Abstract
Aim:
To observe the efficacy and safety of Rocaltrol (calcitriol) and/or Caltrate D (calicum carbonate plus vitamin D) in elderly Chinese women with osteopenia or osteoporosis.
Methods:
One hundred fifty Chinese women aged over 65 years with osteopenia or osteoporosis from three centers were randomly divided into two groups. Seventy-six participants received Caltrate D as one pill daily; the other 74 participants received 0.25 μg Caltrate D plus Rocaltrol daily. The changes in bone mineral density (BMD) served as primary end-points. Height changes, the presence of new vertebral fractures, muscle strength and balance were evaluated.
Results:
The following are the mean percentage changes (and SD) in BMD over 12 months: at L2-L4, 0.83±3.88 in the Caltrate D group and 2.84±4.04 in the Rocaltrol+Caltrate D group (P=0.003, by ANCOVA); at the femoral neck, 0.04±3.94 in the Caltrate D group and 2.01±5.45 in the Rocaltrol+Caltrate D group (P=0.085, by ANCOVA); and in the trochanter, 1.59±4.57 in the Caltrate D group and 3.76±6.25 in the Rocaltrol+Caltrate D group (P=0.053, by ANCOVA). The stand and maximal forward reach test (SMFRT) was significantly enhanced in both groups during the 12 months of treatment, but no significant differences were found between these two groups. No severe adverse event related to these medications occurred throughout the study.
Conclusion:
Treatment with Rocaltrol plus Caltrate D or Caltrate D for 12 months in elderly Chinese postmenopausal women effectively increased BMD at the lumbar spine. Rocaltrol plus Caltrate D was more effective at the lumbar spine than Caltrate D alone.
Similar content being viewed by others
Introduction
Osteoporosis, a common disease among aging people, is characterized by decreased bone mass and microarchitecture deterioration of bone tissue, leading to increased bone fragility and fracture1. Many age-related factors contribute to osteoporosis, such as a decline of gonadal function, a reduction in intestinal calcium absorption, vitamin D insufficiency, decreased production of 1,25(OH)2D3 and secondary hyperparathyroidism2.
Vitamin D insufficiency is common in older people3. If untreated, it leads to bone loss and consequently to an increased risk of fracture. Vitamin D insufficiency may progress to a clinical deficiency, which is associated with severe muscle weakness in elderly adults and often results in a marked disability4, 5 that can be ameliorated within several weeks of vitamin D supplementation. Metabolites of vitamin D not only can increase the intestinal absorption of calcium but also possess other cellular effects6. Calcitriol [1,25 (OH)2 vitamin D, the most active metabolite] has been thought to play an important role in promoting bone formation, since it increases osteocalcin production by osteoblasts7. Calcitriol has been advocated for treatment of osteoporosis, but its effects in such use are inconsistent8, 9. In this randomized, multi-center, open-label, placebo-controlled clinical trial, the effects of 12 months of treatment with Rocaltrol plus Caltrate D or Caltrate D alone on bone mass, muscle function and balance have been investigated in elderly Chinese women.
Materials and methods
Subjects and methods
This was a multi-center, randomized, open-label, controlled clinical trial, conducted by the Endocrinology Department of Peking Union Medical College Hospital, the Department of Gynaecology and Obstetrics of the Chinese PLA General Hospital and the Radiology Institute of Medical Research of Shanghai Fudan University. The study protocol was approved by the institutional review boards at each study site. All subjects gave signed informed consent.
