Abstract
On the basis of the benefits of frontline radiation in early-stage, extranodal natural killer (NK)/T-cell lymphoma (ENKTL), we conducted the trial of concurrent chemoradiotherapy (CCRT) followed by three cycles of gemcitabine, dexamethasone and cisplatin (GDP). Thirty-two patients with newly diagnosed, stage IE to IIE, nasal ENKTL received CCRT (that is, all patients received intensity-modulated radiotherapy 56 Gy and cisplatin 30 mg/m2 weekly, 3–5 weeks). Three cycles of GDP (gemcitabine 1000 mg/m2 intravenously (i.v.) on days 1 and 8, dexamethasone 40 mg orally on days 1–4 and cisplatin 75 mg/m2 i.v. on day 1 (GDP), every 21 days as an outpatient were scheduled after CCRT. All patients completed CCRT, which resulted in 100% response that included 24 complete responses (CRs) and eight partial responses. The CR rate after CCRT was 75.0% (that is, 24 of 32 responses). Twenty-eight of the 32 patients completed the planned three cycles of GDP, whereas four patients did not because they withdrew (n=1) or because they had an infection (n=3). The overall response rate and the CR rate were 90.6% (that is, 29 of 32 responses) and 84.4% (that is, 27 of 32 responses), respectively. Only two patient experienced grade 3 toxicity during CCRT (nausea), whereas 13 of the 30 patients experienced grade 4 neutropenia. The estimated 3-year overall survival and progression-free rates were 87.50% and 84.38%, respectively. In conclusion, CCRT followed by GDP chemotherapy can be a feasible and effective treatment strategy for stage IE to IIE nasal ENKTL.
Similar content being viewed by others
Introduction
Nasal extranodal natural killer/T-cell lymphoma (ENKT), which is a highly aggressive disease frequently involving the nasal cavity and upper aerodigestive tract, is more frequently seen in Asia than in Western countries. No consensus has been reached on the management of the disease.1, 2 The use of radiotherapy as initial treatment was reported to produce a greater complete response (CR) rate than chemotherapy.3, 4 However, when ENKTL is treated with radiation alone, local and systemic failures are observed frequently, even when the disease is in the early stage. Some studies have demonstrated that early stage, localized nasal disease is highly curable with combination therapy, but the optimal dose, combination, and sequence of radiotherapy and chemotherapy are still undefined, and better methods of treatment are needed.5 Other studies have demonstrated that concurrent chemoradiotherapy (CCRT) using multidrug resistance-nonrelated agents is a safe and effective treatment for localized nasal NK/T-cell lymphoma and that patients with newly diagnosed, stage IE to IIE, nasal ENKTL are best treated with frontline CCRT.6, 7, 8 Yet, nasal type NK/T-cell lymphoma showed a poor response to the conventional anthracycline-based chemotherapy, such as cyclophosphamide, doxorubicin, vincristine and prednisone (CHOP) or CHOP-like regimens, thereby an investigation for a novel therapy is urgently needed to improve survival.9 Some studies have demonstrated that the gemcitabine-based combination regimen, especially gemcitabine, dexamethasone and cisplatin (GDP) regimen, is safe and well tolerated with promising clinical activity in patients with peripheral T-cell lymphomas. Gemcitabine alone and/or containing chemotherapy is efficient in refractory or relapsed NK/T-cell lymphoma. Gemcitabine was effective in a subset of pretreated ENKL patients and can be considered as a good option.10, 11
Thus, we designed a CCRT regimen with 56 Gy of radiation and weekly administration of cisplatin as a radiosensitizer. In addition, in consideration of the risk of systemic relapse after CCRT, we added systemic chemotherapy that consisted of GDP. We report here the results of our phase II, prospective study that consisted of CCRT followed by systemic chemotherapy.
