured in the presence of the indicated doses of IL-2 or IL-15. (a and b) Dose response of caspase activity as measured by DEVD-rhodamine after treatment with increasing amounts of IL-2 or IL-15 for 2 days. Data are representative of three independent experiments. Values are mean±S.E.M. The significance of differences observed in caspase activity within each cytokine group was analyzed by one-way ANOVA followed by Bonferroni multiple comparisons test (overall P-value <0.0001 in a and not significant in b). (c–e) On day 5, T cells recultured in IL-2 (50 U/ml) or IL-15 (20 ng/ml) were either not restimulated (white bars) or anti-CD3 restimulated (black bars) for 4 h, and analyzed for (c) caspase activity by DEVD-rhodamine assay and (d and e) cell death by TUNEL assay. Data are representative of three independent experiments. Values are mean±S.E.M. The significance of differences observed in caspase activity was analyzed by two-way ANOVA followed by Bonferroni multiple comparisons test (*P<0.0001)
Further investigation showed that the CD3-induced cell death was caspase-3 dependent, as IL-2-cultured T cells lacking caspase-3 did not demonstrate an increase in caspase activity or cell death upon CD3 restimulation (Figure 2). To assess why IL-2-cultured T cells manifested robust levels of AICD, we initially examined the expression of cell surface molecules known to influence susceptibility to cell death, including the death receptor Fas (CD95), CD3, and TCR. However, no differences in the surface expression of these molecules were observed among the CD4+ population, whereas there was an increase in surface Fas and TCR found among the CD8+ population cultured in IL-15 (Supplementary Figure S1).
IL-2-dependent activation-induced cell death is mediated by caspase-3. Day 5 T-cell blasts from wild-type or caspase-3-deficient mice were cultured with or without anti-CD3 for 4 h and assessed for (a) caspase activity or (b and c) proportion of dead cells by TUNEL staining. Values are mean±S.E.M. The significance of differences observed in caspase activity was analyzed by two-way ANOVA followed by Bonferroni multiple comparisons test (*P<0.0001)
IL-15 regulates caspase-3 activity independently of caspase-8 and caspase-9
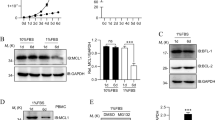
To further define which caspases were activated in day 5 effector T cells grown with each of the two cytokines, we examined upstream caspase-8 and caspase-9 that are known to participate in cleavage of procaspase-3 to active caspase-3 (p18/20). Active caspases in the cell lysates were selectively labeled with biotin-VAD-fmk (bVAD), precipitated using streptavidin-sepharose beads, and then examined by immunoblot for specific active caspases. Of interest was that the levels of total and active caspase-8 and caspase-9 were comparable between IL-2 and IL-15-cultured T cells (Figures 3a–c). In striking contrast, the levels of active caspase-3 were dramatically higher in T cells grown in IL-2 versus IL-15 despite similar levels of total caspase-3 in the whole cell lysates (Figures 3a and d). Thus, the upstream caspases were not responsible for the augmented caspase-3 activity in IL-2-cultured T cells. This raised the question of how IL-15 is able to maintain low levels of active caspase-3 despite robust levels of active initiator caspase-8 and caspase-9.
IL-15 regulates caspase-3 activity independently of caspase-8 and caspase-9. (a) Day 5 IL-2- and IL-15-cultured T cells were lysed in buffer containing biotin-VAD (bVAD) to selectively label active caspases. Then, 450 μg protein lysate was selectively precipitated with streptavidin-sepharose beads. Precipitates were resolved by SDS-PAGE and immunoblotted for caspase-8, caspase-9, and caspase-3, and compared with whole-cell lysates (WCLs; 30 μg) from the same cells. (b–d) Densitometry of bVAD precipitates immunoblotted for (b) caspase-8, (c) caspase-9, and (d) caspase-3. Values are the combined readings of three independent experiments. Values are mean±S.E.M. Data were analyzed by Student’s t-test for IL-2- versus IL-15-cultured T cells (*P<0.05)
As a possible explanation for the differences in active caspase-3 levels between IL-2- and IL-15-cultured T cells, proteins known to influence mitochondrial integrity and caspase-3 activity were examined. Examination of Bcl-2 family members revealed increased expression of Bcl-2 in IL-15-cultured T cells, but no difference in the levels of Bcl-xL (Figure 4a). In addition, although levels of the pro-apoptotic BH3-only member, Bim, were equivalent, IL-15-cultured T cells manifested a higher proportion of the upper band-shifted form of Bim (Figure 4a) that has been reported to represent a phosphorylated form of Bim that dissociates from Bcl-2 and also undergoes more rapid proteosomal degradation.23 Investigation of apoptosis regulators downstream of the Bcl-2 family, including the inhibitor of apoptosis (IAP) family, XIAP, cIAP, and survivin,24 as well as the pro-apoptotic proteins, cytochrome C and second mitochondria-derived activator of caspases/direct IAP binding protein with low pI (Smac/DIABLO),25 revealed no differences in the levels of these proteins between T cells cultured in IL-2 or IL-15 (Figure 4b). Furthermore, there were no differences in the levels of RIPK1 or RIPK3, the recently described members of the death-promoting Ripoptosome (Figure 4c).26 Collectively, these findings suggested that caspase-3 activity was being regulated by events at or below the level of the mitochondria. Consistent with this, evidence of cleavage and activation of downstream caspase-6 and caspase-727 was greater in IL-2-cultured T cells than with IL-15 (Figure 4d).
Levels of protein for molecules that regulate apoptosis proximal to caspase-3. Equal amounts of whole-cell lysates from day 5 IL-2- and IL-15-cultured T cells were resolved by SDS-PAGE, and immunoblotted for (a) Bcl-2, Bcl-xL, and BimEL, (b) XIAP, cIAP-1, survivin, Smac/DIABLO, and cytochrome C, (c) RIPK1 and RIPK3, and (d) caspase-6 and caspase-7. Data are representative of at least two independent experiments
Reduced caspase activity in homeostatically proliferating T cells, Treg, and memory T cells
As IL-15 is involved with homeostatic proliferation and survival of T cells,28 we examined the levels of caspase activity in T cells undergoing homeostatic proliferation using the established model of lymphopenia-induced proliferation. Lymph node cells from wild-type mice were adoptively transferred to Rag1−/− mice. After 14 days, donor CD4+ and CD8+ cells were recovered by cell sorting and analyzed for caspase activity. Homeostatically expanded T cells manifested very low levels of caspase activity, nearly as low as naive T cells (Figure 5a). We further examined caspase activity in freshly isolated Treg, naive, and memory T cells. FoxP3GFP mice were used to sort CD4+ cells that were either FoxP3+ or FoxP3− (Figure 5b). Both subsets had low levels of caspase activity compared with IL-2 T-cell blasts (Figure 5c). Similarly, CD4+ and CD8+ T cells from wild-type mice were sorted into CD44high (memory) and CD44low (naive) T cells (Figure 5d) and each subset manifested low levels of caspase activity (Figure 5e).
