Abstract
Calcium signaling depends on a tightly regulated set of pumps, exchangers and channels that are responsible for controlling calcium fluxes between the different subcellular compartments of the eukaryotic cell. We have recently reported that two members of the highly-conserved UPF0016 family, human TMEM165 and budding yeast Gdt1p, are functionally related and might form a new group of Golgi-localized cation/Ca2+ exchangers. Defects in the human protein TMEM165 are known to cause a subtype of Congenital Disorders of Glycosylation. Using an assay based on the heterologous expression of GDT1 in the bacterium Lactococcus lactis, we demonstrated the calcium transport activity of Gdt1p. We observed a Ca2+ uptake activity in cells expressing GDT1, which was dependent on the external pH, indicating that Gdt1p may act as a Ca2+/H+ antiporter. In yeast, we found that Gdt1p controls cellular calcium stores and plays a major role in the calcium response induced by osmotic shock when the Golgi calcium pump, Pmr1p, is absent. Importantly, we also discovered that, in the presence of a high concentration of external calcium, Gdt1p is required for glycosylation of carboxypeptidase Y and the glucanosyltransferase Gas1p. Finally we showed that glycosylation process is restored by providing more Mn2+ to the cells.
Similar content being viewed by others
Introduction
As a closed compartment, the cell must adapt to environmental changes and has therefore developed intracellular signaling systems that can identify these stimuli and implement cellular responses to counteract the stress. In eukaryotic cells, calcium ions play a key role in the transduction of external signals into the cytosol. Upon stimulation, the signal is generated by a sudden, transient and massive calcium influx into the cytosol from the external medium and/or internal stores. In the yeast Saccharomyces cerevisiae, large increases in the cytosolic calcium concentration have been observed in response to hypo- or hypertonic shock, sugar refeeding, mating pheromone α factor, or Ca2+-depletion of the secretory pathway1. Depending on the stress, calcium can either flow from the external medium through the low-affinity Ca2+ influx system2 and the high-affinity Ca2+ influx system (HACS), composed of three interacting proteins, Cch1p, Mid1p and Ecm73, or be released from the vacuole via the mechanosensitive Ca2+ channel Yvc1p4. The low basal cytosolic calcium concentration [in the range of 50 to 200 nM5] is then rapidly restored by specific transporters that actively pump Ca2+ out of the cytosol.
Accumulation of calcium in the cytosol is directly sensed by different Ca2+-binding proteins, of which calmodulin (CaM) is the best studied. The Ca2+-CaM complex activates several Ca2+-responsive signaling pathways, including the serine/threonine protein phosphatase calcineurin pathway. Calcineurin inhibits the vacuolar Ca2+/H+ exchanger Vcx1p through a, as yet, poorly understood post-translational regulatory mechanism6 and induces expression of PMR1 and PMC1 via dephosphorylation of the transcription factor Crz1p and its subsequent mobilization into the nucleus7. PMR1 encodes a high affinity, low capacity, P-type Ca2+/Mn2+-ATPase primarily required for maintaining a suitable calcium concentration in the Golgi apparatus (around 200 μM) and, indirectly, in the endoplasmic reticulum (ER) (around 10 μM)8. Maintenance of an appropriate calcium concentration in secretory pathway organelles is essential for the activity of many Golgi- and ER-resident enzymes involved in the retention of luminal proteins, export of secretory proteins and protein folding, degradation and maturation8,9. Together with the vacuolar Ca2+-ATPase Pmc1p, Pmr1p also plays a crucial role in detoxifying the cytosol when high calcium concentrations are encountered in the environment, allowing the maintenance of low [Ca2+]cyt levels6 and pmr1 and pmc1 mutants therefore show increased sensitivity to high external Ca2+ 10,11.
Although the calcium transport system has been intensively studied, the molecular identity of some transporters remains unknown. For instance, Miseta et al.10 suggested the existence of an unidentified Ca2+/H+ exchanger that, in the absence of VCX1, is activated upon Ca2+ stress . Other well-known examples are the putative transporters X and M responsible for the influx of external Ca2+ through the plasma membrane which have not yet been identified12.