Materials and methods
Eligibility criteria
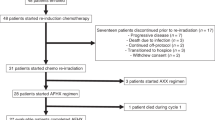
Thirty-two patients were enrolled from March 2009 to June 2010. To be eligible for enrollment, patients were required to be at least 18 years old, to have a biopsy-proven diagnosis of nasal ENKTL, to have disease classified as Ann Arbor stage IE or IIE, to have measurable disease, to have a life expectancy >12 weeks and to have an Eastern Cooperative Oncology Group performance status of 0–2. Patients also had to have adequate hepatic function (that is, aspertate aminotransferase ⩽3 times upper limit of normal and total bilirubin ⩽2 times the upper limit of normal; renal function (that is, creatinine clearance ⩾50 ml/min, serum creatinine ⩽1.5 mg/dl) and hematologic function (that is, hemoglobin ⩾9.0 g/dl, absolute neutrophil count >1500/l and platelets >100 000/l). Diagnosis of ENKTL was based on the presence of histologic features and immunophenotypes compatible with ENKTL (for example, positive for cytotoxic molecules, cytoplasmic CD3+, CD20−, CD56+). The exclusion criteria were prior or concomitant malignant tumors and any coexisting medical problems of sufficient severity to prevent full compliance with the study protocol. The subtypes of non-Hodgkin’s lymphoma, including myeloid/NK cell precursor acute leukemia, blastic NK cell lymphoma/precursor NK cell lymphoblastic leukemia, aggressive NK cell leukemia and peripheral T-cell lymphoma unspecified, were excluded. ENKTL with non-nasal sites, such as skin or gastrointestinal tract, was excluded even if it was localized. All patients provided written informed consent. This study was approved by the institute review board of each hospital.
Treatment and dose modifications
All patients received intensity-modulated radiotherapy (IMRT) 56 Gy and cisplatin 30 mg/m2 weekly, 3–5 weeks. After immobilization of the patient, computed tomography (CT) simulation was performed to determine the appropriate radiation therapy doses for each patient. The radiation target volume covered the gross clinical lesions plus adequate margins. All patients received IMRT by using 6 MV photons generated from a linear accelerator. The addition of elective lymphatic irradiation was determined on an individual basis. The median total dose to the gross lesion was 56 Gy, and the daily dose was 2 Gy for most patients. Four weeks after the completion of CCRT, GDP chemotherapy was given every 3 weeks for up to three cycles. Gemcitabine 1000 mg/m2 intravenously on days 1 and 8, dexamethasone 40 mg orally on days 1–4 and cisplatin 75 mg/m2 intravenously on day 1 GDP. All drugs were administered only if the platelet count was >75 000/l and the absolute neutrophil count was >1500/l before each cycle. If either the platelets or neutrophils were lower than these levels, treatment was delayed for 7 days. Granulocyte colony-stimulating factor was administered for occurrences of grade 3–4 neutropenia. Dosages of gemcitabine, and cisplatin were reduced by 25% if the platelet counts and absolute neutrophil count recovered to ⩾50 000/l and to 1000–1499/l, respectively, after a 1-week delay. If thrombocytopenia (that is, platelets <50 000/l) and neutropenia (that is, absolute neutrophil count <1 000/l) persisted for 2 weeks, the patient was withdrawn from this study. If patients experienced grade 2 or higher nonhematologic toxicities, treatment was interrupted until the toxicity resolved to grade 0 to 1. If creatinine clearance was >40 ml/min before a cycle, GDP treatment was delayed for up to 2 weeks. GDP was restarted after the creatinine clearance recovered to >40 ml/min. If the creatinine clearance did not recover, cisplatin was omitted from the next GDP.
Evaluation
Baseline evaluation were performed 14 days or less before enrollment, and all patients were staged according to the Cotswold modification of the Ann Arbor staging system, which included history taking, physical examination, trephine biopsy, endoscopic examination of the nasal and oral cavities by otorhinolaryngologists, complete blood count, serum biochemistry with lactate dehydrogenase, bone marrow aspiration and CT scanning or magnetic resonance imaging of the involved lesions, CT scanning of the chest and abdomen-pelvis, and/or positron emission tomography. All of these studies were performed before treatment and after completion of CCRT and GDP, and they were repeated every 3–6 months thereafter to monitor the disease progression. The median follow-up time is 38.3 months.
