Abstract
Background:
Multiple myeloma (MM) is an incurable clonal plasma cell malignancy. The constitutive expression of HIF-1α in MM suggests that inhibition of HIF-1α-mediated transcription represents an interesting target in MM.
Methods:
As p300 is a crucial co-activator of hypoxia-inducible transcription, disrupting the complex HIF-1α/p300 to target HIF activity appears to be an attractive strategy.
Results:
We reported that chetomin, an inhibitor of HIF-1α/p300 interaction, exhibits antitumour activity in human myeloma cell lines and primary MM cells from patients.
Conclusions:
Our data suggest that chetomin may be of clinical value in MM and especially for patients characterised by a high EP300/HIF -1α expression and a poor prognosis.
Similar content being viewed by others
Main
Multiple myeloma (MM), characterised by the clonal expansion of plasma cells within the bone marrow, is the second most common haematological malignancy. MM murine models have shown that hypoxia is a specific feature of MM with a significant increased hypoxia marker hypoxia-inducible factor-1 (HIF-1) α in the bone morrow of MM tumour-bearing mice (Hu et al, 2012). HIF1-α was reported to be expressed in 95.4% of CD138-purified myeloma cell samples from 329 previously untreated myeloma patients (Hose et al, 2010). Myeloma cells of patients with t(4;14) translocations showed a significantly higher HIF-1α expression, whereas a significant downregulation of HIF-1α expression was described in hyperdiploid patients (Hose et al, 2010). Two independent studies (Colla et al, 2007; Martin et al, 2011) also confirmed the expression of HIF in bone marrow biopsy specimens from MM patients. HIF-1α is overexpressed in MM cells growing within the bone marrow hypoxic environment. Recent studies have explored the effects of HIF-1α inhibition in MM. Using HIF-1α shRNA, Storti et al (2013) reported a limited impact on MM cell proliferation and survival in vitro. However, a significant inhibition of MM-induced angiogenesis and bone lesions was reported in vivo (Storti et al, 2013). Treatment of MM cells with a small antisense oligonucleotide against HIF-1α (EZN-2968) was shown to induce cell cycle arrest and a metabolic shift with a significant increased of ATP production by oxidative phosphorylation (Borsi et al, 2014).
HIF-1 is a heterodimeric transcription factor constituted of O2-regulated HIF-1α and constitutively expressed HIF-1β subunits. In hypoxic conditions, HIF-1α is translocated into nucleus, heterodimerises with HIF-1β and binds to hypoxia response element DNA sequence. Then, p300/CBP co-activators are recruited to initiate the transcription of a diverse group of genes involved in hypoxia response (Semenza, 2010).
According to these data, disrupting the complex formed by HIF-1α and p300 appeared to be a promising approach to target HIF (Colla et al, 2007; Storti et al, 2013; Reece et al, 2014).
Chetomin, a metabolite complex, produced by several fungi of the genus Chaetomium, disrupts the ability of tumours to adapt to hypoxia by blocking the HIF pathway (Kung et al, 2004). Chetomin targets p300, a transcriptional co-activator, by disrupting the structure of its CH1 domain. By this way, chetomin makes impossible the interaction between p300 and HIF-1α, which results, in a mitigation of hypoxia-inducible transcription (Kung et al, 2004; Reece et al, 2014). The aim of this study was to investigate the interest of chetomin as a strategy for MM treatment.
Materials and methods
Human myeloma cell lines (HMCL, n=10) XG-1, XG-2, XG-6, XG-7, XG-11, XG-13, XG-19, RPMI8226, LP1 and SKMM2 were obtained as previously described (Moreaux et al, 2011) or purchased from Deutsche Sammlung von Mikroorganismen und Zellkulturen GmbH (Braunschweig, Germany) and American Type Culture Collection (Rockville, MD, USA). Microarray data are deposited in the ArrayExpress public database (accession numbers E-TABM-937 and E-TABM-1088). Human myeloma cell lines were cultured with graded chetomin (Sigma, St Louis, MO, USA) concentrations. Human myeloma cell lines’ cell growth was quantified with a Cell Titer Glo Luminescent Assay (Promega, Madison, WI, USA) and the 50% inhibitory concentration (IC50) was determined using GraphPad Prism software (http://www.graphpad.com/scientific-software/prism/).