We recently suggested that Gdt1p is a novel putative Golgi-localized Ca2+/cation antiporter in yeast13. Gdt1p belongs to the UPF0016 family, a highly conserved family of membrane proteins, the members of which display topological similarities with members of the cation/Ca2+ (CaCA) exchanger superfamily14,15. Like the Golgi Ca2+/Mn2+-ATPase Pmr1p, Gdt1p is involved in tolerance to high external Ca2+ concentrations. We previously showed that a strain lacking either of these transporters is sensitive to an increase in the external Ca2+ and that this sensitivity is increased by the loss of both transporters13, suggesting that Gdt1p and Pmr1p are involved in high Ca2+ stress tolerance by two distinct pathways and that one pathway can compensate for the absence of the other. Interestingly, the Ca2+ sensitivity of the gdt1 mutant was subsequently shown to be suppressed by the expression of bacterial orthologs or the human ortholog TMEM16513,15, indicating conservation of function throughout evolution. A defect in the TMEM165 gene is known to cause a subtype of Congenital Disorder of Glycosylation (CDG), a group of rare diseases associated with impaired protein glycosylation16. At the cellular level, we have shown that TMEM165-deficient patients display acidification of the late endosomes and lysosomes and, using patch-clamp analysis in HeLa cells, have observed TMEM165-dependent cation transport13. Based on these data, we suggested that Gdt1p, TMEM165 and other members of the UPF0016 family could form a new group of Ca2+/cation antiporters regulating Ca2+ homeostasis13,15. The defects of glycosylation observed in TMEM165-deficient patients might be the result of an unbalanced Ca2+ concentration in organelles involved in the secretory pathway.
In this report, we present direct evidence that the budding yeast family member Gdt1p transports calcium. Using an in vivo transport assay in Lactococcus lactis cells expressing Gdt1p, we observed that Gdt1p promoted Ca2+ influx into the cytosol. Interestingly, Ca2+ influx was enhanced as the external pH increased, suggesting that Gdt1p couples calcium transport to proton transport and probably acts as a Ca2+/H+ antiporter. Furthermore, we showed in yeast that Gdt1p is involved in the Ca2+ response to environmental osmotic stress when Pmr1p, the major Ca2+ pump under normal conditions, is absent. The amplitude of the Ca2+ response was also found to increase with an increase in the cellular calcium stores. Importantly, we also showed that GDT1 is required for glycosylation of carboxypeptidase Y and the glucanosyltransferase Gas1p, probably by maintaining an appropriate Ca2+ concentration in organelles involved in protein glycosylation. Strikingly we found that this defect was restored by the addition of Mn2+ in the external medium.
Results
Expression of yeast GDT1 in Lactococcus lactis
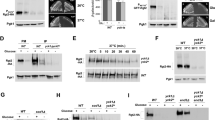
In order to investigate whether Gdt1p was involved in Ca2+ transport, we developed an in vivo functional transport assay based on the heterologous expression of Gdt1p in L. lactis, an organism that has been shown to be a valuable host for expressing eukaryotic membrane proteins17,18. The choice of this expression system was further supported by the absence of Gdt1p orthologs in L. lactis. As shown in Fig. 1A, Western blotting analysis showed that 10His-Strep-TEV-Δ23GDT1, a tagged version of Gdt1p lacking the first 23 amino acids predicted to be a signal peptide, could be expressed under the control of the nisin-inducible promoter in the wild type (WT) NZ9000 strain of L. lactis. We previously observed that the signal peptide of Gdt1p is not essential for the function of the protein and that this tagged version of Gdt1p is functional in yeast (data not shown). Nisin concentration and induction time were optimized to obtain the highest yield of Gdt1p (Fig. S1). Levels of expression were also compared in the WT strain and the “evolved” DML1 strain, which has been shown to be an efficient host for enhanced production of eukaryotic membrane proteins19. As shown in Fig. 1A, Gdt1p expression was clearly higher in the DML1 strain than in the WT and DML1 was therefore chosen to set up the Ca2+ transport assay.
Gdt1p mediates calcium influx in L. lactis cells and this is strongly dependent on the external Ca2+ concentration and pH.