Subjects and study design
Volunteers were recruited through notices posted in the communities surrounding the three centers. Postmenopausal women aged over 65 years were eligible for the study when both of the following criteria were met: a) a bone mineral density (BMD) T score of -1.0 or less at the lumbar spine (meaning that fractures did not involve more than two lumbar vertebrae) and the anatomical structures were suitable for DXA scanning or femoral neck, and b) body mass index (BMI) between 18 and 30 kg/m2. Exclusion criteria included the use of bisphosphonates within the previous 12 months or fluoride within the previous 24 months; tibolone, parathyroid hormone or any derivative, or systemic glucocorticoids (more than 5 mg of prednisone equivalent daily for more than 10 days); inhaled glucocorticoids (more than 2000 μg daily for more than 10 days); anabolic steroids or testosterone within 6 months; and estrogens, selective estrogen receptor modulators, calcitonin, or calcitriol within 3 months of enrollment. Other causes of secondary osteoporosis were excluded, including hyperparathyroidism or hypoparathyroidism, hyperthyroidism or hypothyroidism, hypocalcemia, rheumatoid arthritis, Paget's disease, osteomalacia, and serum creatinine greater than 1.7 mg/dL.
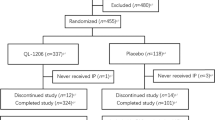
There were 150 subjects enrolled in the study, and 142 of them completed the 12 months of treatment. The study subjects were assigned to either the Caltrate D group or the Rocaltrol+Caltrate D group using randomized permuted blocks. Seventy-six subjects were allocated to the Caltrate D group and received oral treatment of 1 pill/day Caltrate D (containing 600 mg calcium and 125 IU vitamin D, Wyeth Pharmaceuticals, Health Care Products, Suzhou, China). Seventy-four subjects were placed in the Rocaltrol+Caltrate D group, receiving 0.25 μg/day Rocaltrol (Calcitriol, Roche Pharmaceuticals Co, Shanghai, China) and 1 pill/day Caltrate D. At the end of the study 72 women were available for statistical processing in the Caltrate D group and 70 patients in the Rocaltrol+Caltrate D group.
The primary efficacy end points were the percent changes in bone mineral density at the lumbar spine (lumbar vertebrae 2 to 4) and hip after 12 months of treatment. Secondary efficacy end points were changes in height, new vertebral and nonvertebral fractures, grip strength of both hands, the time to perform the five-times-sit-to-stand test (FTSST) and the stand maximal forward reach test (SMFRT).
Measurement of bone mineral density
The BMD of the lumbar spine (L2–L4), right femoral neck (Neck) and trochanter (Troch) was measured at baseline and at 6- and 12-month follow-up using dual-energy X-ray absorptiometry (DXA) (Mode GE Lunar Prodigy, Madison, WI). Scanner software version 5.0 was used for acquisition and analysis. The coefficients of variation (CV) for the DXA measurements were 1.7% (L2–L4), 0.9% (femoral neck) and 1.1% (trochanter). Bone mineral density was expressed as milligrams per square centimeter.
Radiographic analysis
Radiography of the lateral lumbar and thoracic spine was performed at baseline, at 12 months and whenever study subjects presented with new back pain. All radiographs were centrally assessed in a blinded fashion by two trained observers — a radiologist and an independent reader — according to the method of Genant et al10. This method consists of visual inspection of the spine from thoracic vertebra 4 through lumbar vertebra 5, comparing each vertebra with those below and above. If an abnormally shaped vertebra is identified, the anterior, middle, and posterior heights of this vertebra are measured with a ruler and scored as follows: grade 0, normal; grade 1, a decrease in the height of 20 to 25 percent; grade 2, a decrease of 25 to 40 percent; and grade 3, a decrease of 40 percent or more. A new fracture was defined as a decrease in height of at least 15 percent on vertebra graded 0 at baseline and with a grade on the semiquantitative scale of 1, 2, or 3. A new vertebral deformity on a radiograph obtained consequent to new back pain was defined as a symptomatic vertebral fracture.
Nonvertebral fractures were confirmed by radiologic evaluation or by a hospital report. Standing height was measured with a stadiometer at baseline and every six months. The measurement was made three times at each visit, and the mean value of the three measurements was recorded.
Evaluation of muscle function and balance
Hand-grip strength was measured with the WCS-100 digital Hand Dynamometer (Jianming, XinDongHuaDeng Sports Instrument Co, Beijing, China) according to the manufacturer's instruction. Briefly, the participants stood upright, put their arms straight down and gripped the dynamometer. Three trials with brief pauses were allowed for each hand alternately. Subjects were encouraged to exert their maximal grip. The best result from each side was chosen for the analyses11. The CV for the hand dynamometer was 2.8%.