Low levels of caspase activity in homeostatically proliferating, regulatory, naive, and memory T cells. (a) Caspase activity of T cells undergoing homeostatic expansion (HP) was compared with day 5 IL-2- or IL-15-cultured T cells and freshly isolated naive (CD44low) T cells. Homeostatically proliferating T cells were generated through adoptive transfer of wild-type lymphocytes into Rag1−/− mice and on day 14, donor CD4+ and CD8+ T cells were purified from Rag1−/− mice by cell sorting. (b and c) Freshly isolated CD4+ T cells from FoxP3GFP mice were sorted for FoxP3− and FoxP3+ cells and their caspase activity was compared with day 5 IL-2-cultured T cells. Numbers in flow cytometry quadrants indicate the percent positive cells. (d and e) Freshly isolated T cells from wild-type mice were sorted for CD4+CD44high, CD4+CD44low, CD8+CD44high, and CD8+CD44low cells and their caspase activity was compared with day 5 IL-2-cultured T cells
IL-15 inactivates caspase-3 by S-nitrosylation
Because total levels of caspase-3 were similar in T cells cultured in IL-2 versus IL-15, we considered the possibility that differences in the metabolic states of these T cells might alter the posttranslational modifications to caspase-3 and, consequently, its activity. It has been previously observed that S-nitrosylation of caspase-3 at the critical Cys163 in the enzymatic pocket inhibits its activity.18, 29 We thus considered that differences in S-nitrosylation might exist between IL-2- and IL-15-cultured T cells that could influence the level of active caspase-3. To examine this possibility, we assessed the level of S-nitrosylation of caspase-3 in T cells cultured in the presence of IL-2 versus IL-15 using the biotin switch technique (BST) that converts nitrosylation sites on proteins to biotinylation sites (Figure 6a). In brief, free thiols were first blocked with methylmethanethiosulfonate (MMTS), a thiol-specific methylthiolating agent, in the presence of sodium dodecyl sulfate (SDS) that denatures proteins to allow MMTS to interact with buried cysteines. Subsequently, ascorbate was added to specifically reduce nitrosothiol bonds to thiols. Finally, N-[6-(biotinamido)hexyl]-3′-(2′-pyridyldithio) propionamide (biotin–HPDP), a sulfydryl-specific biotinylating reagent, was added to the secondarily formed free thiols.30, 31 Biotinylated proteins were then precipitated using streptavidin-sepharose beads, separated by gel electrophoresis, and examined by immunoblot for caspase-3. Compared with IL-2, IL-15 promoted considerably greater S-nitrosylation of caspase-3 (Figures 6b and c). Although IL-2-cultured T cells lacked detectable S-nitrosylated caspase-3, this could be induced by the addition of the transnitrosylating agent S-nitrosylated-cysteine (SNOC) (Figures 6b and d). We did not observe detectable S-nitrosylation of either caspase-6 or caspase-7 with either cytokine (data not shown).
IL-15 inactivates caspase-3 by S-nitrosylation. (a) Model of the biotin switch technique (BST) that converts S-nitrosylated thiols to biotinylated thiols. In step 1, free thiols are blocked with a thiol-specific methylthiolating agent, methylmethanethiosulfonate (MMTS). In step 2, ascorbate specifically reduces nitrosothiol bonds to thiols. In step 3, N-[6-(biotinamido)hexyl]-3′-(2′- pyridyldithio)propionamide (bHPDP; biotin-HPDP), a sulfydryl-specific biotin linker, biotinylates secondarily formed free thiols. Biotinylated proteins are then precipitated using streptavidin-sepharose beads, separated by gel electrophoresis, and examined by immunoblot for protein of interest. (b) S-nitrosylation of caspase-3 (caspase-3-SNO) was detected by BST in T cells expanded in IL-2 or IL-15 for 5 days. Equal loading of protein was verified by immunoblotting for actin. Caspase-3-SNO was detected by BST. The −MMTS, −bHPDP, and −ascorbate represent controls without thiol blocking agent, biotin linker, or reducing agent, respectively. (c and d) Densitometry of IL-2- and IL-15-cultured T cells immunoblotted for total caspase-3-SNO. Values are mean±S.E.M. Statistical analysis was based on two independent experiments combined using Student’s t-test (*P<0.05). (e) Measurement of caspase activity by DEVD-rhodamine assay of IL-2-cultured T cells treated with medium, vehicle control HCl/EDTA, or SNOC/NAc. Results are based on two combined independent experiments. Values are mean±S.E.M. Data were analyzed by Student’s t-test (*P<0.05)
S-transnitrosylation in vivo can also occur via GSNO, produced by the reaction of ONOO− with glutathione (GSH).12, 17, 18 Endogenous GSNO may serve as a pool of NO groups to S-nitrosylate proteins.17 Consistent with this, formation of GSNO by SNOC in combination with N-acetylcysteine (NAc), which replenishes GSH stores, abrogated levels of caspase activity in IL-2-cultured T cells by 40.5% compared with vehicle control (Figure 6e). These findings demonstrated that caspase-3 activity was negatively regulated by S-nitrosylation in effector T cells.
The reduction-oxidation (redox) state of the cell, reflected by the levels of reactive species and GSH, regulates nitrosylation of proteins.32 The products formed by NO reacting with ROS have been shown to nitrosylate caspase-3 both directly through ONOO− and indirectly through transnitrosylation by GSNO.12, 18 ROS was thus measured by 5-(and -6)carboxy-20,70-dichlorodihydrofluorescein diacetate (DCFDA) using flow cytometry. IL-15-cultured T cells exhibited considerably higher levels of ROS compared with IL-2-cultured T cells (Figure 7a). NO levels, as assessed by total levels of nitrate (NO3−) and nitrite (NO2−), were determined using 2,3-Diamino-naphthalene (DAN). Similar to ROS, T cells in the presence of IL-15 accumulated considerably higher levels of NO compared with T cells cultured in IL-2 (Figure 7b). Because reduced GSH scavenges ROS33 and thus indirectly reduces S-nitrosylation, GSH levels were measured by o-phthalaldehyde (OPA). T cells cultured in IL-15 exhibited considerably less GSH, GSSG, and total levels of GSH compared with IL-2 (Figures 7c–e).
Increased reactive species in IL-15-cultured T cells. Day 5 wild-type T cells cultured in the presence of either IL-2 (50 U/ml) or IL-15 (20 ng/ml) were analyzed for (a) ROS measured by DCFDA and flow cytometry. Data are representative of three independent experiments. (b) Nitric oxide as a measurement of total nitrate+nitrite by DAN probe. (c) Reduced glutathione (GSH), (d) oxidized glutathione (GSSG), or (e) GSH+GSSG were independently assayed. Data are based on three independent experiments combined. Values are mean±S.E.M. The significance of differences observed was analyzed by Student’s t-test (*P<0.05; **P<0.001)
Given that S-nitrosylation correlates with the levels of reactive species and that reactive species are by-products of metabolism, we considered that S-nitrosylation of caspase-3 might reflect the metabolic state of T cells. GSH represents the largest component of the endogenous thiol buffer and is kept in its reduced state by the NADPH-dependent enzyme, GSH disulfide reductase.33 NADPH is generated by the pentose phosphate pathway and promotes reduced forms of GSH. We thus assessed levels of NADP(H) (total NADP+ and NADPH) and observed that NADPH levels were markedly reduced in IL-15-cultured T cells (Figure 8a), suggesting reduced activity of the pentose phosphate pathway.