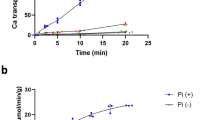
(A) Wild type and evolved DML1 L. lactis cells expressing 10His-Strep-TEV-Δ23GDT1 were grown to an OD600 of 0.4–0.5 and Gdt1p expression was induced with nisin (2.5 μg/L). After 3 h of induction, the total membrane fraction was prepared and Gdt1p expression analyzed by SDS-PAGE followed by Western blotting with anti-Gdt1p antibodies. The negative control (C) consisted of the total membrane fraction from cells containing the empty pNZ8048 vector. (B) Calcium influx time course measurements performed in Fura-2-loaded DML1 cells expressing 10His-Strep-TEV-Δ23GDT1 or transformed with the empty vector pNZ8048 (C). After 3 h of induction, the cells were washed and resuspended in Ca2+-free assay medium pH 7.4. The fluorescence ratio (340/380) was recorded every 10 sec and converted into the [Ca2+]cyt using the equation derived by Liao et al.41. The arrow indicates addition of 0.5 mM CaCl2. (C) Effect of the external Ca2+ concentration (right axis; mM) on Ca2+ accumulation in DML1 L. lactis cells expressing 10His-Strep-TEV-Δ23GDT1 at an extracellular pH of 7.4. (D) Effect of the external pH on Ca2+ accumulation in DML1 cells expressing 10His-Strep-TEV-Δ23GDT1 or transformed with the empty vector pNZ8048 (C) after addition of 0.5 mM CaCl2.
Gdt1p promotes Ca2+ influx into L. lactis in a pH-dependent manner
To determine whether Gdt1p can function as a Ca2+ transporter, we used the Ca2+-sensitive fluorescent probe, Fura-2, to measure changes in the intracellular calcium concentration ([Ca2+]cyt) in L. lactis DML1 cells expressing GDT1 or containing the empty vector. As shown in Fig. 1B, addition of 0.5 mM CaCl2 to DML1 cells expressing GDT1 resulted in a marked increase in the [Ca2+]cyt, whereas no increase was seen in control (C) cells lacking GDT1. Moreover, we observed that the [Ca2+]cyt increased as the extracellular Ca2+ concentration was increased from 0.1 to 2 mM (Fig. 1C). Again, no increase was observed in control (C) cells lacking GDT1 (data not shown). These results demonstrate that Gdt1p mediates Ca2+ transport across the plasma membrane when expressed in L. lactis. As mentioned above, Gdt1p shows striking similarities to members of the CaCA exchanger superfamily which transport Ca2+ across membranes against their electrochemical gradient by utilizing the downhill gradient of other cations, such as H+ or Na+ 14. Furthermore, mutation in the human ortholog TMEM165 has been shown to impair lysosomal and endosomal pH homeostasis13. For those reasons, we previously proposed that TMEM165 and Gdt1p might function as Ca2+/H+ antiporters, using the proton gradient as the driving force for Ca2+ influx across the Golgi membrane. To test this hypothesis, Fura-2-loaded L. lactis cells expressing tagged-Δ23Gdt1p were resuspended in assay buffer at different pH values (7.0 and 8.0) and Ca2+ accumulation was measured after addition of 0.5 mM CaCl2. As shown in Fig. 1D, Gdt1p clearly displayed Ca2+ influx activity that was dependent on the extracellular pH, with Ca2+ transport increasing as the external pH was increased from 7.0 to 8.0. This could be explained by the fact that proton extrusion from the cell is energetically more favorable at a higher pH and Gdt1p can use this energetically favorable condition to couple Ca2+ influx to the H+ efflux. No marked difference in the cell density of the Fura-2-loaded L. lactis cells was seen before and after CaCl2 addition, indicating that CaCl2 addition did not cause cell lysis, regardless of the external pH (Table S1). In addition, centrifugation of the cells after incubation with calcium and examining the fluorescence of the pellet and supernatant demonstrated that Fura-2 was not released into the extracellular medium, as less than 5% of the fluorescence was found in the supernatant and more than 95% in the pellet (data not shown). Together, these results demonstrate that Gdt1p mediates calcium influx in L. lactis and that this is regulated by the pH gradient, meaning that Gdt1p could be a Ca2+/H+ antiporter in yeast.
