Abstract
Background:
In order to improve therapy for HNSCC patients, novel methods to predict and combat local and/or distant tumour relapses are urgently needed. This study has been dedicated to the hypothesis that Rac1, a Rho GTPase, is implicated in HNSCC insensitivity to chemo-radiotherapy resulting in tumour recurrence development.
Methods:
Parental and radiation-resistant (IRR) HNSCC cells were used to support this hypothesis. All cells were investigated for their sensitivity to ionising radiation and cisplatin, Rac1 activity, its intracellular expression and subcellular localisation. Additionally, tumour tissues obtained from 60 HNSCC patients showing different therapy response were evaluated for intratumoral Rac1 expression.
Results:
Radiation-resistant IRR cells also revealed resistance to cisplatin accompanied by increased expression, activity and trend towards nuclear translocation of Rac1 protein. Chemical inhibition of Rac1 expression and activity resulted in significant improvement of HNSCC sensitivity to ionising radiation and cisplatin. Preclinical results were confirmed in clinical samples. Although Rac1 was poorly presented in normal mucosa, tumour tissues revealed increased Rac1 expression. The most pronounced Rac1 presence was observed in HNSCC patients with poor early or late responses to chemo-radiotherapy. Tissues taken at recurrence were characterised not only by enhanced Rac1 expression but also increased nuclear Rac1 content.
Conclusions:
Increased expression, activity and subcellular localisation of Rac1 could be associated with lower early response rate and higher risk of tumour recurrences in HNSCC patients and warrants further validation in larger independent studies. Inhibition of Rac1 activity can be useful in overcoming treatment resistance and could be proposed for HNSCC patients with primary or secondary chemo-radioresistance.
Similar content being viewed by others
Main
Despite continuous efforts towards the improvement of therapy results in head and neck squamous carcinoma (HNSCC) patients, the overall survival rate is ⩽50% and remains unchanged during the past 30 years (Haddad and Shin, 2008; Begg, 2012). As existing therapeutic approaches are effective only in a limited number of HNSCC patients, the use of a ‘one-size-fits-all’ approach to cancer treatment is being strongly criticised. Therefore it may be unnecessary and unethical to continue the practice of treating unselected patients, understanding that only a limited number of them will benefit from the proposed therapy. The capacity to either predict patients’ insensitivity to the traditional therapy and higher risk of tumour relapses or to find more effective approaches could improve treatment outcome and avoid recurrence development in HNSCC patients.
Locoregional and distant relapses of the tumour are considered to be the main causes of death in HNSCC patients (Vokes et al, 1993; Lambrecht et al, 2009). The median overall survival is only 6–9 months for patients with recurrent tumours and 3–4 months for patients with metastatic disease progression (Argiris et al, 2004; Gold et al, 2009; Schaaij-Visser et al, 2010). The management of recurrent and metastatic tumours is also an important clinical problem. A minority of HNSCC patients with locoregional recurrences may be salvaged by surgery or re-irradiatation (Vermorken and Specenier, 2010). Re-irradiation alone is not particularly effective, because recurrent tumours arise from radiation-resistant carcinoma cells. Concomitant administration of chemotherapeutics and radiation therapy slightly improves tumour response in relapsed patients. However, this treatment approach also showed limited overall survival and was accompanied by pronounced toxic side effects (Baghi et al, 2006; Vermorken and Specenier, 2010; Tselis et al, 2011). Undoubtedly, treatment outcome in patients with HNSCC recurrences could be significantly improved if new treatment strategies could be designed.
We have recently reported on the possible role of Rac1 protein in HNSCC recurrence development after radiotherapy (Skvortsov et al, 2011). Rac1 is a member of the Rho family of small GTPases acting as a signalling molecule regulating various cellular processes, including cell division, proliferation, differentiation (Michaelson et al, 2008; Gastonguay et al, 2012), survival, motility (Skvortsov et al, 2011; Gastonguay et al, 2012), vesicle transport, nuclear assembly and control of cytoskeleton (Yalovsky et al, 2008). Rac1 was also described as a protein controlling receptor-associated intracellular signalling (Koh and Moon, 2011), ROS production (Iwashima et al, 2008; Ma et al, 2009) and NF-κB activation (Gastonguay et al, 2012). Our findings demonstrated that increased expression of Rac1 is closely associated with radiation resistance of carcinoma cells. It was additionally shown that Rac1 could be proposed as a putative biomarker to predict enhanced ability of the tumour for metastatic spread.
This study aimed to evaluate the possibility to use Rac1 as a potential therapeutic target to combat primary or secondary HNSCC resistance to conventional chemo-radiotherapy. Additionally, this study was planned to determine whether Rac1 expression could be associated with limited tumour response to chemo-radiotherapy in HNSCC patients.
Materials and methods
Cell culture
FaDu, SCC25 and CAL27 HNSCC cell lines were purchased from the American Type Culture Collection (Wesel, Germany). FaDu cells were grown in minimum essential medium (Eagle) with Earle’s BSS (PAA Laboratories GmbH, Linz, Austria) supplemented with 2 mM L-glutamine, 1.5 g l−1 sodium bicarbonate, 0.1 mM non-essential amino acids, 50 U ml−1 penicillin, 50 μg ml−1 streptomycin and 10% fetal calf serum (FCS, Sigma-Aldrich Handels GmbH, Vienna, Austria). SCC25 cells were maintained in a DMEM/Ham’s F-12 medium (1 : 1, Invitrogen GmbH, Lofer, Austria) containing 2.5 mM L-glutamine, 50 U ml−1 penicillin, 50 μg ml−1 streptomycin, 400 ng ml−1 hydrocortisone and supplemented with 10% FCS. CAL27 cells were grown in advanced DMEM (Invitrogen GmbH) containing 2 mM L-glutamine, 50 U ml−1 penicillin, 50 μg ml−1 streptomycin and 10% FCS. Cultures were incubated in a 5% CO2 humidified atmosphere.
Radiation-resistant cells, FaDu-IRR, SCC25-IRR and CAL27-IRR (IRR cells) were derived from parental HNSCC cells after repeated exposure to ionising radiation (10 Gy) (16 MV X-rays) using an Elekta Precise Linear Accelerator (Elekta Oncology Systems, Crawley, UK) at a dose rate of approximately 1.8 Gy min−1. Cells received this treatment 10 times every 2–3 weeks (resulting in a total dose of 100 Gy). The HNSCC cell clones recovering after exposure to ionising radiation were collected for further experiments. The newly received cell lines maintained resistance to ionising radiation even after 3 years of passaging.