Gene expression profiling (GEP) of MM cells (MMCs) were obtained from two independent large patients’ cohorts: the Heidelberg–Montpellier (HM, N=206) cohort (ArrayExpress public database under accession number E-MTAB-362) (Hose et al, 2011; Moreaux et al, 2012) and the publicly available cohort from University of Arkansas for Medical Sciences (UAMS, Little Rock, AR, USA, cohort treated with total therapy 2, N=345) (GEO, http://www.ncbi.nlm.nih.gov/geo/, accession number GSE2658). Patients presenting with previously untreated MM (N=206) at the university hospitals of Heidelberg and Montpellier have been included in the study approved by the ethics committee of Montpellier (DC-2008-417) and Heidelberg after written informed consent in accordance with the Declaration of Helsinki. Clinical parameters and treatment regimens of the MM patients included in the HM cohort were previously described (Moreaux et al, 2012).
Bone marrow of patients presenting with previously untreated MM (N=6) at the university hospital of Montpellier was obtained after patients’ written informed consent in accordance with the Declaration of Helsinki and agreement of the Montpellier University Hospital Centre for Biological Resources (DC-2008-417). Primary myeloma cells of patients were cultured with or without graded concentrations of chetomin and MMC cytotoxicity evaluated using anti-CD138-phycoerythrin monoclonal antibody (Immunotech, Marseille, France) as described (Moreaux et al, 2012).
Results
A combined high HIF-1α and EP300 expression, defined using maxstat R package (Kassambara et al, 2012), in MMCs could predict for shorter overall survival (OS) in two independent cohorts of patients (P=0.007 in the HM cohort N=206 and P=0.0001 in the UAMS-TT2 cohort N=345) (Figure 1). Low-risk patients accounted for 85.4% with a not reached median OS and high-risk patients for 14.6% with a median OS of 54.9 months in the HM cohort (Figure 1). In the UAMS TT2 cohort, a significant better survival in EP300low/HIF-1αlow group (median OS not reached) compared with EP300high/HIF-1αhigh group (median OS of 56 months; P=0.0001) was also found (Figure 1). Patients with EP300high/HIF-1αhigh expression have also a median event-free survival significantly decreased compared with those of the EP300low/HIF-1αlow group (P=0.004 in the HM cohort and P=0.0003 in the UAMS TT2 cohort).
High HIF1-α and EP300 expression in MMCs could predict for shorter overall and event-free survival (EFS) in two independent cohorts of patients. (A) Patients of the HM cohort (n=206) were ranked according to increasing EP300 and HIF-1α expression and a maximum difference in OS was obtained using the Maxstat R function. High EP300 and HIF-1α expression is also associated with a shorter EFS in the HM cohort (n=206). (B) High EP300 and HIF1α expression is associated with a poor prognosis (OS and EFS) in an independent cohort of 345 patients (LR-TT2 cohort).
Gene set enrichment analysis was performed to compare gene expression profiles of patients myeloma cells with EP300high/HIF-1αhigh and EP300low/HIF-1αlow expression. Genes overexpressed in MM molecular subgroups CD2 and LB (low bone disease) were significantly enriched in EP300low/HIF-1αlow group (Supplementary Figure S1A and Supplementary Tables S1 and S2). Patients of the EP300high/HIF-1αhigh group were characterised by a significant enrichment of genes related to embryonic stem cells, DNMT1 targets and proliferation (Supplementary Figure S1B and Supplementary Tables S3–S5). According to these data, we investigated the correlation between HIF-1 α /EP300 expression and MM plasma cell labelling index or GEP-based growth proliferation index (GPI) (Hose et al, 2011). No significant correlation between HIF-1α/EP300 expression and myeloma cell proliferation or GPI was identified (Supplementary Figures S2A and B).
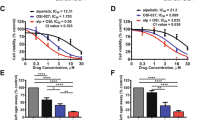
The effect of chetomin was investigated in 10 different HMCL representative of the patients’ molecular heterogeneity (Moreaux et al, 2011). Chetomin induced a dose-dependent inhibition of cell growth in all investigated HMCL, independently of the their molecular heterogeneity (Moreaux et al, 2011) with a median IC50 of 4.1 nM (range: 2.29–6.89 nM) (Figure 2A). Chetomin treatment induced a significant downregulation of HIF-1α target gene expression (Storti et al, 2013) including VEGF, IL-8 and CLL3 proangiogenic genes (Supplementary Figure S3).