Assessment
Treatment response was assessed according to the WHO criteria.12 CR was defined as disappearance of all previously measurable lesions and absence of any new tumor lesions. Partial response (PR) was defined as a decrease of at least 50% in the product of two perpendicular diameters of each measurable lesion. Stable disease was defined as a decrease of <50% or an increase of <25% in tumor size, and progressive disease (PD) was defined as >25% increase in the product of the two diameters of at least one tumor or as the presence of a newly developed lesion. Every patient received a laryngoscopic exam before and after CCRT and after completions of GDP to confirm the pathologic CR. The primary end point was the response rate, which was calculated as the proportion of patients classified as CR and PR. The secondary end points were toxicity, overall survival (OS) and progression-free survival (PFS). Toxicity was evaluated before each treatment cycle according to the National Cancer Institute Common Terminology Criteria of Adverse Events, version 3.0. OS was measured from the date of enrollment to the date of death as a result of any cause, and it was censored at the date of the last follow-up visit. PFS was defined as the time from the date of enrollment to the date of documented disease progression, and it was censored at the date of the last follow-up visit.
Statistical analysis
All statistical analyses were conducted using the SPSS (Chicago, IL, USA) 13.0 statistical package for Windows. The Kaplan–Meier method was used to calculate PFS and OS, and the survival curves were compared by the log-rank test.
Results
Characteristics of patients
The characteristics of the patients are summarized in Table 1. The median age was 47.5 years (range, 21–72 years); 84.4% of all patients were younger than 60 years of age. All patients were in the low- or low-intermediate risk categories of the International Prognostic Index.
Response
The primary end point was the response rate, which was calculated as the proportion of patients classified as CR and PR. In the first stage, eight patients were entered into the study, and seven of these experienced CR. After this preliminary result, 32 patients were registered. After CCRT, the overall response rate, which included 24 CRs and 8 PRs, was 100%. After GDP chemotherapy, 24 patients retained CRs, and three patients with PRs to CCRT achieved CR. Twenty-seven patients achieved CR after the completion of GDP (CR rate, 84.4%).
Relapse
Three (9.38%) of the 32 patients experienced disease progression. Distant metastasis was observed in two patients: one patient (a 47-year-old man) was found with new lesions in his right lung, after progression, he received three cycles of etoposide, ifosfamide, cisplatin and dexamethasone (VIPD), and another patient (a 25-year-old man), who was still in PR after the completion of treatment, experienced disease progression in multiple regions of skin all over the body, after progression, he was treated with the solumedrol, methotrexate, ifosfamide, l-asparaginase and etoposide regimen. The two patients remained alive after treatment at the time of this article. Local recurrence was found in one patient: a 23-year-old woman experienced a relapse in the right parotid gland within her previous radiation field, after recurrence, she refused additional treatment, then the disease progressed and she died 6 months later.
Toxicity
Hematologic toxicity was minimal during CCRT; grade 1–2 leukopenia were observed in 11 patients (34.4%, Table 2). Grade 3–4 leukopenia were observed in two patients (6.3%, Table 2). Nonhematologic toxicities were tolerable, and most toxicities were grade 1. Although two patients had grade 3 nausea, they could continue treatment without interruption, because his nausea was controlled by supportive care. The other grade 2, nonhematologic toxicities were managed so there was no delay or interruption of CCRT. However, one patient (a 47-year-old man) dropped out after CCRT and withdrew his consent for personal reasons. Grade 3–4 hematologic toxicities were frequent during GDP. Thrombocytopenia was more severe in patients than was leukopenia (Table 2). Six patients experienced grade 4 thrombocytopenia. There was one death that was associated with complications because of infection: one patient (a 58-year-old man) died as a result of Gram-positive sepsis while not being neutropenic; however, this death might be related to disease progression because the patient was suffering from lower gastrointestinal hemorrhage.