Cell viability
Cell viability was evaluated as previously described (Skvortsova et al, 2008; Skvortsov et al, 2011). Briefly, parental and radioresistant HNSCC cells (1 × 105) were plated in six-well plates and treated either with ionising radiation (0–10 Gy) or cisplatin (0–10 μ M) alone or with their combination with Rac1 inhibitor (20 μ M) (Merck Millipore Austria, Vienna, Austria) or after pre-treatment using siRNA-Rac1 (Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA). Cells were incubated at 37 °C in a humidified atmosphere for 72 h, trypsinised and counted using Beckman Coulter Vi-CELL AS cell viability analyzer (Beckman Coulter, Fullerton, CA, USA). The number of viable cells was determined in untreated and treated HNSCC cells. The cell viability was expressed as percentage relative to the untreated control cells.
Clonogenic cell survival assay
To investigate reproductive abilities of both parental and IRR cells to form large colonies or clones after treatment with ionising radiation, cisplatin and Rac1 inhibitor effects were estimated using a clonogenic cell survival assay. Radiosensitivity of HNSCC cells was determined as recently described (Skvortsov et al, 2011). In brief, exponentially growing cells were either only irradiated (0–10 Gy) or treated with ionising radiation in combination with Rac1 inhibitor (20 μ M) that was given 24 h before radiation exposure. Immediately after irradiation, cells were trypsinised and plated into six-well plates (TPP Techno Plastic Products AG, Trasadingen, Switzerland).
In order to evaluate sensitivity of HNSCC cells to cisplatin alone or to its combination with Rac1 inhibitor or after cell transfection with siRNA-Rac1, cells were incubated either with cisplatin (0–10 μ M) or with cisplatin and Rac1 inhibitor (20 μ M) for 24 h and then trypsinised and plated into six-well plates.
After 14 days, the colonies formed after radiation- or cisplatin-based treatments were stained with 0.5% crystal violet in 20% methanol, and the colonies containing >50 cells were counted with a ColCount colony counter (Oxford Optronix, Oxford, UK). Individual assays were performed in triplicates and repeated at least three times. Surviving fraction (SF) was estimated by the following formula: SF=number of colonies formed/number of cells seeded × plating efficiency of the control group, where plating efficiency was calculated as the ratio between colonies observed and the number of cells plated. Dose–response clonogenic survival curves were plotted on a log-linear scale. To quantify how radiation and cisplatin response of IRR cells was changed, data from the dose–response curve were used to calculate the dose-modifying factor (DMF). DMFs were calculated as follows: DMF=dose to reach the specified survival in IRR cells/dose to reach the same survival in the parental cells.
In order to evaluate the influence of Rac1 inhibitor on cell survival and clonogenic abilities of HNSCC cells after treatment with ionising radiation or cisplatin, DMF was determined for radiation- or cisplatin-induced 50% inhibitory effect (IC50) without Rac1 inhibitor compared with IC50 when radiation or cisplatin were combined with Rac1 inhibitor.
Transfection of short interfering RNA (siRNA) and treatment with ionising radiation or cisplatin
Synthesised Rac1-specific 19–25 nucleotide siRNA (siRNA-Rac1 (h): sc-36351) and scrambled siRNA (negative) control (siRNA-A: sc-37007) were purchased from Santa Cruz Biotechnology, Inc.. Parental and treatment-resistant IRR HNSCC cells (25 × 104) per well were seeded in triplicates and cultured for 24 h in the appropriate culture medium without antibiotics at 37 °C in 5% CO2. Just before transfection, cells were washed with Transfection Medium (Santa Cruz Biotechnology, Inc.) and then transfected with either Rac1-specific (10 μ M) or scrambled siRNA (10 μ M). After 7 h at 37 °C, cells were cultivated in the appropriate medium containing twice the normal concentration of FCS and antibiotics. Twenty-four hours after transfection, the knockdown level of Rac1 was determined by western blotting analysis. Then cells were treated with ionising radiation or cisplatin, and cell viability and clonogenic survival were determined as already described in the appropriate sections.
Nuclear/cytoplasmic fractionation and western blotting analysis
Subcellular fractionation to obtain nuclear and cytoplasmic fractions was performed as previously described (Skvortsov et al, 2007). Briefly, 4 × 107 cells were lysed in the cytoplasmic extract buffer and then centrifuged at 200 g. Cytoplasmic fractions were separated from the pellets, and the nuclei were washed and centrifuged in the nuclear extract buffer at 10 000 g for 10 min at 4 °C. Protein precipitation in both cytoplasmic and nuclear fractions was done using methanol/chloroform. Next, pellets were dissolved in the loading buffer, and protein concentration was determined using the Bradford assay.
Western blotting for whole-cell lysates and nuclear/cytoplasmic fractions was performed as published previously (Skvortsov et al, 2011). In order to evaluate real Rac1 expression in the investigated carcinoma cells, we primarily analysed western blot bands using Abcam ab33186 anti-Rac1 antibody (ab33186, Abcam, Cambridge, UK) described as having slight Rac2 with no Rac3 cross-reactivity and rabbit polyclonal Rac1 antibody (C-11: sc-95, Santa Cruz Biotechnology, Inc.) with no Rac2 and slight Rac3 cross-reactivity. Both antibodies demonstrated very similar western blotting results (data not shown), therefore Abcam ab33186 anti-Rac1 antibodies were selected for further analysis of Rac1 expression. Anti-α-tubulin mouse monoclonal antibody (NeoMarkers, Fremont, CA, USA) was used as a loading control for cytoplasmic and total cell extracts, and anti-histone H3 (FL-136, Santa Cruz Biotechnology, Inc.) was applied for nuclear fractions.
The protein bands were measured with a computerised digital imaging system using the GelScan 5.1 software (Serva Electrophoresis, Heidelberg, Germany). The integrated density value (IDV) was obtained as a ratio of protein band density to α-tubulin band density after background correction.