The FTSST12 was administered as follows. Using a chair of standard height (40–45 cm), subjects stood up and sat down five times, as rapidly as possible. Their arms were folded across their chests. Timing with a digital stopwatch began with the command “go” and ceased when subjects stood up for the final time. Two trials were allowed, and subjects were allowed sufficient time between trials to fully recover. The CV for the FTSST was 5.0%.
In the SMFRT13, subjects stood with their arms extended forward along a yardstick. They were instructed to reach forward as far as possible while keeping their feet flat on the floor and without letting their hand touch the yardstick. The location of the middle finger (in cm) was recorded. The trial distance (in cm) was obtained by recording the distance between the tip of the middle finger and the starting position. One practice trial was performed to ensure that patients understood the instructions, and the next trial was recorded. The CV for the SMFRT was 3.6%.
All three tests were performed initially to establish a baseline and again after 6 and 12 months of treatment.
Biochemical measurement
Measurements of biochemical parameters, including calcium, phosphorus, creatinine, albumin, alanine aminotransferase (ALT), alkaline phosphatase (ALP) and glucose in serum collected at baseline and 6 and 12 months were analyzed using routine assays available at the center laboratory.
Safety criteria and adverse events
At each visit, all adverse events were recorded. The degree of association between each event and the study drugs was scored as “no relation,” “possible,” “probable,” or “definite.” The biochemical parameters of liver and renal function, complete blood count (CBC), urine analysis, and kidney ultrasound were also employed as safety indexes.
Statistical analysis
Quantitative values are expressed as mean±SD with 95% confidence intervals. The analysis was based on the intention-to-treat (ITT) principle. With 70 subjects per treatment group, assuming a 20 percent dropout rate, a sample size of 56 subjects in each group was needed to provide 90 percent power to detect a mean difference of 2 percent at a significance level of 0.05. A common standard deviation of 3.6 percent for each percentage change in BMD was assumed. The two independent-samples t test or Wilcoxon test (if data were found not to be normally distributed) were used to compare baseline measurements between the Caltrate D group and the Rocaltrol+Caltrate D group. Comparison between the two groups was made using analysis of co-variance (ANCOVA), adjusting for baseline measurements. Changes in BMD (expressed as percentage changes from baseline) between groups were compared using ANCOVA, adjusting for baseline BMD. Paired-sample t tests were performed to compare the differences between 6- and 12-month and baseline measurements for each treatment group. For all tests, a P value ≤0.05 was considered to indicate statistical significance. All analyses were performed with the SPSS 13.0 software package.
Results
Baseline characteristics, bone mineral density and the parameters of muscle strength and balance for the two groups are shown in Table 1. There were no significant differences in baseline parameters between the two groups except in the BMD at the femoral neck.
Changes in BMD
The BMD measurements before and after treatment are shown in Table 2. After 12 months of treatment, the BMD values at L2–L4, the femoral neck and the trochanter were significantly higher than baseline in the Rocaltrol+Caltrate D group; the BMD values at L2–L4 and the trochanter were significantly higher than baseline in the Caltrate D group. The mean percentage changes of BMD from baseline are reported in Table 3. The BMD at the lumbar spine (L2–L4) significantly increased from baseline by 2.8% (P<0.01 compared with baseline) in the Rocaltrol+Caltrate D group and 0.8% (P<0.05, compared with baseline) in the Caltrate D group at 12 months, respectively. The percentage changes from baseline of BMD at L2–L4 in the Rocaltrol+Caltrate D group were significantly higher than that in the Caltrate D group (P<0.05). Femoral neck BMD increased by 2.0% (P<0.01) and 0.04% from baseline after 12 months of treatment in the Rocaltrol+Caltrate D group and the Caltrate D group, respectively. No significant difference was found in the percentage changes from the baseline BMD at the neck after adjusting for baseline differences (P>0.05). After 12 months of treatment, the BMD at the trochanter had increased by 3.8% (P<0.01 compared with baseline) and 1.6% (P<0.01 compared with baseline) from baseline in the Rocaltrol+Caltrate D group and the Caltrate D group, respectively. The differences in the percentage changes of BMD at the trochanter between these two groups was not significant after adjusting for baseline BMD (P>0.05).