IL-15 promotes reduced glycolysis, NADPH, and NADH. Day 5 T cells cultured in IL-2 or IL-15 were analyzed for (a) total levels of NADP(H) and NADPH. Data shown are based on the results of three combined independent experiments. Values are mean±S.E.M. Statistical analysis was assessed by Student’s t-test (*P<0.05). (b) Production of lactate as an independent measure of glycolysis using supernatants from cultures for the indicated times. Values are mean±S.E.M. The significance of differences observed was analyzed by two-way ANOVA followed by Bonferroni multiple comparisons test (***P<0.0001). (c) Surface glucose transporter 1 (GLUT 1) levels by flow cytometry. (d and e) Immunoblot and densitometry for hexokinase II (HK II). Data are representative of four independent experiments. Values are mean±S.E.M. Statistical analysis was assessed by Student’s t-test (**P<0.001). (f) Total levels of NAD(H) and NADH. Data shown are based on the results of three combined independent experiments. Values are mean±S.E.M. Statistical analysis was assessed by Student’s t-test (*P<0.05; **P<0.001)
Because the pentose phosphate pathway is a shunt off the glycolytic pathway, we next analyzed whether decreased levels of NADPH were due to overall decreased consumption of glucose. Glycolysis, as measured by lactate production, was considerably reduced in IL-15-cultivated versus IL-2-cultivated T cells (Figure 8b). Consistent with this, surface glucose transporter 1 (GLUT1) and hexokinase II, steps in glycolysis, were dramatically decreased in IL-15 T cells (Figures 8c–e). These findings suggest that IL-15, unlike IL-2, drives a low level of glycolysis in T cells. This is consistent with other reports demonstrating that IL-15 promotes fatty acid oxidation in T cells, whereas IL-2 drives glycolysis.20, 21, 22 Levels of NAD(H) (total NAD+ and NADH) were also examined as a measure of the bioenergetic capacity of the cell. NAD+ is reduced to generate NADH in the glyceraldehyde-3-phosphate dehydrogenase step of glycolysis. We observed that IL-15-cultured T cells manifested reduced NADH levels compared with IL-2 (Figure 8f). Taken together, these data support the model that IL-15 inhibits T-cell apoptosis and contributes to T-cell survival by maintaining low levels of active caspase-3 via S-nitrosylation.
Discussion
The current findings demonstrate that IL-2 promotes active caspase-3 and thus renders T cells sensitive to AICD, whereas IL-15 induces the opposite phenotype. Although IL-2 and IL-15 promote similar levels of active caspase-8 and -9, active caspase-3 levels are decreased in the presence of IL-15. Thus, the level of active caspase-3 in effector T cells is not explained purely by the levels of active upstream caspases. Rather, the relatively low level of glycolysis of IL-15-cultured T cells and parallel increased levels of reactive nitrogen and oxygen species promote the S-nitrosylation of caspase-3. The metabolic state of effector T cells thus emerges as an important regulator of caspase-3 activity and hence susceptibility to cell death.
Although caspases were originally described as mediators of cell death, it is now appreciated that certain caspases, particularly caspase-8, are involved with the maintenance of cell survival and growth of not only T cells,8, 34 but also several other cell types.35, 36, 37 Further studies revealed that in effector T cells low levels of active caspases are maintained on the cell membrane in lipid rafts, with no cleavage of BID to pro-apoptotic tBID, whereas during Fas-induced apoptosis large amounts of caspase activity are observed throughout the cytoplasm, leading to substantial cleavage of BID.38, 39, 40 Thus, both the intensity and location of active caspases are critical to regulating cell growth versus cell death. It is now appreciated that the absence of caspase-8 activity results in the formation of a complex containing RIPK1 and caspase-8 known as the Ripoptosome that promotes necrotic caspase-independent cell death by an as yet undefined mechanism.41, 42 However, the regulation and functions of other caspase activities in effector T cells is largely unexplored.
The initiation of caspase activation in T cells following TCR stimulation raises the question of what determines the level of sustained caspase activity in cycling effector T cells, and how this affects their survival. The current findings demonstrate that the cytokine environment is a major determinant of ambient caspase activity in effector T cells. Despite sharing the same CD122 and CD132 cytokine receptor signaling subunits,1, 4 IL-2 and IL-15 modulate caspase activity differentially. This finding is consistent with previous studies that show IL-2 and IL-15 mediate contrasting fates in T cells.1, 4, 6, 7, 20 IL-2 has been reported to promote both T-cell survival and cell death,5 whereas IL-15 has been reported to primarily support T-cell survival. It has been suggested that IL-15 contributes to T-cell survival in part through increased expression of Bcl-2, which was confirmed in the present study.3, 22, 43, 44 Following T-cell activation, IL-2Rα (CD25) is upregulated resulting in a requirement for IL-2 to maintain T-cell survival.45 In contrast, IL-2 also primes effector T cells for death following TCR restimulation. These paradoxical effects can perhaps be explained by the high ambient level of caspase-3 activity induced in effector T cells by IL-2. As a result, TCR restimulation can more readily further augment caspase-3 activity to a point where it results in cell death. It is therefore essential that activated T cells balance the generation of caspase activity to allow cell cycling while maintaining ambient levels sufficiently low to prevent induction of cell death.
We previously demonstrated in human T-cell clones that caspase-3 activity was likely independent of active caspase-8 and -9.46 The current results are in agreement with these observations as well as findings from an earlier study in Jurkat cells,47 suggesting that upstream initiator caspases were not solely responsible for the level of active caspase-3 in T cells. In these studies, the mechanism for the independent regulation of caspase-3 remained unexplained. More recently, it has been reported that caspase-3 activation can be inhibited by posttranslational modifications at the catalytic site cysteine within the enzymatic pocket of caspase-3.15, 18, 29, 48 Proteins with cysteine thiol side chains can be modified by NO through S-nitrosylation to form S-nitrosothiol.30, 31, 32 Similar to phosphorylation, S-nitrosylation of proteins can switch biological signals on and off and has been reported to function for both innate and adaptive immunity.49 In human B- and T-cell lines, S-nitrosylation blocks activation of caspase-3.14, 15, 29 Our results extend these findings to show that S-nitrosylation of caspase-3 occurs in primary murine T cells in a nonglycolytic metabolic state conferred by IL-15 that also promotes production of reactive species such as ROS and NO.