Rac1 G-protein linked immunosorbent assay (G-LISA)
Rac1 G-protein linked immunosorbent assay (G-LISA Rac Activation Assay Biochem Kit, absorbance based, Cytoskeleton, Inc., Denver, CO, USA) was used to measure constitutive, EGF-induced and Rac1 inhibitor-affected levels of active Rac1 in HNSCC cells. Briefly, HNSCC cells were grown to 75–80% confluence in 100-mm Petri dishes and then incubated for 24 h in FCS-free medium. Serum-starved cells were treated with 20 μ M Rac1 inhibitor for 24 h. After treatments, HNSCC cells were lysed according to the manufacturer’s protocol. The protein concentrations in all samples were determined using the standard Bradford method. Cell lysates containing 20 μg proteins were incubated in Rac-GTP-binding protein-coated 96-well plates. Bound Rac-GTP was detected with a Rac specific primary antibody and a HRP-conjugated secondary antibody. Appropriate positive and negative controls were carried out using Rac1 control protein and lysis buffer alone, respectively. The optical density of each well was read at 490 nm using a microplate reader (Bio-Rad Microplate Reader 680, Bio-Rad Laboratories GmbH, Munich, Germany). All G-LISA measurements were performed in triplicates. Means and s.ds. were calculated with the SigmaPlot 8.0 software (Systat Software Inc., San Jose, CA, USA), and the paired Student’s t-test after Bonferroni correction was performed to determine whether found differences in Rac1 activity were significant.
Immunofluorescence
Parental FaDu, SCC25, CAL27 and radioresistant FaDu-IRR, SCC25-IRR and CAL27-IRR cells were plated at a concentration 1 × 104 cells in 0.5 ml medium onto chambered slides (8-chamber polysterene vessel tissue culture-treated glass slide, Falcon, Becton Dickinson Labware Europe, Le Pont De Claix, France) and incubated in CO2 humidified atmosphere at 37 °C. After 24 h of cell growing, medium and unattached cells were removed and appropriate fresh cell medium was replaced. In order to determine the effects of Rac1 inhibition on Rac1 subcellular localisation and expression, cells were treated with 20 μ M Rac1 inhibitor for 24 h. Then incubation medium was removed, untreated and treated cells were washed with PBS, fixed in −20 °C acetone for 15 min. The further steps of immunocytochemical analysis have been performed as published before Dudas et al, 2011) using anti-Rac1 mouse monoclonal antibody (ab33186, Abcam) at a final dilution of 1 : 50 and Alexa 647-conjugated secondary anti-mouse antibody (Invitrogen, Carlsbad, CA, USA) at a final dilution of 1 : 1200. The chamber slides were then stained with DAPIand visualised in Zeiss laser scanning microscope (LSM 50) (Zeiss, Jena, Germany) at × 200 original magnification. Alexa 647 and DAPI channels were photographed separately and relative mean red fluorescence densities were determined in 30 areas of cytoplasm and cell nucleus in all the experimental settings using the Image J software (NIH, Bethesda, MD, USA). Paired t-test adjusted by Bonferroni correction was used to evaluate differences in Rac1-related fluorescence density.
Patients
This study enrolled 60 patients with HNSCC diagnosed and treated at the Innsbruck Country Hospital (TILAK) during a period between 18 July 2007 and 1 March 2012. The median age was 61.58 (range 44–81) years. The primary tumour biopsies were performed before therapy. The biopsies of the recurrences were done when residual tumours were diagnosed or suspected immediately after therapy or within 8–12 weeks after treatment.
Complete treatment data were available for all patients treated with neoadjuvant approach using radiation therapy (66–70 Gy) combined with mitomycin C or cisplatin, in some cases with cetuximab at standard therapeutic doses. All patients were assessable for objective tumour response. The primary end point was the detection of therapy response for times longer than 12 weeks. Re-staged HNSCC patients demonstrating objective complete response without any signs of the tumour re-growth for >12 weeks after the end of chemo-radiotherapy were considered as responders. Patients revealing partial response or relapsed tumour within 12 weeks after treatment were considered as non-responders. Ethical permission to collect and analyse tumour specimens was obtained from the Ethics Committee at Innsbruck Medical University (UN4428, date: 26.07.2011). Details of the patient characteristics are summarised in Supplementary Table S1. The study was extended by tumour tissue of 10 patients with recurrent HNSCC and 14 normal mucosa samples from uvulopalatopharyngoplasty.
Immunohistochemistry
Five-μm thick paraffin sections were used for immunohistochemical analysis from HNSCC samples. The immunohistochemical staining was performed in a Discovery automated staining system (Ventana, Tucson, AZ, USA) as previously published (Schartinger et al, 2012). Mouse monoclonal anti-Rac1 antibody (ab33186, Abcam) was used at final dilution of 1 : 750 as suggested by the manufacturer.
Uvulopalatopharyngoplasty (UPPP) samples were used for evaluation of Rac1 expression in normal mucosa. Quantitative analysis was performed independently by two blinded observers, who collected 10 areas from each non-malignant or malignant specimen, at sites where the highest Rac1 reactivity was found (Schartinger et al, 2012). These areas were analysed on an Olympus BX50 microscope (Olympus Corporation, Tokyo, Japan). Additionally, cells with clear cytoplasmic or nuclear reactions were counted. The average ratio between the positive cells and whole-cell number was calculated as the percentage of Rac1-positive cells.
Human papilloma virus (HPV) status was also evaluated using immunohistochemical assay for the surrogate HPV marker p16 (Langendijk and Psyrri, 2010; Thomas and Primeaux, 2012). CINTec p16 Histology Kit (mouse monoclonal anti-p16 IgG2a, Roche Austria, Roche, Vienna, Austria) was used to determine the levels and distribution of p16 in tumours obtained from HNSCC patients before therapy.
Results
HNSCC cells response to ionising radiation and cisplatin
To determine the radiation and cisplatin sensitivity of IRR cells as compared with parental HNSCC cells, cell viability and clonogenic survival assays were performed (Figure 1). Cell viability of parental and IRR HNSCC cells was evaluated after treatments with ionising radiation or cisplatin at clinically relevant single doses of 2 Gy and 10 μ M, respectively (Figure 1A and B). Although each IRR cell line demonstrated significantly increased cell viability compared with parental cells after radiation exposure (Figure 1A), cell incubation with cisplatin resulted in similar cell viability in FaDu-IRR and FaDu cells and in SCC25-IRR and SCC25 cells (Figure 1B). Only CAL27-IRR cells showed significantly increased cell survival after cisplatin treatment compared with CAL27 cells (24.15±4.61 cell viability in CAL27-IRR cells vs 13.99±2.79 cell viability in CAL27 cells at 72 h).