Changes in muscle strength and balance
The grip strength of the left hand was significantly decreased in both groups after 6 months of treatment, but this change was not found after 12 months of treatment. There was no significant change in the grip strength of the right hand in either group during the 12 months of treatment. The FTSST time decreased significantly from baseline in the Caltrate D group (P<0.01) but not in the Rocaltrol+Caltrate D group. The SMFRT time significantly increased in both two groups after 12 months of treatment (Table 4).
Vertebral fractures, body height, and nonvertebral fractures
During the 12 months of treatment, 2 and 1 new vertebral fractures occurred in the Caltrate D group and Rocaltrol+Caltrate D group, respectively. All of these vertebral fractures were asymptomatic. At baseline, the mean height measurements were comparable between these two groups [153.4±5.7 cm (Caltrate D group) vs 154.0±5.5 cm (Rocaltrol+Caltrate D group), P>0.05]. The height changes were not statistically different between the two groups after 12 months of treatment [153.4±5.8 cm (Caltrate D group) vs 153.8±5.4 cm (Rocaltrol+Caltrate D group), P>0.05]. Nonvertebral fractures occurred in two patients in the Rocaltrol+Caltrate D group over the study: one patient with a right femoral neck fracture and another with a clavicular fracture. There were no nonvertebral fractures in the Caltrate D group during the 12 months of treatment.
Withdrawal from the study and adverse events
A total of eight participants (four in each group) withdrew from the study. In the Caltrate D group, one patient withdrew owing to lung cancer. In the Rocaltrol group, one patient withdrew because a fracture was attributable to a severe adverse event but not to the use of study medication, according to clinical judgment. The other six patients withdrew for reasons independent of study medication. There were no clinically relevant changes from baseline in blood pressure, pulse rate, or other physical signs during the study. No significant differences in markers of hepatic and renal function were recorded. The concentration of serum calcium and phosphorus concentrations and the total alkaline phosphatase activities in the two groups remained within normal ranges throughout the study. No renal lithiasis or calcification was detected by ultra-sound examination at the end of the 12 months of treatment.
Discussion
The incidence of osteoporotic fractures increases with age. It is projected that by the year 2050 there will be 900 million men and women who are 65 years of age and older in. As a result, while only 30% of all hip fractures in the world occurred in Asia in 1990, more than 50% of all hip fractures will occur in this continent by the year 2050. By then, the total number of individuals with hip fractures in Asia will be approximately 3.2 million per year14. Prevention of osteoporotic fractures in Asia is therefore of paramount importance.
Two major kinds of drugs are used to prevent or treat osteoporosis; one inhibits bone resorption, the other increases bone formation. The antiresorptive treatments have been used most widely; these include calcium, estrogen, calcitonin, bisphosphonate and vitamin D15. Calcium and standard vitamin D are considered secondary treatment for osteoporosis15, but they have been reported to increase BMD in healthy postmenopausal women16. Our results showed that after 12 months of treatment with Caltrate D (600 mg calcium plus 125 IU vitamin D per day), BMD was significantly increased in L2–L4 and the trochanter in elderly women with low bone mass. These results suggest that calcium and vitamin D are effective at preventing bone loss among elderly Chinese women. A 2005 survey of Chinese nutrition indicated that the average daily calcium intake was 486 mg17. The dietary reference intakes (DRIs) for people over 50 years are lower than 1000 mg. With a 600 mg calcium supplement, calcium intake can reach a DRI of 1000 mg, which should be sufficient to maintain body calcium homeostasis. Although the serum 25(OH)D levels of the subjects were not measured before and after treatment, vitamin D insufficiency is known to be common in older people3. Our previous study showed that the average 25(OH) vitamin D level was 18.9 ng/mL in the Beijing area during the summer18, lower than the recommended optimal concentration of 30 ng/mL. The 125 IU of vitamin D in Caltrate D might be helpful in preserving bone mass and muscle.