Gdt1p is involved in calcium response to osmotic stress in yeast
Exposure of yeast cells to saline or osmotic stress triggers a sudden and transient increase in the [Ca2+]cyt that results from Ca2+ influx through the plasma membrane channel Cch1p/Mid1p20 and release from the vacuole via the vacuolar channel Yvc1p4. The resting calcium level is then restored by reabsorption of calcium into the vacuole via the antiporter Vcx1p4.
The role of Gdt1p in the calcium response following saline stress (1.33 M NaCl) was assessed in the WT and the gdt1Δ or pmr1Δ mutant by monitoring changes in the [Ca2+]cyt using the genetically-encoded Ca2+ sensor aequorin. As shown in Fig. 2A, a similar calcium response to saline stress was observed on the WT and the gdt1Δ deletant; both strains had a low resting [Ca2+]cyt of about 0.2 μM and the [Ca2+]cyt increased sharply after exposure to stress, then returned to the basal level within 8 min. This shows that the loss of GDT1 had no effect on the salt-induced calcium response. However, consistent with previous studies3,21, the basal [Ca2+]cyt was markedly higher in the pmr1Δ mutant than in the WT (0.5 versus 0.2 μM). Loss of the Golgi Ca2+-ATPase Pmr1p is known to induce Ca2+-depletion in the secretory compartments that is compensated by a higher Cch1p/Mid1p-mediated Ca2+ influx and a net increase in the resting [Ca2+]cyt is observed in cells grown under normal conditions3. As shown in Fig. 2A, addition of NaCl to pmr1Δ cells led to a higher calcium peak than in WT cells, suggesting either a higher rate of Ca2+ influx into the cytosol or a reduced reabsorption of Ca2+ into the organelles. We then tested whether Gdt1p was involved in the Ca2+ response in the absence of PMR1 by monitoring the [Ca2+]cyt in pmr1Δ cells lacking or overexpressing GDT1. The relative amount of Gdt1p detected in WT, pmr1Δ and pmr1Δ + GDT1 strains is shown in Fig. 2B. As previously reported, the level of Gdt1p is decreased in pmr1Δ cells compared to WT cells while overexpression of GDT1 in pmr1Δ cells increases its level13. Interestingly, as shown in Fig. 2A, deletion of GDT1 in pmr1Δ cells led to a smaller increase in the [Ca2+]cyt than in pmr1Δ cells, while its overexpression (indicated as “ +GDT1”) resulted in a greater increase than in pmr1Δ cells. Similar calcium responses were observed after exposure of the cells to sorbitol (Fig. S2). Together, these results demonstrate that Gdt1p modulates the Ca2+ response in the pmr1Δ strain upon exposure of the cells to saline or osmotic stress. How does Gdt1p alter calcium responses? The simplest answer would be that it modulates the magnitude of the calcium peak by transporting Ca2+ from the Golgi to the cytosol. However, it was also possible that Gdt1p could act in an indirect manner, regulating levels of other Ca2+ transporters or altering internal Ca2+ stores and these possibilities were therefore examined.
Gdt1p is involved in the calcium response to saline stress.
(A) Wild type (WT) or various pmr1 and/or gdt1 yeast mutants expressing apo-aequorin from a plasmid were grown overnight to an OD600 of 1.2 in synthetic medium supplemented with coelenterazine (chromophore) to reconstitute the holoenzyme. Afterwards, 200 μL of each culture was transferred to luminometric tubes. After 2 min, NaCl (saline stress) was added at a final concentration of 1.33 M and the signal monitored for 20 min, then the lumimetric units were converted into the [Ca2+]cyt using the equation from Allen et al.42. All displayed results are representative of those obtained in at least three replicates. (B) Proteins from the membrane-enriched fractions of exponentially growing cells (OD600 = 1.2) of the indicated strains were separated by SDS-PAGE and transferred to nitrocellulose membranes, which were then immunoblotted with antibodies against Gdt1p, Pmc1p, Pmr1p, Vcx1p, or Yvc1p. Coomassie blue-staining of the SDS-polyacrylamide gel indicated equal sample loading (Fig S3). (C) Cultures of the indicated strains were grown in synthetic medium to an OD600 of 3, then the cellular Ca2+ content was measured by ICP-AES on the dry matter. The data were analyzed by one-way analysis of variance (ANOVA) followed by a post-hoc Tukey-Kramer multiple comparisons test. The values are expressed as the mean ± S.E.M (n = 3). Letters not shared in two bars denote a significant difference (p < 0.05). The pmr1Δ+ GDT1 strain corresponds to the pmr1Δ mutant overexpressing GDT1 under the control of the constitutive TPI1 promoter. Extracellular Ca2+ concentration for those three experiments was assessed by ICP-AES to be around 1 mM.