Cell viability and clonogenic survival of HNSCC cells in response to radiation and cisplatin exposure. Parental FaDu, SCC25 and CAL27 and appropriate radiation-resistant IRR cells were seeded in six-well plates, treated with ionising radiation at a single dose of 2 Gy (A) or cisplatin at a clinically relevant single dose of 10 μ M (B) and then incubated for 72 h. Cell viability and number of cells was evaluated using the Beckman Coulter Vi-CELL AS cell viability analyser. Data are given as mean and s.d. obtained from at least three independent experiments. (C and D) Clonogenic cell survival assay. Colonies were counted on the fourteenth day following radiation (C) or cell exposure to cisplatin (D), and surviving fractions were plotted as a function of dose on a log-linear scale. Error bars indicate s.d. from mean of duplicate measurements from at least four independent experiments. **P<0.01; ***P<0.001.
In contrast, clonogenic survival markedly differed in IRR and parental HNSCC cells after treatment with ionising radiation or cisplatin. Figure 1C and D demonstrate significantly increased clonogenic abilities in all three IRR cell lines compared with parental cells. The DMFs for the surviving fraction of 0.1 after radiation exposure were found to be∼1.42 for FaDu-IRR, ∼1.46 for SCC25-IRR and ∼1.98 for CAL27-IRR cells. The DMFs for cisplatin-treated HNSCC cells were ∼1.92, ∼1.73 and ∼1,63 for FaDu-IRR, SCC25-IRR and CAL27-IRR cells, respectively.
Expression, activity and subcellular localisation of Rac1 in treatment-resistant HNSCC cells
As it was suggested that Rac1 can promote development of cell insensitivity to ionising radiation and cisplatin that are usually used in HNSCC treatment, we next determined the levels of Rac1 expression and activity in radio- and cisplatin-resistant IRR cells compared with parental cells (Figure 2). As seen in Figure 2, FaDu-IRR, SCC25-IRR and CAL27-IRR cells revealed increased Rac1 expression by ∼2.2-, ∼2.3- and ∼1.6-fold compared with parental FaDu, SCC25 and CAL27 cells, respectively.
Rac1 expression in HNSCC cells. Western blotting analysis was done using protein extracts from parental FaDu, SCC25, CAL27 and treatment-resistant FaDu-IRR, SCC25-IRR and CAL27-IRR cells, as described in Materials and methods section. α-Tubulin was used as a loading control. IDV was calculated for each protein band and normalised to the α-tubulin band density after background correction. IDV ratio means fold increase of Rac1 band density in IRR cells to those in parental HNSCC cells. Western blots shown are representative of three independent experiments.
Constitutive Rac1 activities were also enhanced in all three FCS-starved IRR cells compared with parental cells by ∼1.7-fold for FaDu-IRR, ∼1.4-fold for SCC25-IRR and ∼1.5-fold for CAL27-IRR cells (Figure 3). The most pronounced Rac1 activity was observed in CAL27 and CAL27-IRR cells and was calculated as two times higher than in parental or resistant FaDu and SCC25 cells. Cell exposure to Rac1 inhibitor markedly decreased Rac1 activity in all parental and IRR cells.
Rac1 activity in parental and treatment-resistant HNSCC cells. Parental and IRR HNSCC cells were serum starved for 24 h and then treated with Rac1 inhibitor (20 μ M). Cell lysates were subjected to the G-LISA assay in triplicates, and absorbance of each well was read at 490 nm. Results are shown as mean and s.d. obtained from three independent experiments. **P<0.01; ***P<0.001.
In order to determine the cytoplasmic and nuclear expression levels of Rac1 in parental and IRR HNSCC cells, fluorescent microscopy (Supplementary Figure S1) and subcellular fractionation followed by western blotting analysis (Figure 4) were performed. After α-tubulin- and histone H3-based evaluation of IDV of the Rac1-specific protein bands, it was found that both fractions demonstrated enhancement of Rac1 expression levels in IRR cells compared with parental cells (Figure 4). The highest nuclear Rac1 signal was observed in CAL27-IRR cells compared with FaDu-IRR and SCC25-IRR cells. It is important to note that HNSCC cell exposure to ionising radiation or cisplatin also amplified Rac1 expression in cytoplasmic and nuclear compartments (Figure 4). Treatment-resistant SCC25-IRR and CAL27-IRR cells were characterised by more pronounced increase of intranuclear Rac1 expression than parental cells after treatment with ionising radiation or cisplatin. Only FaDu cells revealed higher cisplatin-caused enhancement of the intranuclear Rac1 expression than FaDu-IRR cells. However, radiation exposure led to more pronounced increase of Rac1 in FaDu-IRR than in FaDu nuclei.
Effects of ionising radiation, cisplatin or Rac1 inhibitor on cytoplasmic and nuclear Rac1 expression in HNSCC cells. Western blotting analysis of Rac1 expression in cytoplasmic and nuclear fractions obtained from parental and treatment-resistant IRR HNSCC cells before and at 24 h after treatment with ionising radiation (2 Gy), cisplatin (10 μ M) or Rac1 inhibitor (20 μ M). IDV ratio was calculated as ratio between IDV values of treated cells to those in untreated HNSCC. For IDV evaluation, the background corrections have been done for all protein band densities using α-tubulin for cytoplasmic and histone H3 for nuclear fractions.
Rac1 inhibitor decreased Rac1-related fluorescence densities and cytosolic and nuclear Rac1 expression levels in parental and IRR cells, which in case of all IRR cells was significant (Supplementary Figure S1, Figure 4). However, it is interesting to note that chemical inhibition of Rac1 was more successful in cytosolic than in nuclear fraction in IRR cells, whereas parental HNSCC cells showed less pronounced Rac1 repression in cytosolic fraction accompanied by remarkable decrease of nuclear Rac1.
Inhibition of Rac1 improves HNSCC cell sensitivity to ionising radiation and cisplatin
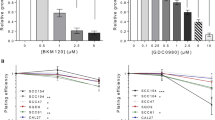
We next determined whether a Rac1 inhibitor could help to overcome radiation and/or cisplatin resistance in HNSCC cells. Cell viability of all investigated cells was markedly decreased when HNSCC cells were treated with ionising radiation or cisplatin in combination with a Rac1 inhibitor (Supplementary Figure S2A and S2B). However, it is necessary to note that anti-survival effects of the Rac1 inhibitor combined with radiation or cisplatin were more pronounced in IRR cells than in parental cells. Furthermore, anti-proliferative activity of combination treatment was more remarkable when the Rac1 inhibitor was combined with radiation than with cisplatin (Supplementary Figure 2A).