Many studies with long-term vitamin D treatment have indicated that hydroxylated form (active vitamin D) has a consistently larger impact on bone density than does standard vitamin D8. The active vitamin D analogs, including 1,25(OH)2D (calcitriol) and 1α(OH)D (alphacalcidol), have been used as a remedy for osteoporosis for many years in developed countries19. The long-term effect of calcitriol treatment on bone mineral density was evaluated in previous studies20, 21. During our 12-month trial, the treatment with calcitriol (Rocaltrol) plus Caltrate D increased the BMD of the lumbar spine, femoral neck and femoral trochanter. The BMD of the femoral neck at baseline in the Rocaltrol+Caltrate D group was significantly lower than in the Caltrate D group, but the difference could no longer be found after 12 months of treatment. There was no significant difference in the percentage changes from the baseline BMD at the neck between the two groups after adjusting for baseline BMD (P>0.05). Papadimitropoulos et al8 concluded that at the lumbar spine and forearm sites, hydroxylated vitamin D doses above 0.5 μg yielded a larger effect than lower doses. The dosage in our study of Calcitriol was smaller — 0.25 μg/d — but the changes of BMD in the lumbar spine were still significant. This suggests that low-dose calcitriol also had a beneficial effect on bone metabolism in elderly Chinese women.
In addition to low bone mineral density, falls are important in the pathogenesis of osteoporotic fractures. Any condition that can increase the risk of falling can also increase the risk of fractures. More than one-third of people aged over 65 years fall each year; one of the most important risk factors is muscle weakness, which may be associated with vitamin D deficiency. A significant correlation between serum 25-hydroxyvitamin D concentration and the occurrence of falls in elderly people was reported in 199922. There is more evidence that supplementation of vitamin D can reduce the incidence of falls. A STOP/IT (Sites Testing Osteoporosis Prevention and Intervention Treatments) trial showed that subjects in the calcitriol group experienced fewer fractures from falls than did the subjects in the estrogen group (odds ratio: 0.78 and 0.94, respectively)23. Interestingly, the FTSST time was significantly shortened in the Caltrate D group but was not significantly different in the Rocaltrol+Caltrate D group. The reason was not clear, but we think it may be related to individual differences. No significant changes in the grip strengths of either hand was found at the end of 12 months. The SMFRT times were markedly lengthened in both the Caltrate D group and the Rocaltrol+Caltrate D group at the end of 12 months of treatment. These data indicate that treatment with either Caltrate D alone or Rocaltrol+Caltrate D may exert beneficial effects on muscle strength and balance in elderly Chinese women.
In Tilyard's study24, patients were treated with 0.25 μg Calcitriol twice a day for three years. The quantity of new vertebral fractures were significantly reduced during the second and third year compared with people receiving calcium alone. Two and one new vertebral fractures, respectively, occurred in the Caltrate D and Rocaltrol D+Caltrate D groups. Two nonvertebral fractures occurred in the Rocaltrol+Caltrate D group, but none occurred in the Caltrate D group.
In several reports, some patients treated with 0.5 μg/d Rocaltrol developed hypercalcinuria, which rarely happened in patients treated with 0.25 μg/d Rocaltrol. In our study, we used 0.25 μg/d Rocaltrol, and no hypercalcemia or renal lithiasis was observed throughout the 12-month treatment. This is consistent with the previous conclusions. Compliance and tolerability of treatments were good; no participant withdrew because of events related to either treatment throughout our 12-month trial.
The main limitations of our study include the small sample size, inadequate power to detect differences in fracture rates, and the limited treatment period. Further studies with larger samples should be done to detect the efficacy of both treatments in fracture reduction. Because this is an open-label, controlled study, but not a double blinded one, the results may have some bias. Some studies showed that calcium or vitamin D may have positive effects on bone mass only within a 1- to 2-year treatment period; the effects may blunt after 2 years. Our study lasted for only 12 months of treatment. The long-term effects and safety of Caltrate D and Rocaltol plus Caltrate D in elderly Chinese postmenopausal women need to be confirmed by further study.