Survival
Three-year OS and PFS rates were 87.50% and 84.38%, respectively. It shows that CCRT followed by three cycles of GDP produced a good OS and PFS (Figure 1). These survival outcomes were similar with Kim’s phase II trial of concurrent radiation and weekly cisplatin followed by VIPD chemotherapy.7
Discussion
Nasal type NK/T-cell lymphoma showed a poor response to the conventional anthracycline-based chemotherapy,9 gemcitabine alone and/or containing chemotherapy is efficient in refractory or relapsed NK/T-cell lymphoma.11 This study applied frontline treatment with CCRT followed by gemcitabine-based chemotherapy for stage IE to IIE nasal ENKTL. For CCRT, we performed a weekly cisplatin administration as a radiosensitizer to augment the efficacy of radiotherapy. The CR rate after CCRT was 75.0%, which was superior to the rates reported in previous studies of chemotherapy followed by radiotherapy, but our radiation doses (median, 50 Gy; range 46–56.0 Gy) were smaller than in previous studies, in which doses >45–70 Gy were used. However, we achieved satisfactory local control with acceptable toxicity by using CCRT because IMRT achieves excellent target coverage and dose conformity, as well as favorable survival and locoregional control rates with acceptable toxicities in patients.1, 13, 14, 15, 16, 17, 18, 19, 20, 21
GDP chemotherapy was added to the regimen for consolidation and to prevent systemic relapse. Five patients who experienced PRs then experienced conversion to CR after GDP chemotherapy, which resulted in a 84.4% CR rate (that is, 29 of 32 patients). Furthermore, only three patients experienced disease progression or relapse during follow-up: one was local, two were systemic, the one local relapse occurred within the previous radiation field, and the other patients had simultaneous systemic relapse, the local relapse rate was only 3.1% (that is, 1 of 32 patients), and the rate of systemic relapse was 6.3% (that is, 2 of 32 patients). Therefore, our treatment strategy may reduce local relapse. Although we observed two occurrences of systemic relapse, this rate was lower than that found in previous reports. Our systemic relapse rate (6.3%; that is, 2 of 32 patients) was lower. We conclude that GDP chemotherapy as an adjuvant to CCRT was effective for reducing relapse.
There were frequent grade 3–4 hematologic toxicities during GDP chemotherapy, even though we reduced the doses of gemcitabine. Although one patient died during GDP, the death was not clearly associated with treatment toxicity because the patient did not have neutropenia. We believe this death may have been related to disease progression because the patient was suffering from lower gastrointestinal hemorrhage.
In this study, the 3-year OS and PFS rates were ~87.50% and 84.38%, respectively. The result is similar with Kim’s phase II trial of concurrent radiation and weekly cisplatin followed by VIPD chemotherapy, its estimated 3-year, progression-free and OS rates were 85.19% and 86.28%, respectively.7
At present, we still did not find any form of long-term toxicity among survivors, although our follow-up period was relatively short. However, our cohort should be observed to monitor the development of long-term toxicity including secondary malignancy. Thus, the survival outcomes that we observed attest the effectiveness of CCRT followed by GDP chemotherapy. In conclusion, CCRT followed by GDP chemotherapy can be a feasible and effective treatment strategy for stage IE to IIE nasal ENKTL.
References
Vazquez A, Khan MN, Blake DM, Sanghvi S, Baredes S, Eloy JA . Extranodal natural killer/T-Cell lymphoma: a population-base comparison of sinonasal and extranasal disease. Laryngoscope 2013; 124: 888–895.
Lee J, Cho SG, Chung SM, Ryu MR, Kim SH, Jang HS et al. Retrospectiveanalysis of treatment outcomes for extranodal NK/T-cell lymphoma (ENKL), nasal type, stage I-IIE: single institute experience of combined modality treatment for early localized nasal extranodal NK/T-cell lymphoma (ENKL). Ann Hematol 2013; 92: 333–343.
Li YX, Wang H, Jin J, Wang WH, Liu QF, Song YW et al. Radiotherapy alone with curative intent in patients with stage I extranodal nasal-type NK/T-cell lymphoma. Int J Radiat Oncol Biol Phys 2012; 82: 1809–1815.
Chauchet A, Michallet AS, Berger F, Bedgedjian I, Deconinck E, Sebban C et al. Complete remission after first-line radio-chemotherapy as predictor of survival in extranodal NK/T cell lymphoma. J Hematol Oncol 2012; 5: 27.
Au WY . Current management of nasal NK/T-cell lymphoma. Oncology (Williston Park) 2010; 24: 352–358.
Yamaguchi M, Tobinai K, Oguchi M, Ishizuka N, Kobayashi Y, Isobe Y et al. Phase I/II study of concurrent chemoradiotherapy for localized nasal natural killer/T-cell lymphoma: Japan Clinical Oncology Group Study JCOG0211. J Clin Oncol 2009; 27: 5594–5600.
Kim SJ, Kim K, Kim BS, Kim CY, Suh C, Huh J et al. Phase II trial of concurrent radiation and weekly cisplatin followed by VIPD chemotherapy in newly diagnosed, stage IE to IIE, nasal, extranodal NK/T-Cell Lymphoma: Consortium for Improving Survival of Lymphoma study. J Clin Oncol 2009; 27: 6027–6032.