Very similar results were observed when the clonogenic abilities of HNSCC cells were evaluated after application of radiation or cisplatin in combination with Rac1 inhibitor (Supplementary Figure S3A and S3B). As seen in Table 1 representing Rac1 blocker-caused DMFs for ionising radiation and cisplatin, inhibition of Rac1 results in marked decreased doses of agents to reach 50% inhibition of clone formation in all the cell lines. However, DMFs after application of the Rac1 inhibitor were more pronounced in treatment-resistant cells than in parental cells.
In order to determine whether attenuation of Rac1 expression using siRNA-Rac1 can, similarly to Rac1 inhibitor, modulate cell viability and clonogenic survival in reponse to radiation or cisplatin exposure, parental and IRR HNSCC cells were transfected with specific siRNA-Rac1 and scrambled control siRNA (siRNA-A) (Supplementary Figure S4). Transfection with scrambled siRNA-A had no effect on cell viability or clonogenic survival after treatment with ionising radiation or cisplatin when compared with non-transfected HNSCC cells (Supplementary Figures 5A, B and 6A, B). Treatment with siRNA-Rac1 resulted in the significant enhancement of cytotoxic effects of ionising radiation and cisplatin in both parental and IRR cells. The most prominent effects were observed at 24 h after treatments of siRNA-Rac1-transfected cells. Only SCC25 cells after siRNA-Rac1 transfection did not visibly change their cell viabilities to either irradiation or cisplatin (Supplementary Figure S5A and B).
HNSCC cells transfected with siRNA-Rac1 also demonstrated diminished clonogenic cell survival in response to radiation or cisplatin exposure compared with non-transfected cells. Sensitivity to irradiation was not enhanced only in siRNA-Rac1-transfected SCC25 cells (Supplementary Figure S6A). Clonogenic survival was not affected in FaDu and SCC25 cells after transfection in response to cisplatin. All other siRNA-Rac1-transfected parental and IRR cells showed significant repression of clone formation after treatment with ionising radiation or cisplatin (Supplementary Figure S6A and B).
Rac1 representation in normal and in HNSCC tissue
All tumour samples obtained from HNSCC patients before treatment were evaluated for HPV status using a surrogate marker p16 (Langendijk and Psyrri, 2010; Thomas and Primeaux, 2012). As shown in the Supplementary Table S1, 29 out of the 60 (48.3%) HNSCC patients had no detectable p16 expression in their tumours, 8 out of the 60 (13.3%) demonstrated focal p16 reaction, 6 out of the 60 (10%) and 17 out of the 60 (28.3%) revealed moderate and high diffuse p16 intratumoral reactions, respectively.
Rac1 protein expression was investigated in normal epithelium from UPPP tissues, in tumour tissues obtained from therapy responders and non-responders and also in a limited number of HNSCC recurrence tissues. In normal epithelium (Figure 5A), Rac1 showed intracellular nuclear reaction in the scattered basal and suprabasal cells. In addition, Rac1 was also detected in the subepithelial cells in the wall of the vessels. If one compares HPV-negative patients without intratumoral p16 expression and HPV-positive patients with high p16 intratumoral reactivity for their Rac1 intratumoral expression, it is possible to see a slightly higher number of HNSCC patients with concomitant p16 and Rac1 expression (Table 2). However, there was no significant difference in Rac1 representation in p16-positive vs p16-negative tumours (P=0.094).
Rac1 immunohistochemical (IHC) reaction in normal mucosa and in HNSCC tissue samples. IHC representation of Rac1 expression in normal epithelium (A); in radio-chemotherapy HNSCC early responder (B); in chemo-radiotherapy HNSCC non-responder (C), in primary tumour of therapy responder without signs of tumour relapse (D) and in the tumour tissue obtained from relapsed HNSCC patient (E). Left panels of images: × 40 magnification, middle panels: × 100 magnification, right panels: × 200 magnification; bars: 100 μm. (F) Graphical representation of percentage of Rac1-positive cells in normal tissue and tumours obtained from therapy responders and non-responders. (G) Differences in Rac1 expression in tumours from HNSCC patients with positive therapy response and from relapsed HNSCC patients. *P<0.05, **P<0.01, ***P<0.001.
HNSCC patients with positive early response possessed tumours that were characterised by heterogeneous slight nuclear Rac1 reactions in the cells belonging to tumour cell nests and stroma (Figure 5B and D). In contrast, tumours from early non-responders demonstrated pronounced and intensive total and nuclear Rac1 expression in nearly all cells of tumour cell nests and stroma (Figure 5C). Recurrent tumours revealed an immunohistochemical picture that was similar to those observed in malignant tissues in HNSCC non-responders. Thus, intense overall and especially nuclear expression of Rac1 was also detected in relapsed tumours (Figure 5E). The percentage of Rac1-positive cells in the tumour epithelium or tumour cell nests was significantly higher than in normal mucosae of UPPP (Figure 5F). There was no significant difference in the content of Rac1-positive cells in tumour tissues obtained from therapy non-responders and responders (Figure 5F). However, the intensity of overall and nuclear staining was more pronounced in tumour tissue from therapy non-responders compared with therapy responders (Figure 4B and C). Tumours from relapsed HNSCC patients demonstrated significantly higher Rac1 immunohistochemical staining in comparison with primary tumours (Figure 5G). Nevertheless, one relapsed HNSCC patient (10%) showed lower Rac1 immunoreactivity than other investigated patients with tumour recurrences (Figure 5G).
Discussion
Unfortunately, currently existing conventional therapeutic approaches such as chemotherapy and radiotherapy have limited effectiveness, resulting in recurrence development in HNSCC patients. Despite all efforts to find the most adequate treatments for recurrent HNSCC, only surgical salvage, re-irradiation and chemotherapy are proposed as a standard care. However, only a few patients could be treated with surgery owing to the poor performance status. Therefore, only re-irradiation and chemotherapy are considered as treatment of choice. Re-irradiation has two main problems: first, recurrent tumours grow from radiation-resistant carcinoma cells, therefore it is possible to predict relapse insensitivity to ionising radiation; and second, re-irradiation is accompanied by pronounced normal tissue toxicity that is registered in 30–50% of HNSCC patients (Baghi et al, 2006). Similar problems, such as side effects and treatment resistance resulting in no long-term survival, are observed when HNSCC patients with relapsed tumours were treated with currently existing chemotherapy protocols (Baghi et al, 2006). Therefore, combination of radiotherapy and chemotherapy has been proposed as more effective treatment approaches. However, concurrent administration of various cytostatics with ionising radiation has limited anti-tumour activity accompanied by increased normal tissue reactions. Median overall survival in these patients was 6–9 months (Argiris et al 2004; Hitt et al, 2004; Baghi et al, 2006).