Chetomin, induces a dose-dependent inhibition of cell growth in HMCL and has toxic effects on primary myeloma cells. (A) HMCL were cultured for 4 days in 96-well flat-bottom microtitre plates in RPMI 1640 medium, 10% fetal calf serum, 2 ng ml−1 interleukin six culture medium (control), and graded concentrations of chetomin. At day 4 of culture, the viability was assessed by CellTiter-Glo Luminescent Cell Viability Assay. The IC50 (concentration responsible for 50% of the maximal inhibitory effect), was determined using GraphPad PRISM software. Data are mean values±s.d. of five experiments determined on sextuplet culture wells. (B) Mononuclear cells from six patients with MM were cultured for 4 days in the presence of IL-6 (1 ng ml−1) with or without graded concentrations of chetomin. At day 4 of culture, the viability and total cell count were assessed and the percentage of (i) CD138+ viable plasma cells, (ii) CD34+ haematopoietic stem cells and (iii) non-myeloma cells was determined by flow cytometry. Results are median values from six patients.
We investigated the effects of chetomin on primary MM cells of patients (n=6, Supplementary Table S6) co-cultured with their bone marrow microenvironment (Moreaux et al, 2012). After 4 days of treatment, cells were enumerated and the fraction of viable myeloma cells (CD138+), non-myeloma cells (CD138−) and haematopoietic progenitors (CD34+) were determined by flow cytometry (Moreaux et al, 2012). Chetomin induced a dose-dependent toxicity on myeloma cells of patients, with a median IC50 of 1.56 nM without affecting the survival of bone marrow normal cells or CD34+ haematopoietic stem cells (Figure 2B). Interestingly, EP300high/HIF-1αhigh myeloma cells of patient 2 were the most sensitive to low-dose chetomin treatment (Supplementary Table S6 and Supplementary Figure S5).
It was previously demonstrated that HIF-1α suppression enhanced the anti-myeloma activity of melphalan and lenalidomide treatment (Hu et al, 2009; Storti et al, 2013). Low concentration of chetomin (2 nM) significantly enhanced the anti-myeloma activity of lenalidomide and melphalan (Supplementary Figures S4A and B).
Discussion
According to our data, targeting HIF-1α/p300 interaction appears to be a potent strategy to target hypoxia pathway in MM. Studies have demonstrated a positive regulation of HIF1-α by MM growth factors including IL6 and IGF-1 (Borsi et al, 2014), a correlation between HIF1-α expression and MYC deregulation (Zhang et al, 2009) and a role of HIF1-α in MM angiogenesis through stimulation of VEGF (Asosingh et al, 2005; Colla et al, 2007). Interestingly, chetomin treatment induced a significant anti-myelomatous activity without toxicity on bone marrow normal cells and haematopoietic progenitors (Figure 2B).
High HIF-1α and EP300 expression are associated with a poor prognostic value in MM (Figure 1). The prognostic value of EP300high/HIF-1αhigh expression was compared with usual prognostic factors—ISS, t(4;14), del17p, or published GEP-based risk scores including UAMS-HRS, IFM score, GPI and RS score. Using univariate Cox analysis on HM cohort, all these factors had prognostic value (Supplementary Table S6). When these parameters were compared two by two, EP300high/HIF-1αhigh expression tested with β2m, del17p, GPI, HRS, IFM and ISS remained significant (Supplementary Table S6). When tested together, only RS, β2m and t(4;14) kept prognostic value (Supplementary Table S6). EP300high/HIF-1αhigh MM patients are characterised by a significant enrichment of genes related to proliferation, stemness and DNMT1 targets. We previously reported genes signatures shared by MM cells and normal stem cells linked with a prognostic value and that might be important in malignant stem cell biology (Kassambara et al, 2012). Furthermore, HIF-1α represents an interesting target to eradicate cancer stem cells in haematological malignancies including lymphoma and acute myeloid leukemia (Wang et al, 2011). Chetomin could be useful to target MM patients with a high myeloma-stem cell score and aggressive disease (Kassambara et al, 2012). Furthermore, chetomin enhances the toxicity of lenalidomide and melphalan on MM cells as previously reported with HIF-1-α depletion (Hu et al, 2009; Storti et al, 2013).