In conclusion, treatment with Rocaltrol plus Caltrate D or Caltrate D alone for 12 months in elderly Chinese postmenopausal women was found to be effective at increasing BMD in the lumbar spine. Compliance and tolerability of the treatments were good. Rocaltrol plus Caltrate D or Caltrate D is effective and safe for the management of elderly Chinese women with low bone mass.
Author contribution
Wei-bo XIA (designed research, performed research, analyzed data and wrote the paper), Zhong-lan ZHANG (performed research), Hong-fu WANG(designed research and performed research), Xun-wu MENG (designed research and performed research), Ying ZHANG (performed research), Guo-ying ZHU (performed research), Xiao-ping XING (performed research), Jian-li LIU (designed research and performed research), Li-hua WANG (performed research), Yan JIANG (performed research), Shi-fang WENG (performed research), Tao XU (statistical analysis), Ying-ying HU (sample collection), Wei YU (performed research), Jun-ping TIAN (BMD measurement).
References
NIH Consensus Development Panel on Osteoporosis Prevention, Diagnosis, and Therapy. Osteoporosis prevention, diagnosis, and therapy. JAMA 2001; 285: 785–95.
Boonen S, Vanderschueren D, Haentjens P, Lips P . Calcium and vitamin D in the prevention and treatment of osteoporosis — a clinical update. J Intern Med 2006; 59: 539–52.
Chapuy MC, Schott AM, Garnero P, Hans D, Delmas PD, Meunier PJ . Healthy elderly French women living at home have secondary hyperparathyroidism and high bone turnover in winter. EPIDOS Study Group. J Clin Endocrinol Metab 1996; 81: 1129–33.
Mingrone G, Greco AV, Castagneto M, Gasbarrini G . A woman who left her wheel chair. Lancet 1999; 353: 806.
Prabhala A, Garg R, Dandona P . Severe myopathy associated with vitamin D deficiency in western New York. Arch Intern Med 2000; 160: 1199–203.
Holick MF . Vitamin D deficiency. N Engl J Med 2007; 357: 266–81.
Pei Y, Zhou XY, Meng XW, Xia WB, Xing XP, Liu HC, et al. Interaction effects between 1,25-dihydroxyvitamin D3 and transforming growth factor-beta1 on the proliferation and differentiation of human embryonic osteoblasts. Zhonghua Yi Xue Za Zhi 2003; 83: 1084–8.(Chinese).
Papadimitropoulos E, Wells G, Shea B, Gillespie W, Weaver B, Zytaruk N, et al. Osteoporosis Methodology Group and The Osteoporosis Research Advisory Group. Meta-analyses of therapies for postmenopausal osteoporosis. VIII: Meta-analysis of the efficacy of vitamin D treatment in preventing osteoporosis in postmenopausal women. Endocr Rev 2002; 23: 560–9.
Bischoff-Ferrari HA, Willett WC, Wong JB, Giovannucci E, Dietrich T, Dawson-Hughes B . Fracture prevention with vitamin D supplementation: a meta-analysis of randomized controlled trials. JAMA 2005; 293: 2257–64.
Genant HK, Jergas M, Palermo L, Nevitt M, Valentin RS, Black D, et al. Comparison of semiquantitative visual and quantitative morphometric assessment of prevalent and incident vertebral fractures in osteoporosis. The Study of Osteoporotic Fractures Research Group. J Bone Miner Res 1996; 11: 984–96.
Rantanen T, Masaki K, Foley D, Izmirlian G, White L, Guralnik JM . Grip strength changes over 27 yr in Japanese-American men. J Appl Physiol 1998; 85: 2047–53.
Whitney SL, Wrisley DM, Marchetti GF, Gee MA, Redfern MS, Furman JM . Clinical measurement of sit-to-stand performance in people with balance disorders: validity of data for the five-times-sit-to-stand test. Phys Ther 2005; 85: 1034–45.