ROS and NO can react to form peroxynitrite that can donate an NO group to the cysteine thiol of caspase-3.12, 18 Our findings of increased ROS coupled with decreased GSH and NADPH in IL-15-cultured T cells are consistent with the role of NADPH-dependent GSH in neutralizing ROS.33 NADPH is a by-product of the pentose phosphate pathway, by which glucose is consumed to create the ribose ring of nucleic acids and other intermediates in cellular biosynthesis needed for replication.50 It is likely that the increased levels of reactive species in IL-15-cultured T cells are thus sustained by low glucose utilization and compensatory increased mitochondrial fatty acid oxidation compared with IL-2.22
The ability of IL-15 to limit apoptosis is crucial for T-cell memory. However, if left unchecked, T-cell accumulation in an IL-15 environment could lead to autoimmune conditions.51 It is thus paramount that clonally expanded T cells undergo apoptosis at the conclusion of an immune response to avoid development of autoimmunity. Reports have indicated an association of increased levels of IL-15 with autoimmune diseases. Elevated serum IL-15 levels have been described in patients with chronic progressive multiple sclerosis,52 type I diabetes,53 and systemic lupus erythematosus.54 Elevated local amounts of IL-15 have also been observed at sites of inflammation,55, 56 including the synovium in rheumatoid arthritis,57, 58 and anti-IL-15 therapy is effective in rheumatoid arthritis patients.59 Consistent with this, rheumatoid arthritis is also associated with elevated serum and synovial fluid NO.60 Migita et al.60 further suggested that synovial hyperplasia in rheumatoid arthritis results from resistance to Fas-mediated apoptosis in the presence of NO, as Fas-mediated apoptosis is suppressed by the addition of a NO donor to synovial cells. Their study further implicates NO in the inhibition of caspase-3 cleavage to its active form. Based on these studies and our current findings, new strategies can be envisioned for eliminating pathogenic T cells that arise in an IL-15-rich environment by increasing AICD through either manipulation of the metabolic state of T cells or more directly through denitrosylation of caspase-3-SNO.
Materials and Methods
Mice
Original C57BL/6, Rag1−/−, and caspase3−/− breeding mice were obtained from Jackson Laboratory (Bar Harbor, ME, USA) and C57BL/6 FoxP3GFP mice were a kind gift from Dr. Vijay Kuchroo, Harvard Medical School (Boston, MA, USA). Mice were housed and bred in the American Association for Accreditation of Laboratory Animal Care (AAALAC)-approved animal facility at The University of Vermont College of Medicine and were used at 2–6 months of age. Protocols were approved by the Institutional Animal Care and Use Committee.
T-cell purification
Inguinal, brachial, axillary, cervical, and popliteal lymph nodes were isolated and disrupted through nylon mesh in RPMI 1640 (MediaTech, Herndon, VA, USA) containing 5% (v/v) bovine calf serum (BCS). Total T cells were isolated by negative selection. Lymph node cells were incubated with anti-MHC class II (M5/114/15/2; a kind gift of M Rincon, University of Vermont, Burlington, VT, USA), anti-CD11b (M1/70), and anti-B220 (RA3-6B2) on ice for 30 min. Cells were washed three times and rocked with goat anti-rat conjugated magnetic beads in a 10 : 1 ratio of beads to cell (Qiagen, Valencia, CA, USA) at 4°C for 45 min. Magnetic depletion was used to remove bead-bound cells, routinely yielding >90% T cells. Cells were washed and resuspended in culture medium consisting of RPMI-1640 supplemented with 25 mM HEPES, 2.5 mg/ml glucose (Sigma-Aldrich, St. Louis, MO, USA), 10 μg/ml folate (Invitrogen Life Technologies, Grand Island, NY, USA), 110.04 μg/ml pyruvate (Invitrogen Life Technologies), 5 × 10−5 M 2-ME (Sigma-Aldrich), 292.3 μg/ml glutamine (Invitrogen Life Technologies), 100 U/ml penicillin–streptomycin (Invitrogen Life Technologies), and 5% v/v BCS (Thermo Scientific Hyclone, Logan, UT, USA).
T-cell culture
T cells were activated in culture medium by plate-bound anti-CD3 clone 145-2C11 (10 μg/ml), soluble anti-CD28 ascites clone 37–51 (1 : 1000), and human recombinant IL-2 (50 U/ml; Cetus, Emeryville, CA, USA) for 2 days. Cells were then removed from anti-CD3 and fed with fresh medium plus IL-2. On day 3, cells were washed 3 times and recultured in either 50 U/ml IL-2 or 20 ng/ml human recombinant IL-15 (kind gift of Amgen, Thousand Oaks, CA, USA) for an additional 2 days. Where indicated, culture conditions included anti-CD3, 0.1 mM SNOC (Sodium nitrite (Acros Organics, Geel, Belgium) with L-cysteine hydrochloride monohydrate (Sigma-Aldrich)), or 10 mM N-acetylcysteine (Sigma-Aldrich).
Lymphopenia-induced proliferation model of T-cell homeostatic proliferation
For adoptive transfer of lymphocytes, 5 × 106 lymph node cells from C57BL/6 (CD90.1) mice were transferred intravenously via tail vein into Rag1−/− (CD90.2) mice. After 14 days, single-cell suspensions of spleen were prepared in RPMI 1640 (Mediatech, Inc.) containing 25 mM Hepes, 5% (v/v) BCS, 5 × 10−5 M β-mercaptoethanol, 100 U/ml penicillin, and 100 U/ml streptomycin (RPMI/5% BCS). Erythrocytes in splenic suspensions were lysed with Gey’s solution. Purified CD4+ and CD8+ donor T cells were obtained by cell sorting and analyzed for caspase activity.
Caspase activity assay
Relative caspase activities were determined using the Apo-ONE Assay (Promega, Madison, WI, USA), which measures the cleavage of DEVD-rhodamine, according to the manufacturer’s recommendations. Spectrophotometric readings were taken using a Fluorescence reader (Biotek Instruments, Winooski, VT, USA).