Even preliminary and early efforts in the development of personalised medicine and application of novel targeted therapeutics (e.g., EGFR blockers) in combination with chemo-radiotherapy did not significantly improve treatment outcome in HNSCC patients (Raben et al, 2004; Ang et al, 2011; Levy et al, 2011). Therefore, further investigations of mechanisms leading to unresponsiveness to conventional therapy approaches, development of locoregional and distant tumour recurrences are urgently needed. Additional knowledge about relapse-associated molecular perturbations in carcinoma cells could help in the development of novel strategies to overcome primary and/or secondary HNSCC resistance.
Recently, we have reported that HNSCC cells lacking response to radiotherapy are characterised by increased expression of Rac1 protein (Skvortsov et al, 2011). Therefore it was decided to evaluate whether Rac1 can be considered as a molecule associated with tumour insensitivity to chemo-radiotherapy and may be used in HNSCC patients as a predictive marker and possible therapeutic target.
Preclinical data presented here have clearly demonstrated that radiation-resistant IRR cells are also resistant to cisplatin. In spite of the pronounced cisplatin-caused reduction in cell viability, cisplatin-treated IRR cells had higher abilities to build colonies than parental cells did. A clinically relevant dose of cisplatin (10 μ M) more effectively affected cell viability of all the investigated HNSCC cells than a clinically relevant single dose of irradiation (2 Gy). It was also seen that enhancement of cell resistance to ionising radiation and cisplatin depended on the increased intracellular Rac1 expression and activity. All three investigated IRR cell lines were characterised by either slight or either significantly or markedly increased nuclear Rac1 expression compared with parental cells. It was recently reported that nuclear expression of Rac1 associates with carcinogenesis, malignant transformation and enhancement of malignant aggressiveness of cells (Mendoza-Catalan et al, 2012). Thus, Rac1 was not detected in nuclei of non-malignant human keratinocytes, whereas cervix carcinoma cells demonstrated nuclear localisation of Rac1. These data are fully supported by our findings (Figure 4F) describing lower Rac1 presence in normal mucosa compared with tumour tissue. It was also reported that nuclear import of Rac1 requires Rac1 activation (Lanning et al, 2003). Indeed, all three treatment-resistant HNSCC IRR cells revealed enhancement of nuclear Rac1 localisation accompanied by concurrent Rac1 activation in comparison with parental cells. Furthermore, cell exposure to ionising radiation or cisplatin markedly enhanced cytoplasmic and nuclear Rac1 expression in all HNSCC cells. However, treatment-caused intranuclear content was more pronounced in treatment-resistant IRR cells compared with parental cells. It is assumed this radiation- or cisplatin-caused amplification of intranuclear Rac1 expression could also contribute to therapy resistance in IRR HNSCC cells.
It was additionally noted that cell viability after cell exposure to either ionising radiation or cisplatin was less dependent on Rac1 expression or activation than clonogenic abilities of HNSCC cells. Clonogenic survival was significantly increased in cells with Rac1 upregulation and enhanced constitutive Rac1 activity. This fact has clinical relevance: although Rac1-overexpressing carcinoma cells can reveal decreased cell viability in response to cytotoxic treatment resulting in the early tumour response, clinicians could observe either tumour re-growth or relapse already in the near future after treatment owing to Rac1-associated enhancement of cell clonogen survival.
Clinical data presented here confirm our pre-clinical findings. HNSCC patients demonstrating early tumour response to chemo-radiotherapy revealed lower overall and nuclear Rac1 staining intensity in the tumours. Despite the fact that the content of Rac1-positive cells did not significantly differ in tumours obtained from therapy responders and non-responders, the levels of Rac1 intratumoral and nuclear expression were higher in HNSCC patients with poor treatment response and tumour relapses. Therefore we hypothesize that evaluation of the intensity of intratumoral Rac1-specific reactions accompanied by increased nuclear content of Rac1 can be used in the clinical practice as a potential sign for lower therapy responsiveness and higher risk of tumour recurrences in HNSCC patients. Additionally, we assume that evaluation of the intratumoral activity of Rac1 protein could also provide important information in helping oncologists to expect relapse development.
It is currently known that HPV/p16-positive HNSCC patients demonstrate better therapy response and better clinical outcome than HPV/p16-negative patients (Langendijk and Psyrri, 2010). However, it is still unknown whether HPV/p16-positive HNSCC patients should be treated using less aggressive or standard therapeutic approaches. A number of different studies have concluded that HPV/p16 status has been used at least for patient stratification, but also other molecular characteristics of malignant tumours should be investigated in parallel, suggesting that p16-positive and p16-negative tumours are different in their molecular properties (Gillison et al, 2008; Langendijk and Psyrri, 2010; Chen et al, 2013). Our own data have tended to show a concomitant expression of Rac1 and p16. This co-expression was not significantly documented perhaps owing to the low number of tumour samples investigated. However, it is impossible to exclude that predictive value of HPV/p16 status can be diminished in HNSCC patients, if Rac1 is concomitantly expressed in HPV/p16-positive tumours. Although p16 was previously described as a negative regulator of carcinoma cell migration and invasion (Fåhraeus and Lane, 1999), more recent reports have shown that p16 could be involved in the positive regulation of cell migration (Chen et al, 2013). Furthermore, along with Rac1 protein, p16 is described as a molecule contributing to TGFβ and Notch signalling associated with functional activities of the most aggressive and treatment-resistant carcinoma cell subpopulation – carcinoma stem cells (Koch and Radtke, 2007; Massagué, 2008; Chen et al, 2013). Perhaps therefore a less aggressive therapy is still not justified for p16-positive HNSCC patients (Langendijk and Psyrri, 2010). Additional clinical and pathological data highlighting molecular properties of p16-positive HNSCC patients are required and urgently needed.