Newton RA . Validity of the multi-directional reach test: a practical measure of limits of stability in older adults. J Gerontol A Biol Sci Med Sci 1997; 56A: M248–252.
Cooper C, Campion G, Melton LJ . Hip fractures in the elderly: a world-wide projection. Osteoporos Int 1992; 2: 25–9.
Marcus R, Wong M, Heath H 3rd, Stock JL. Antiresorptive treatment of postmenopausal osteoporosis: comparison of study designs and outcomes in large clinical trials with fracture as an endpoint. Endocrine Rev 2002; 23: 16–37.
Baeksgaard L, Anderson KP, Hyldstrup L . Calcium and vitamin D supplementation increase spinal BMD in healthy postmenopausal women. Osteoporos Int 1998; 8: 255–60.
Liu XY, Zhao XH, Xu L . Food sources of calcium and iron in the diet of Beijing elderly. J Hygiene Res 2004; 33: 336–8. (Chinese).
Zhou XY, Meng XW, Liu SQ, Chen SS, Song LQ . The study of serum 25-hydroxyvitamin D levels between the people living in Lhasa area in Tibet and Beijing area. Natl Med J China 1995; 75: 261. (Chinese).
Häuselmann HJ, Rizzoli R . A comprehensive review of treatments for postmenopausal osteoporosis. Osteoporos Int 2003; 14: 2–12.
Sairanen S, Kärkkäinen M, Tähtelä R, Laitinen K, Mäkelä P, Lamberg-Allardt C, et al. Bone mass and markers of bone and calcium metabolism in postmenopausal women treated with 1,25-dihydroxyvitamin D (Calcitriol) for four years. Calcif Tissue Int 2000; 67: 122–7.
Shah D, Shroff S, Jankharia B, Parihar A . Effects of calcitriol (Rocaltrol) on spine and femoral neck bone mineral density in postmenopausal osteoporosis. Bombay Hosp J 2002; 44: 236–41.
Venning G . Recent developments in vitamin D deficiency and muscle weakness among elderly people. BMJ 2005; 330: 524–6.
Dawson-Hughes B, Harris SS, Krall EA, Dallal GE . Effect of calcium and vitamin D supplementation on bone density in men and women 65 years of age or older. N Engl J Med 1997; 337: 670–6.
Tilyard MW, Spears GF, Thomson J, Dovey S . Treatment of postmenopausal osteoporosis with calcitriol or calcium. N Engl J Med 1992; 326: 357–62.
Acknowledgements
This work was supported by the Key Technologies R & D Program under grant No 2006BAI02B03 and the National Natural Science Foundation of China (NSFC) under grant No 30370781.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Xia, Wb., Zhang, Zl., Wang, Hf. et al. The efficacy and safety of calcitriol and/or Caltrate D in elderly Chinese women with low bone mass. Acta Pharmacol Sin 30, 372–378 (2009). https://doi.org/10.1038/aps.2009.12
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/aps.2009.12
Keywords
This article is cited by
-
US-guided microwave ablation for primary hyperparathyroidism: a safety and efficacy study
European Radiology (2019)
-
The three different types of paleokarstification and reservoir distribution of Leikoupo Formation, Middle Triassic in the northern Sichuan Basin, China
Carbonates and Evaporites (2018)
-
Differences between the platform-margin shoal reservoirs and the platform-interior shoal reservoirs of the Middle Triassic Leikoupo Formation, Sichuan Basin, China
Carbonates and Evaporites (2014)
-
Comparison of the effects of cholecalciferol and calcitriol on calcium metabolism and bone turnover in Chinese postmenopausal women with vitamin D insufficiency
Acta Pharmacologica Sinica (2012)
-
General depositional features of the carbonate platform gas reservoir of the Lower Triassic Jialingjiang Formation in the Sichuan Basin of southwest China: Moxi gas field of the central basin
Carbonates and Evaporites (2011)