Altogether, these data suggest that chetomin or HIF-1-α/p300 inhibitors may be of clinical value in MM and especially for patients characterised by a high EP300 and HIF-1α expression.
Accession codes
Change history
01 March 2016
This paper was modified 12 months after initial publication to switch to Creative Commons licence terms, as noted at publication
References
Asosingh K, de Raeve H, de Ridder M, Storme GA, Willems A, van Riet I, van Camp B, Vanderkerken K (2005) Role of the hypoxic bone marrow microenvironment in 5T2MM murine myeloma tumor progression. Haematologica 90 (6): 810–817.
Borsi E, Perrone G, Terragna C, Martello M, Dico AF, Solaini G, Baracca A, Sgarbi G, Pasquinelli G, Valente S, Zamagni E, Tacchetti P, Martinelli G, Cavo M (2014) Hypoxia inducible factor-1 alpha as a therapeutic target in multiple myeloma. Oncotarget 5 (7): 1779–1792.
Colla S, Tagliaferri S, Morandi F, Lunghi P, Donofrio G, Martorana D, Mancini C, Lazzaretti M, Mazzera L, Ravanetti L, Bonomini S, Ferrari L, Miranda C, Ladetto M, Neri TM, Neri A, Greco A, Mangoni M, Bonati A, Rizzoli V, Giuliani N (2007) The new tumor-suppressor gene inhibitor of growth family member 4 (ING4) regulates the production of proangiogenic molecules by myeloma cells and suppresses hypoxia-inducible factor-1 alpha (HIF-1alpha) activity: involvement in myeloma-induced angiogenesis. Blood 110 (13): 4464–4475.
Hose D, Reme T, Hielscher T, Moreaux J, Messner T, Seckinger A, Benner A, Shaughnessy JD Jr ., Barlogie B, Zhou Y, Hillengass J, Bertsch U, Neben K, Mohler T, Rossi JF, Jauch A, Klein B, Goldschmidt H (2011) Proliferation is a central independent prognostic factor and target for personalized and risk-adapted treatment in multiple myeloma. Haematologica 96 (1): 87–95.
Hose D, Seckinger A, Goldschmidt H, Meissner T, Leber B, Neben K, Hillengass J, Bertsch U, Janssen B, Klein B, Lewis J, Vanderkerken K, Schultes C (2010) A novel class of sulfonanilides entering clinical trials for targeted treatment of multiple myeloma: dual-mechanism compounds inhibiting hif1a-signaling and inducing apoptosis. ASH Annual Meeting Abstracts 116 (21): 2987.
Hu J, Van Valckenborgh E, Menu E, De Bruyne E, Vanderkerken K (2012) Understanding the hypoxic niche of multiple myeloma: therapeutic implications and contributions of mouse models. Dis Model Mech 5 (6): 763–771.
Hu Y, Kirito K, Yoshida K, Mitsumori T, Nakajima K, Nozaki Y, Hamanaka S, Nagashima T, Kunitama M, Sakoe K, Komatsu N (2009) Inhibition of hypoxia-inducible factor-1 function enhances the sensitivity of multiple myeloma cells to melphalan. Mol Cancer Ther 8 (8): 2329–2338.
Kassambara A, Hose D, Moreaux J, Reme T, Torrent J, Rossi JF, Goldschmidt H, Klein B (2012) Identification of pluripotent and adult stem cell genes unrelated to cell cycle and associated with poor prognosis in multiple myeloma. PLoS One 7 (7): e42161.
Kung AL, Zabludoff SD, France DS, Freedman SJ, Tanner EA, Vieira A, Cornell-Kennon S, Lee J, Wang B, Wang J, Memmert K, Naegeli HU, Petersen F, Eck MJ, Bair KW, Wood AW, Livingston DM (2004) Small molecule blockade of transcriptional coactivation of the hypoxia-inducible factor pathway. Cancer Cell 6 (1): 33–43.
Martin SK, Diamond P, Gronthos S, Peet DJ, Zannettino AC (2011) The emerging role of hypoxia, HIF-1 and HIF-2 in multiple myeloma. Leukemia 25 (10): 1533–1542.