Avilés A, Neri N, Fernández R, Huerta-Guzmán J, Nambo MJ . Combined therapy in untreated patients improves outcome in nasal NK/T lymphoma: results of a clinical trial. Med Oncol 2013; 30: 637.
Youssef YB, Bougmiza I, Bouabid Z, Achour B, Regaieg H, Sriha B et al. Nasopharyngeal/nasal type NK/T lymphoma: analysis of 23 cases and current review of the literature. Kulak Burun Bogaz Ihtis Derg 2012; 22: 275–283.
Dong M, He XH, Liu P, Qin Y, Yang JL, Zhou SY et al. Gemcitabine-based combination regimen in patients with peripheral T-cell lymphoma. Med Oncol 2013; 30: 351.
Ahn HK, Kim SJ, Hwang DW, Ko YH, Tang T, Lim ST et al. Gemcitabine alone and/or containing chemotherapy is efficient in refractory or relapsed NK/T-cell lymphoma. Invest New Drugs 2013; 31: 469–472.
Miller AB, Hoogstraten B, Staquet M, Winkler A . Reporting results of cancer treatment. Cancer 1981; 47: 207–214.
Shen Q, Ma X, Hu W, Chen L, Huang J, Guo Y . Intensity-modulated radiotherapy versus three-dimensional conformal radiotherapy for stage I-II natural killer/T-cell lymphoma nasal type: dosimetric and clinical results. Radiat Oncol 2013; 8: 152.
Wang H, Li YX, Wang WH, Jin J, Dai JR, Wang SL et al. Mild toxicity and favorable prognosis of high-dose and extended involved-field intensity-modulated radiotherapy for patients with early-stage nasal NK/T-cell lymphoma. Int J Radiat Oncol Biol Phys 2012; 82: 1115–1121.
Bi XW, Li YX, Fang H, Jin J, Wang WH, Wang SL et al. high-dose and extended-field intensity modulated radiation therapy for early-stage NK/T-Cell lymphoma of Waldeyer's ring: dosimetric analysis and clinical outcome. Int J Radiat Oncol Biol Phys 2013; 87: 1086–1093.
Xu PP, Wang Y, Shen Y, Wang L, Shen ZX, Zhao WL . Prognostic factors of Chinese patients with T/NK-cell lymphoma: a single institutionstudy of 170 patients. Med Oncol 2012; 29: 2176–2182.
Tse E, Kwong YL . How I treat NK/T-cell lymphomas. Blood 2013; 121: 4997–5005.
Li S, Feng X, Li T, Zhang S, Zuo Z, Lin P et al. Extranodal NK/T-cell lymphoma, nasal type: a report of 73 cases at MD Anderson Cancer Center. Am J Surg Pathol 2013; 37: 14–23.
Lin N, Song Y, Zheng W, Tu M, Xie Y, Wang X et al. A prospective phase II study of L-asparaginase- CHOP plus radiation in newly diagnosed extranodal NK/T-cell lymphoma, nasal type. J Hematol Oncol 2013; 6: 44.
Jo JC, Yoon DH, Kim S, Lee BJ, Jang YJ, Park CS et al. Clinical features and prognostic model for extranasal NK/T-cell lymphoma. Eur J Haematol 2012; 89: 103–110.
Huang H, Lin Z, Lin X, Cai Q, Xia Z, Jiang W . Long-term outcomes of patients with newly diagnosed extranodal natural killer/T-cell lymphoma treated by etoposide, prednisone, vincristine, cyclophosphamide, and doxorubicin regimen: a single-institution experience. Leuk Lymphoma 2011; 52: 1041–1048.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-nd/4.0/
About this article
Cite this article
Ke, QH., Zhou, SQ., Du, W. et al. Concurrent IMRT and weekly cisplatin followed by GDP chemotherapy in newly diagnosed, stage IE to IIE, nasal, extranodal NK/T-Cell lymphoma. Blood Cancer Journal 4, e267 (2014). https://doi.org/10.1038/bcj.2014.88
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/bcj.2014.88
This article is cited by
-
Treatment of localized extranodal NK/T cell lymphoma, nasal type: a systematic review
Journal of Hematology & Oncology (2018)
-
The optimal timing of radiotherapy in the combined modality therapy for limited-stage extranodal NK/T cell lymphoma (ENKTL): a systematic review and meta-analysis
Annals of Hematology (2018)