Immunoblot analysis
Viable cells were lysed in buffer containing 0.2% Nonidet P-40, 20 mM Tris-HCl (pH 7.4), 2 mM sodium orthovanadate, 10% glycerol, 150 mM NaCl, and complete protease inhibitor (Roche Diagnostics, Indianapolis, IN, USA). Protein concentration was determined by Bradford assay (Bio-Rad, Hercules, CA, USA). Protein lysates were boiled for 5 min in loading buffer containing 2-ME and were separated by SDS-PAGE using 10 or 12.5% gels, transferred onto polyvinylidene difluoride (PVDF) membranes (Bio-Rad), and blocked using 4% milk in Tris-buffered saline plus 0.1% Tween-20 (American Bioanalytical, Natick, MA, USA) at room temperature for 1 h. Membranes were incubated at 4°C overnight in milk/Tris-buffered saline (TBS)-Tween containing mouse anti-caspase-8 monoclonal antibody (a kind gift of A Strasser, The Walter and Eliza Hall Institute of Medical Research, Melbourne, Australia), rabbit anti-caspase-3 585 polyclonal antibody (the kind gift of Dr. Yuri Lazebnik, Cold Spring Harbor Laboratories, Cold Spring Harbor, NY, USA), mouse anti-caspase-9 monoclonal antibody (5B4) (Stressgen Assay Designs, Ann Arbor, MI, USA), rabbit anti-XIAP polyclonal antibody (Lifespan Biosciences, Seattle, WA, USA), rabbit anti-cIAP polyclonal antibody (Cell Signaling Technology, Danvers, MA, USA), rabbit anti-survivin monoclonal antibody (Cell Signaling Technology), rabbit anti-Smac/DIABLO polyclonal antibody (BioVision, Milpitas, CA, USA), mouse anti-CytC monoclonal antibody (BD, San Jose, CA, USA), rabbit anti-hexokinase II monoclonal antibody (Cell Signaling Technology), or mouse anti-actin monoclonal antibody (Sigma-Aldrich), rabbit anti-caspase-6 (Cell Signaling), rabbit anti-caspase-7 (Cell Signaling), mouse anti-RIPK1 (BD Transduction Laboratories), rabbit anti-RIPK3 (ProSci Incorporated, Poway, CA, USA), rat anti-Bim (Enzo Life Sciences, Farmingdale, NY, USA), rabbit anti-Bcl-xL (Cell Signaling), or mouse anti-Bcl-2 (Santa Cruz Biotechnology, Santa Cruz, CA, USA). Immunoreactive proteins were visualized using species-specific secondary antibodies conjugated to horseradish peroxidase (Santa Cruz Biotechnology; Southern Biotech, Birmingham, AL, USA; Biomeda, Foster City, CA, USA; Jackson Immunoresearch, West Grove, PA, USA) and developed using LumiGLO (KPL, Gaithersburg, MD, USA) or SuperSignal West Femto Chemiluminescent Substrate (ThermoScientific, Rockford, IL, USA). Quantitative assessment of band densities on immunoblots were performed using the Quantity-One software (Bio-Rad) and are displayed as the sum of the intensities of the pixels inside the volume boundary × area of a single pixel (in mm2) after correction for background signal.
Biotin-VAD-fmk active caspase precipitation assay
Cells were washed once with phosphate-buffered saline (PBS), and lysed in lysis buffer containing 20 μM biotin-VAD-fmk (bVAD) (MP Biomedicals, Solon, OH, USA). Then, 450 μg of protein lysate in 300 μl lysis buffer was precleared by rocking with 40 μl Sepharose 6B agarose beads (Sigma-Aldrich) at 4°C for 2 h. Supernatants were then rocked with 60 μl streptavidin-sepharose beads (Invitrogen Life Technologies) at 4°C overnight. Beads were washed 3 times in lysis buffer without protease inhibitor, then boiled in Laemmli loading buffer. Beads were removed by centrifugation and immunoblot analysis was performed on supernatants.
Flow cytometry
For direct surface staining, single-cell suspensions (1 × 106) were washed in cold (4°C) PBS containing 1% w/v bovine serum albumin (PBS/1% BSA) and then incubated with PE-Texas-Red-conjugated anti-CD4 Ab (Invitrogen/Caltag Laboratories, Carlsbad, CA, USA), PE-Cy5.5-conjugated anti-CD8α Ab (Invitrogen/Caltag), Alexa 647-conjugated CD3ε (Invitrogen/Caltag), PE-conjugated Fas (BD Biosciences, San Jose, CA, USA), or APC-conjugated TCR-β (BD Biosciences) diluted in PBS/1% BSA for 30 min on ice (4°C). Cells were then washed with cold PBS/1% BSA and fixed using freshly made 1% v/v methanol-free formaldehyde (Ted Pella, Redding, CA, USA) in PBS/1% BSA. To assess apoptosis, DNA strand breaks were measured by TUNEL staining. Briefly, single-cell suspensions were treated with or without plate-bound anti-CD3 for 4 h, and then washed twice with cold PBS. For TUNEL assay, surface staining was first completed as above, with the exception that cells were fixed on ice for 15 min using 1% formaldehyde in PBS. Cells were then washed twice with PBS, fixed in 70% ice-cold ethanol for 15 min, and then washed twice with PBS. Nicked DNA was labeled by incubating cells with labeling mix (1 × terminal deoxynucleotidyl transferase (TdT) buffer, 10 U TdT, 2.5 mM CoCl2, and 0.2 pmol/μl FITC-dUTP (Roche Diagnostics) in a total volume of 50 μl) at 37°C for 1 h. Cells were then washed twice with 1% BSA in PBS and fixed with 1% formaldehyde in PBS/1% BSA. Samples were then analyzed by flow cytometry.
For DCFDA staining (Invitrogen Life Technologies), T cells were resuspended in warm (37°C) PBS containing 1 μM DCFDA and incubated in a 37°C, 5% CO2 incubator for 30 min. Labeling was stopped by the addition of cold (4°C) PBS/1% BSA. The cells were then pelleted, resuspended in cold PBS/1% BSA, and analyzed without delay by flow cytometry.
For GLUT1 staining, T cells were stained for cell surface proteins for 30 min on ice, fixed with 1% formaldehyde in PBS for 15 min, and permeabilized with 0.03% saponin in PBS/1% BSA. All subsequent steps were done with 0.03% saponin in PBS/1% BSA. The cells were then incubated with or without anti-GLUT1 (MitoSciences, Eugene, OR, USA), washed, and then incubated with goat anti-rabbit Alexa 488 (Invitrogen/Molecular Probes). After washing, the cells were fixed with 1% formaldehyde in PBS/1% BSA and collected on an LSR II flow cytometer (BD) and analyzed by FlowJo software (Tree Star, Ashland, OR, USA).
For FoxP3+ cell sorting, the inguinal, brachial, axillary, cervical, and popliteal lymph nodes, and the spleen of C57BL/6 FoxP3GFP mice were harvested. Red blood cells were lysed with Gey’s solution and CD4+ T cells were purified by negative selection using the EasySep mouse CD4+ T cell isolation kit (STEMCELL Technologies, Vancouver, BC, Canada). The purified cells were stained for CD4 by incubating with APC-conjugated anti-CD4 antibody (Invitrogen/Caltag). Cells were passed through a 40 μm cell strainer (BD) before sorting for CD4+GFP+ and CD4+GFP- cells on a FACS Aria cell sorter (BD Biosciences).
For CD44+ cell sorting, T cells were purified from the lymph nodes and spleen of C57BL/6 mice by negative selection. The cells were stained with APC-conjugated anti-CD4 antibody (Invitrogen/Caltag), PE-conjugated anti-CD8 antibody (Invitrogen/Caltag), and FITC-conjugated anti-CD44 antibody (Invitrogen/Caltag). Cells were passed through a 40 μm cell strainer (BD Biosciences) before sorting for CD4+CD44low, CD4+CD44high, CD8+CD44low, and CD8+CD44high cells.