There are some limitations in our study: first, there are no well clinically annotated HNSCC tumour databases to be used in our study; second, as the majority of our patients’ samples have been collected for only 1–3 years, we have no data describing the relationship between Rac1 expression, HPV status and disease-free and overall survivals in HNSCC patients. Further analysis of a larger number of samples is required and is being currently organised in our clinic. However, based on the recent data confirming that overexpression of Rac small GTPases Rac1 and Rac3 is associated with poor prognosis in breast cancer patients (Katz et al, 2012), it is necessary to continue the research work on the role of Rac1 to predict therapy outcome in HNSCC patients.
As Rac1 is considered as a potential biomarker and therapeutic target, it is necessary to know how Rac1 inhibition could change HNSCC cell behaviour. Previously published articles reported on the successful inhibition of carcinoma cell viability and proliferation after application of Rac1 inhibitor (Iwashima et al, 2008; Gastonguay et al, 2012). However, there are no data about Rac1 targeting in carcinoma cells with resistance to conventional therapeutic approaches. It is suggested that these novel data could open new opportunities to use Rac1 inhibitor in the treatment of resistant or relapsed HNSCCs. Here we presented preclinical data that clearly show that combination of radiation or cisplatin with Rac1 inhibitor could be effectively used to reach better clinical outcomes in HNSCC patients. It is interesting to note that Rac1 inhibitor more actively blocked clonogenic survival in HNSCC cells with more pronounced Rac1 expression and activity. Rac1 inhibitor allows a reduction in dosage of ionising radiation or cisplatin by∼1.5–3.0-fold in order to reach the same cell treatment effects as was observed with administration of radiation or cisplatin alone (Table 1). Therefore, these combinations of Rac1 inhibitor with either radiation therapy or cytostatics could be proposed to treat patients with tumour recurrences or primarily resistant tumours possessing Rac1 overexpression or activation.
Based on these results, we conclude that increased expression, activity and subcellular localisation of Rac1 contribute to the limited response rate and higher risk of tumour recurrences in HNSCC patients. Inhibition of Rac1 activity and expression can be useful in overcoming treatment resistance and could be proposed for HNSCC patients with primary or secondary chemo-radioresistance.
Change history
27 May 2014
This paper was modified 12 months after initial publication to switch to Creative Commons licence terms, as noted at publication
References
Ang KK, Zhang QE, Rosenthal DI, Nguyen-Tan P, Sherman EJ, Weber RS, Galvin JM, Schwartz DL, El-Naggar AK, Gillison ML, Jordan R, List MA, Konski AA, Thorstad WL, Trotti A, Beitler JJ, Garden AS, Spanos WJ, Yom SS, Axelrod RS (2011) A randomized phase III trial (RTOG 0522) of concurrent accelerated radiation plus cisplatin with or without cetuximab for stage III-IV head and neck squamous cell carcinomas (HNC). J Clin Oncol 29 (suppl): abstr 5500.
Argiris A, Li Y, Forastiere A (2004) Prognostic factors and long-term survivorship in patients with recurrent or metastatic carcinoma of the head and neck. Cancer 101: 2222–2229.
Baghi M, Hambek M, Wagenblast J, May A, Gstoettner W, Knecht R (2006) A phase II trial of docetaxel, cisplatin and 5-fluorouracil in patients with recurrent squamous cell carcinoma of the head and neck (SCCHN). Anticancer Res 26: 585–590.
Begg AC (2012) Predicting recurrence after radiotherapy in head and neck cancer. Semin Radiat Oncol 22: 108–118.
Chen YW, Chu HC, Lin ZS, Shiah WJ, Chou CP, Klimstra DS, Lewis BC (2013) p16 stimulates CDC42-dependent migration of hepatocellular carcinoma cells. PLoS One 8: e69389.
Dudas J, Fullar A, Bitsche M, Schartinger V, Kovalszky I, Sprinzl GM, Riechelmann H (2011) Tumor-produced, active interleukin-1beta regulates gene expression in carcinoma-associated fibroblasts. Exp Cell Res 317: 2222–2229.
Fåhraeus R, Lane DP (1999) The p16(INK4a) tumour suppressor protein inhibits alphavbeta3 integrin-mediated cell spreading on vitronectin by blocking PKC-dependent localization of alphavbeta3 to focal contacts. EMBO J 18: 2106–2118.
Gastonguay A, Berg T, Hauser AD, Schuld N, Lorimer E, Williams CL (2012) The role of Rac1 in the regulation of NF-kappaB activity, cell proliferation, and cell migration in non-small cell lung carcinoma. Cancer Biol Ther 13: 647–656.
Gillison ML, D'Souza G, Westra W, Sugar E, Xiao W, Begum S, Viscidi R (2008) Distinct risk factor profiles for human papillomavirus type 16-positive and human papillomavirus type 16-negative head and neck cancers. J Natl Cancer Inst 100: 407–420.
Gold KA, Lee HY, Kim ES (2009) Targeted therapies in squamous cell carcinoma of the head and neck. Cancer 115: 922–935.
Haddad RI, Shin DM (2008) Recent advances in head and neck cancer. N Engl J Med 359: 1143–1154.
Hitt R, Jimeno A, Rodriguez-Pinilla M, Rodriguez-Peralto JL, Millan JM, Lopez-Martin A, Brandariz A, Peña C, Cortés-Funes H (2004) Phase II trial of cisplatin and capecitabine in patients with squamous cell carcinoma of the head and neck, and correlative study of angiogenic factors. Br J Cancer 91: 2005–2011.
Iwashima F, Yoshimoto T, Minami I, Sakurada M, Hirono Y, Hirata Y (2008) Aldosterone induces superoxide generation via Rac1 activation in endothelial cells. Endocrinology 149: 1009–1014.
Katz E, Sims AH, Sproul D, Caldwell H, Dixon MJ, Meehan RR, Harrison DJ (2012) Targeting of Rac GTPases blocks the spread of intact human breast cancer. Oncotarget 3: 608–619.
Koch U, Radtke F (2007) Notch and cancer: a double-edged sword. Cell Mol Life Sci 64: 2746–2762.
Koh MS, Moon A (2011) Activation of H-Ras and Rac1 correlates with epidermal growth factor-induced invasion in Hs578T and MDA-MB-231 breast carcinoma cells. Biochem Biophys Res Commun 406: 25–29.
Lambrecht M, Dirix P, Van den BW, Nuyts S (2009) Incidence of isolated regional recurrence after definitive (chemo-) radiotherapy for head and neck squamous cell carcinoma. Radiother Oncol 93: 498–502.