Moreaux J, Klein B, Bataille R, Descamps G, Maiga S, Hose D, Goldschmidt H, Jauch A, Reme T, Jourdan M, Amiot M, Pellat-Deceunynck C (2011) A high-risk signature for patients with multiple myeloma established from the molecular classification of human myeloma cell lines. Haematologica 96 (4): 574–582.
Moreaux J, Reme T, Leonard W, Veye JL, Requirand G, Goldschmidt H, Hose D, Klein B (2012) Development of gene expression-based score to predict sensitivity of multiple myeloma cells to DNA methylation inhibitors. Mol Cancer Ther 11 (12): 2685–2692.
Reece KM, Richardson ED, Cook KM, Campbell TJ, Pisle ST, Holly AJ, Venzon DJ, Liewehr DJ, Chau CH, Price DK, Figg WD (2014) Epidithiodiketopiperazines (ETPs) exhibit in vitro antiangiogenic and in vivo antitumor activity by disrupting the HIF-1alpha/p300 complex in a preclinical model of prostate cancer. Mol Cancer 13: 91.
Semenza GL (2010) Defining the role of hypoxia-inducible factor 1 in cancer biology and therapeutics. Oncogene 29 (5): 625–634.
Storti P, Bolzoni M, Donofrio G, Airoldi I, Guasco D, Toscani D, Martella E, Lazzaretti M, Mancini C, Agnelli L, Patrene K, Maiga S, Franceschi V, Colla S, Anderson J, Neri A, Amiot M, Aversa F, David Roodman G, Giuliani N (2013) Hypoxia-inducible factor (HIF)-1alpha suppression in myeloma cells blocks tumoral growth in vivo inhibiting angiogenesis and bone destruction. Leukemia 27 (8): 1697–1706.
Wang Y, Liu Y, Malek SN, Zheng P, Liu Y (2011) Targeting HIF1alpha eliminates cancer stem cells in hematological malignancies. Cell Stem Cell 8 (4): 399–411.
Zhang J, Sattler M, Tonon G, Grabher C, Lababidi S, Zimmerhackl A, Raab MS, Vallet S, Zhou Y, Cartron MA, Hideshima T, Tai YT, Chauhan D, Anderson KC, Podar K (2009) Targeting angiogenesis via a c-Myc/hypoxia-inducible factor-1alpha-dependent pathway in multiple myeloma. Cancer Res 69 (12): 5082–5090.
Acknowledgements
This work was supported by grants from French INCA (Institut National du Cancer) Institute (2012-109/087437), Languedoc Roussillon CRLR (R14026FF), Fondation de France (201400047510), ITMO Cancer (MM&TT) and AXLR SATT (30041633). EV is supported by a grant from Guillaume Espoir association (Saint- Genis-Laval, France).
Author contributions
EV performed research, bioinformatic studies and participated in the writing of the paper. CG participated in the research. HG, AS and DH participated in clinical data analysis and writing of the paper. BK participated in the research and in the writing of the paper. JM supervised the research, bioinformatic studies and the writing of the paper.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies this paper on British Journal of Cancer website
Rights and permissions
From twelve months after its original publication, this work is licensed under the Creative Commons Attribution-NonCommercial-Share Alike 4.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-sa/4.0/
About this article
Cite this article
Viziteu, E., Grandmougin, C., Goldschmidt, H. et al. Chetomin, targeting HIF-1α/p300 complex, exhibits antitumour activity in multiple myeloma. Br J Cancer 114, 519–523 (2016). https://doi.org/10.1038/bjc.2016.20
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/bjc.2016.20
Keywords
This article is cited by
-
Novel cancer treatment paradigm targeting hypoxia-induced factor in conjunction with current therapies to overcome resistance
Journal of Experimental & Clinical Cancer Research (2023)
-
Myoglobin expression by alternative transcript in different mesenchymal stem cells compartments
Stem Cell Research & Therapy (2022)
-
HLA-DPA1 gene is a potential predictor with prognostic values in multiple myeloma
BMC Cancer (2020)
-
Glial type specific regulation of CNS angiogenesis by HIFα-activated different signaling pathways
Nature Communications (2020)
-
Overexpression of HIF-1α contributes to melphalan resistance in multiple myeloma cells by activation of ERK1/2, Akt, and NF-κB
Laboratory Investigation (2019)