Metabolism and redox assays
NO, GSH, NADP(H), lactate, and NAD(H) measurements were performed with Quantification Kits from BioVision according to the manufacturer’s protocols. For NADP(H) assay, cells were washed with cold PBS, pelleted, and NADP(H) extracted with NADP/NADPH Extraction Buffer by freeze/thaw. Samples were incubated with NADP cycling enzyme and developer at room temperature on a shaker to detect total NADP/NADPH (NADPt). To detect NADPH only, NADP was decomposed by heating samples to 60°C for 30 min in a heating block. Readings were taken using a plate reader at OD 450 nm. For NAD(H) assay, cells were washed with cold PBS, pelleted, and NAD(H) extracted with NAD/NADH Extraction Buffer by freeze/thaw. Samples were incubated with NAD cycling enzyme and developer at room temperature on a shaker to detect total NAD/NADH (NADt). To detect NADH only, NAD was decomposed by heating samples to 60°C for 30 min in a heating block. Readings were taken using a plate reader at OD 450 nm. For GSH assay, cells were washed with cold PBS, pelleted, and resuspended in Glutathione Assay Buffer containing perchloric acid, 6N (PCA). Supernatants were neutralized with ice-cold 6N potassium hydroxide (KOH) for 5 min on ice and spun down to precipitate PCA. GSH was detected by the addition of OPA probe, which reacts with GSH and not GSSG to generate fluorescence, and readings were taken at Ex/Em=340/420 nm by Fluorescence reader (Biotek). Total GSH levels were determined by adding a reducing agent to convert GSSG to GSH. GSSG was quantified by adding a GSH quencher to remove GSH and then adding reducing agent to destroy excess quencher and convert GSSG to GSH. For NO assay, NO was measured based on quantitation of nitrite (NO2−) and nitrate (NO3−). Nitrate is converted to nitrite by nitrate reductase at room temperature for 4 h, probed with DAN (2,3-diaminonaphthalene), and read by a fluorometer (Biotek) at Ex/Em=360/450 nm. For lactate assay, cells were cultured at 5 × 106 cells/ml and supernatant samples collected between 1 and 4 h. Relative lactate measurements were determined according to the manufacturer’s protocol by using the Lactate Assay II Kit that measures the product generated by oxidation of lactate by lactate dehydrogenase at 450 nm via plate reader.
Biotin switch technique
S-Nitrosylation of caspase-3 was assessed by the biotin switch technique as previously described.17, 30, 31 In brief, day 5 lymphocytes cultured in either IL-2 or IL-15 were lysed in BST lysis buffer (25 mM HEPES (Sigma-Aldrich), 50 mM NaCl (Sigma-Aldrich), 0.1 mM EDTA, 1% NP-40, and 0.5 mM PMSF plus protease inhibitors, pH 7.4). Samples were quantitated by Bradford assay and 1.0 mg of protein was diluted with HEN buffer (100 mM HEPES, 1 mM EDTA, 0.1 mM neocuproine (Acros Organics), pH 8.0), 2.5% SDS, and 0.1% MMTS (Sigma-Aldrich) (prepared in dimethylformamide (DMF) (Sigma-Aldrich)) in a total volume of 2.0 ml and incubated at 50°C in the dark for 20 min with frequent vortexing to block free thiols by their methylation. Excess MMTS was removed by addition of three volumes of cold acetone (Mallincrodkt Chemicals, Hazelwood, MO, USA) for 20 min at −20°C followed by centrifugation and washing with 70% acetone four times. Samples were resuspended in HENS buffer (HEN buffer with 1% SDS (w/v)) in the presence of 200 mM sodium ascorbate to reduce nitrosothiols to free thiols and then the free thiols were biotinylated using biotin-HPDP (2.5 mg/ml) (Invitrogen Life Technologies) at room temperature in the dark by rocking for 1 h. DMSO was used as vehicle control for biotin-HPDP and 200 mM NaCl was used as an ascorbate-free control. Samples were precipitated in three volumes of cold acetone for 20 min at −20°C, spun down, and washed four times with 70% acetone to remove residual biotin-HPDP and ascorbate. Subsequently, samples were resuspended in 0.25 ml HENS/10 buffer and 0.75 ml of neutralization buffer (20 mM HEPES pH 7.7, 100 mM NaCl, 1 mM EDTA, and 0.5% Triton X-100), and rotated with 60 μl of prewashed streptavidin-sepharose beads (Invitrogen Life Technologies) at 4°C overnight. Beads were washed 4 times with neutralization buffer containing 600 mM NaCl and eluted with HENS/10 buffer containing Laemmli loading buffer with 2-ME by boiling. Beads were removed by centrifugation and the samples were resolved by SDS-PAGE and transferred for immunoblotting. For input samples, 10 μl of samples resuspended in HENS/10 and neutralization buffer were added to Laemmli buffer and run on SDS-PAGE.
Statistical analyses
Statistical analyses as indicated in the figure legends were conducted using GraphPad Prism 6 software (GraphPad Prism, Inc., La Jolla, CA, USA).
Abbreviations
- AICD:
-
activation-induced cell death
- BCS:
-
bovine calf serum
- biotin-HPDP:
-
N-[6-(biotinamido)hexyl]-3′-(2′-pyridyldithio) propionamide
- BSA:
-
bovine serum albumin
- BST:
-
biotin switch technique
- bVAD:
-
biotin-VAD-fmk
- DAN:
-
2,3-Diamino-naphthalene
- DCFDA:
-
5-(and -6)carboxy-20,70-dichlorodihydrofluorescein diacetate
- DMF:
-
dimethylformamide
- GLUT1:
-
glucose transporter 1
- GSH:
-
glutathione
- GSNO:
-
S-nitrosoglutathione
- IAP:
-
inhibitor of apoptosis
- IL-15:
-
interleukin-15
- IL-2:
-
interleukin-2
- iNOS:
-
inducible nitric oxide synthase
- KOH:
-
potassium hydroxide
- MMTS:
-
methylmethanethiosulfonate
- NAc:
-
N-acetylcysteine
- NO:
-
nitric oxide
- NO2−:
-
nitrite
- NO3−:
-
nitrate
- ONOO−:
-
peroxynitrite
- OPA:
-
o-phthalaldehyde
- PBS:
-
phosphate-buffered saline
- PCA:
-
perchloric acid
- Redox:
-
reduction-oxidation
- ROS:
-
reactive oxygen species
- SDS:
-
sodium dodecyl sulfate
- SNOC:
-
S-nitrosylated-cysteine
- TBS:
-
Tris-buffered saline
- TCR:
-
T cell receptor
References
Malek TR . The biology of interleukin-2. Annu Rev Immunol 2008; 26: 453–479.