Langendijk JA, Psyrri A (2010) The prognostic significance of p16 overexpression in oropharyngeal squamous cell carcinoma: implications for treatment strategies and future clinical studies. Ann Oncol 21: 1931–1934.
Lanning CC, Ruiz-Velasco R, Williams CL (2003) Novel mechanism of the co-regulation of nuclear transport of SmgGDS and Rac1. J Biol Chem 278: 12495–12506.
Levy AR, Johnston KM, Sambrook J, Donato B, Penrod JR, Corral M, Chasen M (2011) Indirect comparison of the efficacy of cetuximab and cisplatin in squamous cell carcinoma of the head and neck. Curr Med Res Opin 27: 2253–2259.
Ma Q, Cavallin LE, Yan B, Zhu S, Duran EM, Wang H, Hale LP, Dong C, Cesarman E, Mesri EA, Goldschmidt-Clermont PJ (2009) Antitumorigenesis of antioxidants in a transgenic Rac1 model of Kaposi’s sarcoma. Proc Natl Acad Sci USA 106: 8683–8688.
Massagué J (2008) TGFbeta in cancer. Cell 134: 215–230.
Mendoza-Catalan MA, Cristobal-Mondragon GR, me-Gomez J, del Valle-Flores HN, Coppe JF, Sierra-Lopez L, Romero-Hernández MA, del Carmen Alarcón-Romero L, Illades-Aguiar B, Castañeda-Saucedo E (2012) Nuclear expression of Rac1 in cervical premalignant lesions and cervical cancer cells. BMC Cancer 12: 116.
Michaelson D, Abidi W, Guardavaccaro D, Zhou M, Ahearn I, Pagano M, Philips MR (2008) Rac1 accumulates in the nucleus during the G2 phase of the cell cycle and promotes cell division. J Cell Biol 181: 485–496.
Raben D, Bianco C, Milas L, Ang KK (2004) Targeted therapies and radiation for the treatment of head and neck cancer: are we making progress? Semin Radiat Oncol 14: 139–152.
Schaaij-Visser TB, Brakenhoff RH, Leemans CR, Heck AJ, Slijper M (2010) Protein biomarker discovery for head and neck cancer. J Proteomics 73: 1790–1803.
Schartinger VH, Falkeis C, Laimer K, Sprinzl GM, Riechelmann H, Rasse M, Virgolini I, Dudás J (2012) Neuroendocrine differentiation in head and neck squamous cell carcinoma. J Laryngol Otol 126: 1261–1270.
Skvortsov S, Jimenez CR, Knol JC, Eichberger P, Schiestl B, Debbage P, Skvortsova I, Lukas P (2011) Radioresistant head and neck squamous cell carcinoma cells: intracellular signaling, putative biomarkers for tumor recurrences and possible therapeutic targets. Radiother Oncol 101: 177–182.
Skvortsov S, Skvortsova I, Stasyk T, Schiefermeier N, Neher A, Gunkel AR, Bonn GK, Huber LA, Lukas P, Pleiman CM, Zwierzina H (2007) Antitumor activity of CTFB, a novel anticancer agent, is associated with the down-regulation of nuclear factor-kappaB expression and proteasome activation in head and neck squamous carcinoma cell lines. Mol Cancer Ther 6: 1898–1908.
Skvortsova I, Skvortsov S, Stasyk T, Raju U, Popper BA, Schiestl B, von Guggenberg E, Neher A, Bonn GK, Huber LA, Lukas P (2008) Intracellular signaling pathways regulating radioresistance of human prostate carcinoma cells. Proteomics 8: 4521–4533.
Thomas J, Primeaux T (2012) Is p16 immunohistochemistry a more cost-effective method for identification of human papilloma virus-associated head and neck squamous cell carcinoma? Ann Diagn Pathol 16: 91–99.
Tselis N, Ratka M, Vogt HG, Kolotas C, Baghi M, Baltas D, Fountzilas G, Georgoulias V, Ackermann H, Zamboglou N (2011) Hypofractionated accelerated CT-guided interstitial (1)(2)Ir-HDR-brachytherapy as re-irradiation in inoperable recurrent cervical lymphadenopathy from head and neck cancer. Radiother Oncol 98: 57–62.
Vermorken JB, Specenier P (2010) Optimal treatment for recurrent/metastatic head and neck cancer. Ann Oncol 21 (Suppl 7): vii252–vii261.
Vokes EE, Weichselbaum RR, Lippman SM, Hong WK (1993) Head and neck cancer. N Engl J Med 328: 184–194.
Yalovsky S, Bloch D, Sorek N, Kost B (2008) Regulation of membrane trafficking, cytoskeleton dynamics, and cell polarity by ROP/RAC GTPases. Plant Physiol 147: 1527–2543.
Acknowledgements
We are grateful to the EORTC Charitable Trust for providing core support to the EORTC. This study was supported in part by Austrian Cancer Society/Tyrol and by Austrian Science Fund (FWF, P22287-B13).
Author information
Authors and Affiliations
Consortia
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
This work is published under the standard license to publish agreement. After 12 months the work will become freely available and the license terms will switch to a Creative Commons Attribution-NonCommercial-Share Alike 3.0 Unported License.
Supplementary Information accompanies this paper on British Journal of Cancer website
Supplementary information
Rights and permissions
From twelve months after its original publication, this work is licensed under the Creative Commons Attribution-NonCommercial-Share Alike 3.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-sa/3.0/
About this article
Cite this article
Skvortsov, S., Dudás, J., Eichberger, P. et al. Rac1 as a potential therapeutic target for chemo-radioresistant head and neck squamous cell carcinomas (HNSCC). Br J Cancer 110, 2677–2687 (2014). https://doi.org/10.1038/bjc.2014.221
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/bjc.2014.221
Keywords
This article is cited by
-
Unique vulnerability of RAC1-mutant melanoma to combined inhibition of CDK9 and immune checkpoints
Oncogene (2024)
-
Identification of potent and novel inhibitors against RAC1: a Rho family GTPase
In Silico Pharmacology (2022)
-
Rac1 activation in human breast carcinoma as a prognostic factor associated with therapeutic resistance
Breast Cancer (2020)
-
Rho GTPases in cancer radiotherapy and metastasis
Cancer and Metastasis Reviews (2020)
-
VAV2 signaling promotes regenerative proliferation in both cutaneous and head and neck squamous cell carcinoma
Nature Communications (2020)