Tagaya Y, Bamford RN, DeFilippis AP, Waldmann TA . IL-15: a pleiotropic cytokine with diverse receptor/signaling pathways whose expression is controlled at multiple levels. Immunity 1996; 4: 329–336.
Oh S, Perera LP, Terabe M, Ni L, Waldmann TA, Berzofsky JA . IL-15 as a mediator of CD4+ help for CD8+ T cell longevity and avoidance of TRAIL-mediated apoptosis. Proc Natl Acad Sci USA 2008; 105: 5201–5206.
Waldmann T . The contrasting roles of IL-2 and IL-15 in the life and death of lymphocytes: implications for the immunotherapy of rheumatological diseases. Arthritis Res 2002; 4 (Suppl 3): S161–S167.
Lenardo MJ . Interleukin-2 programs mouse alpha beta T lymphocytes for apoptosis. Nature 1991; 353: 858–861.
Brincks EL, Woodland DL . Novel roles for IL-15 in T cell survival. F1000 Biol Rep 2010; 2: 67.
Marks-Konczalik J, Dubois S, Losi JM, Sabzevari H, Yamada N, Feigenbaum L et al. IL-2-induced activation-induced cell death is inhibited in IL-15 transgenic mice. Proc Natl Acad Sci USA 2000; 97: 11445–11450.
Kennedy NJ, Kataoka T, Tschopp J, Budd RC . Caspase activation is required for T cell proliferation. J Exp Med 1999; 190: 1891–1896.
Chun HJ, Zheng L, Ahmad M, Wang J, Speirs CK, Siegel RM et al. Pleiotropic defects in lymphocyte activation caused by caspase-8 mutations lead to human immunodeficiency. Nature 2002; 419: 395–399.
Salmena L, Lemmers B, Hakem A, Matysiak-Zablocki E, Murakami K, Au PY et al. Essential role for caspase 8 in T-cell homeostasis and T-cell-mediated immunity. Genes Dev 2003; 17: 883–895.
Fuentes-Prior P, Salvesen GS . The protein structures that shape caspase activity, specificity, activation and inhibition. Biochem J 2004; 384 (Pt 2): 201–232.
Mannick JB . Regulation of apoptosis by protein S-nitrosylation. Amino Acids 2007; 32: 523–526.
Lane P, Hao G, Gross SS . S-nitrosylation is emerging as a specific and fundamental posttranslational protein modification: head-to-head comparison with O-phosphorylation. Sci STKE 2001; 2001: re1.
Mitchell DA, Morton SU, Fernhoff NB, Marletta MA . Thioredoxin is required for S-nitrosation of procaspase-3 and the inhibition of apoptosis in Jurkat cells. Proc Natl Acad Sci USA 2007; 104: 11609–11614.
Mannick JB, Schonhoff C, Papeta N, Ghafourifar P, Szibor M, Fang K et al. S-Nitrosylation of mitochondrial caspases. J Cell Biol 2001; 154: 1111–1116.
Kaur N, Naga OS, Norell H, Al-Khami AA, Scheffel MJ, Chakraborty NG et al. T cells expanded in presence of IL-15 exhibit increased antioxidant capacity and innate effector molecules. Cytokine 2011; 55: 307–317.
Jaffrey SR, Erdjument-Bromage H, Ferris CD, Tempst P, Snyder SH . Protein S-nitrosylation: a physiological signal for neuronal nitric oxide. Nat Cell Biol 2001; 3: 193–197.
Mitchell DA, Marletta MA . Thioredoxin catalyzes the S-nitrosation of the caspase-3 active site cysteine. Nat Chem Biol 2005; 1: 154–158.
Warburg O . On the origin of cancer cells. Science 1956; 123: 309–314.
Cornish GH, Sinclair LV, Cantrell DA . Differential regulation of T-cell growth by IL-2 and IL-15. Blood 2006; 108: 600–608.
Pearce EL, Walsh MC, Cejas PJ, Harms GM, Shen H, Wang LS et al. Enhancing CD8 T-cell memory by modulating fatty acid metabolism. Nature 2009; 460: 103–107.
van der Windt GJ, Everts B, Chang CH, Curtis JD, Freitas TC, Amiel E et al. Mitochondrial respiratory capacity is a critical regulator of CD8+ T cell memory development. Immunity 2012; 36: 68–78.
Hubner A, Barrett T, Flavell RA, Davis RJ . Multisite phosphorylation regulates Bim stability and apoptotic activity. Mol Cell 2008; 30: 415–425.
Deveraux QL, Reed JC . IAP family proteins—suppressors of apoptosis. Genes Dev 1999; 13: 239–252.
Srinivasula SM, Datta P, Fan XJ, Fernandes-Alnemri T, Huang ZW, Alnemri ES . Molecular determinants of the caspase-promoting activity of Smac/DIABLO and its role in the death receptor pathway. J Biol Chem 2000; 275: 36152–36157.
Feoktistova M, Geserick P, Kellert B, Dimitrova DP, Langlais C, Hupe M et al. cIAPs block Ripoptosome formation, a RIP1/caspase-8 containing intracellular cell death complex differentially regulated by cFLIP isoforms. Mol Cell 2011; 43: 449–463.
Denault JB, Salvesen GS . Human caspase-7 activity and regulation by its N-terminal peptide. J Biol Chem 2003; 278: 34042–34050.
Wherry EJ, Becker TC, Boone D, Kaja MK, Ma A, Ahmed R . Homeostatic proliferation but not the generation of virus specific memory CD8 T cells is impaired in the absence of IL-15 or IL-15Ralpha. Adv Exp Med Biol 2002; 512: 165–175.
Mannick JB, Hausladen A, Liu LM, Hess DT, Zeng M, Miao QX et al. Fas-induced caspase denitrosylation. Science 1999; 284: 651–654.
Forrester MT, Foster MW, Benhar M, Stamler JS . Detection of protein S-nitrosylation with the biotin-switch technique. Free Radic Biol Med 2009; 46: 119–126.
Jaffrey SR, Snyder SH . The biotin switch method for the detection of S-nitrosylated proteins. Sci STKE 2001; 2001: pl1.
Martinez-Ruiz A, Lamas S . Signalling by NO-induced protein S-nitrosylation and S-glutathionylation: convergences and divergences. Cardiovasc Res 2007; 75: 220–228.
Dickinson DA, Forman HJ . Glutathione in defense and signaling: lessons from a small thiol. Ann NY Acad Sci 2002; 973: 488–504.
Misra RS, Jelley-Gibbs DM, Russell JQ, Huston G, Swain SL, Budd RC . Effector CD4+ T cells generate intermediate caspase activity and cleavage of caspase-8 substrates. J Immunol 2005; 174: 3999–4009.
Beisner DR, Ch'en IL, Kolla RV, Hoffmann A, Hedrick SM . Cutting edge: innate immunity conferred by B cells is regulated by caspase-8. J Immunol 2005; 175: 3469–3473.
Kang TB, Ben-Moshe T, Varfolomeev EE, Pewzner-Jung Y, Yogev N, Jurewicz A et al. Caspase-8 serves both apoptotic and nonapoptotic roles. J Immunol 2004; 173: 2976–2984.
Liadis N, Salmena L, Kwan E, Tajmir P, Schroer SA, Radziszewska A et al. Distinct in vivo roles of caspase-8 in beta-cells in physiological and diabetes models. Diabetes 2007; 56: 2302–2311.
Bidere N, Snow AL, Sakai K, Zheng L, Lenardo MJ . Caspase-8 regulation by direct interaction with TRAF6 in T cell receptor-induced NF-kappaB activation. Curr Biol 2006; 16: 1666–1671.
Misra RS, Russell JQ, Koenig A, Hinshaw-Makepeace JA, Wen R, Wang D et al. Caspase-8 and c-FLIPL associate in lipid rafts with NF-kappaB adaptors during T cell activation. J Biol Chem 2007; 282: 19365–19374.
Koenig A, Russell JQ, Rodgers WA, Budd RC . Spatial differences in active caspase-8 defines its role in T-cell activation versus cell death. Cell Death Differ 2008; 15: 1701–1711.
Weinlich R, Dillon CP, Green DR . Ripped to death. Trends Cell Biol 2011; 21: 630–637.
Green DR, Oberst A, Dillon CP, Weinlich R, Salvesen GS . RIPK-dependent necrosis and its regulation by caspases: a mystery in five acts. Mol Cell 2011; 44: 9–16.
Berard M, Brandt K, Bulfone-Paus S, Tough DF . IL-15 promotes the survival of naive and memory phenotype CD8+ T cells. J Immunol 2003; 170: 5018–5026.
Sandau MM, Kohlmeier JE, Woodland DL, Jameson SC . IL-15 regulates both quantitative and qualitative features of the memory CD8 T cell pool. J Immunol 2010; 184: 35–44.
Cantrell DA, Smith KA . Transient expression of interleukin 2 receptors. Consequences for T cell growth. J Exp Med 1983; 158: 1895–1911.
Thai PT, Collins CC, Fortner KA, Koenig A, Hayes SM, Budd RC . Increased caspase activity primes human Lyme arthritis synovial gammadelta T cells for proliferation and death. Hum Immunol 2011; 72: 1168–1175.
Aouad SM, Cohen LY, Sharif-Askari E, Haddad EK, Alam A, Sekaly RP . Caspase-3 is a component of Fas death-inducing signaling complex in lipid rafts and its activity is required for complete caspase-8 activation during Fas-mediated cell death. J Immunol 2004; 172: 2316–2323.
Rossig L, Fichtlscherer B, Breitschopf K, Haendeler J, Zeiher AM, Mulsch A et al. Nitric oxide inhibits caspase-3 by S-nitrosation in vivo. J Biol Chem 1999; 274: 6823–6826.
Hernansanz-Agustin P, Izquierdo-Alvarez A, Garcia-Ortiz A, Ibiza S, Serrador JM, Martinez-Ruiz A . Nitrosothiols in the immune system: signaling and protection. Antioxid Redox Signal 2013; 18: 288–308.
Kruger NJ, von Schaewen A . The oxidative pentose phosphate pathway: structure and organisation. Curr Opin Plant Biol 2003; 6: 236–246.
Bobbala D, Chen XL, Leblanc C, Mayhue M, Stankova J, Tanaka T et al. Interleukin-15 plays an essential role in the pathogenesis of autoimmune diabetes in the NOD mouse. Diabetologia 2012; 55: 3010–3020.
Kivisakk P, Matusevicius D, He B, Soderstrom M, Fredrikson S, Link H . IL-15 mRNA expression is up-regulated in blood and cerebrospinal fluid mononuclear cells in multiple sclerosis (MS). Clin Exp Immunol 1998; 111: 193–197.
Kuczynski S, Winiarska H, Abramczyk M, Szczawinska K, Wierusz-Wysocka B, Dworacka M . IL-15 is elevated in serum patients with type 1 diabetes mellitus. Diabetes Res Clin Pract 2005; 69: 231–236.
Aringer M, Stummvoll GH, Steiner G, Koller M, Steiner CW, Hofler E et al. Serum interleukin-15 is elevated in systemic lupus erythematosus. Rheumatology (Oxford) 2001; 40: 876–881.
Liu Z, Geboes K, Colpaert S, D'Haens GR, Rutgeerts P, Ceuppens JL . IL-15 is highly expressed in inflammatory bowel disease and regulates local T cell-dependent cytokine production. J Immunol 2000; 164: 3608–3615.
Maiuri L, Ciacci C, Auricchio S, Brown V, Quaratino S, Londei M . Interleukin 15 mediates epithelial changes in celiac disease. Gastroenterology 2000; 119: 996–1006.
McInnes IB, al-Mughales J, Field M, Leung BP, Huang FP, Dixon R et al. The role of interleukin-15 in T-cell migration and activation in rheumatoid arthritis. Nat Med 1996; 2: 175–182.
Thurkow EW, van der Heijden IM, Breedveld FC, Smeets TJ, Daha MR, Kluin PM et al. Increased expression of IL-15 in the synovium of patients with rheumatoid arthritis compared with patients with Yersinia-induced arthritis and osteoarthritis. J Pathol 1997; 181: 444–450.
Baslund B, Tvede N, Danneskiold-Samsoe B, Larsson P, Panayi G, Petersen J et al. Targeting interleukin-15 in patients with rheumatoid arthritis: a proof-of-concept study.[see comment]. Arthritis Rheum 2005; 52: 2686–2692.
Migita K, Yamasaki S, Kita M, Ida H, Shibatomi K, Kawakami A et al. Nitric oxide protects cultured rheumatoid synovial cells from Fas-induced apoptosis by inhibiting caspase-3. Immunology 2001; 103: 362–367.
Acknowledgements
We thank Ms. Colette Charland and Dr. Roxana del Rio Guerra for technical assistance with flow cytometry. This work was supported by grants from the National Institutes of Health (AI36333, AR43520, AI45666, and AI055402).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Edited by G Salvesen
Supplementary Information accompanies this paper on Cell Death and Differentiation website
Supplementary information
Rights and permissions
About this article
Cite this article
Saligrama, P., Fortner, K., Secinaro, M. et al. IL-15 maintains T-cell survival via S-nitrosylation-mediated inhibition of caspase-3. Cell Death Differ 21, 904–914 (2014). https://doi.org/10.1038/cdd.2014.10
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/cdd.2014.10
Keywords
This article is cited by
-
Mitochondrial reactive oxygen is critical for IL-12/IL-18-induced IFN-γ production by CD4+ T cells and is regulated by Fas/FasL signaling
Cell Death & Disease (2022)
-
Glycolysis promotes caspase-3 activation in lipid rafts in T cells
Cell Death & Disease (2018)
-
HIV-1 pathogenicity and virion production are dependent on the metabolic phenotype of activated CD4+ T cells
Retrovirology (2014)