Gdt1p slightly modifies Vcx1p levels without affecting Pmr1p, Pmc1p, or Yvc1p levels
To assess the effect of Gdt1p on Pmr1p, Pmc1p, Yvc1p and Vcx1p levels, we carried out Western blotting analysis on total membrane extracts from WT cells and cells expressing various mutants using specific antibodies. A specific signal was seen in the different strains analyzed, except in those strains in which the corresponding gene was deleted. As shown in Fig. 2B, Pmr1p and Yvc1p levels were similar in the different strains analyzed. In contrast, Pmc1p levels were higher in the PMR1-deleted strains (pmr1Δ, pmr1Δ/gdt1Δ and pmr1Δ + GDT1). This result confirms the findings of Marchi et al.22, who reported calcineurin-dependent compensatory induction of the PMC1 gene due to the loss of PMR1. This higher level of PMC1 expression in pmr1Δ cells was unaffected by the level of expression of GDT1 (lanes 6–8). Finally, Vcx1p levels were higher in the gdt1Δ cells (lane 4) than in the WT cells (lane 1) and were further increased in pmc1Δ cells (lane 5) and pmr1Δ cells (lane 6). Note that, in the pmr1Δ strain, Vcx1p levels were further increased after deletion of GDT1 (lane 7) and decreased when GDT1 was overexpressed (lane 8). These results rule out an indirect effect of Yvc1p, Pmr1p and Pmc1p levels on the Gdt1p-dependent modulation of the Ca2+ response to osmotic stress, as their levels were not modified by deletion or overexpression of GDT1. In addition, the observed changes in Vcx1p levels cannot be linked to the Ca2+ responses, as, regardless of its level of expression in the pmr1Δ strains, Vcx1p should be inhibited as a result of calcineurin activation6. The activation of calcineurin in PMR1-deleted strains is suggested by the overexpression of PMC1 (Fig. 2B and Fig. S4B) and by the observation that steady-state [Ca2+]cyt is higher in these strains (pmr1Δ, pmr1Δ/gdt1Δ and pmr1Δ + GDT1) when treated with FK506, an inhibitor of calcineurin (Fig. S4A).
Gdt1p modulates total calcium content in the pmr1Δ mutant
To assess the effect of Gdt1p on the total Ca2+ content, we used inductively coupled plasma atomic emission spectroscopy (ICP-AES) to measure Ca2+ levels in the WT and the four mutants that we used in the aequorin-based assay. As shown in Fig. 2C, there was no significant difference in Ca2+ content between the WT and the gdt1Δ strain, whereas, in line with previous findings21, the whole-cell Ca2+ content of the pmr1Δ strain was significantly higher than that in the WT (10.6 versus 6.2 mmole/kg dry weight in WT cells, p < 0.05). In a pmr1Δ mutant, Ca2+ depletion of the secretory pathway occurs and is compensated by entry of calcium into the cell. As a result, the [Ca2+]cyt increases, resulting in activation of the calcineurin signaling pathway, leading to overexpression of Pmc1p and, therefore, higher sequestration of Ca2+ into the vacuole22. Interestingly, as shown in Fig. 2C, the cellular Ca2+ concentration in the pmr1Δ mutant was dependent on GDT1 expression, showing a decrease to 8.5 mmole/kg dry weight when GDT1 was deleted and an increase to 13.2 mmole/kg dry weight when Gdt1p was overproduced. These results demonstrate that Gdt1p controls Ca2+ stores in yeast. As the yeast vacuole accumulates over 95% of the total cellular Ca2+ 23, the observed differences mainly reflect modifications of the vacuolar stock and the variations observed in cellular Ca2+ content in pmr1Δ mutants expressing different levels of GDT1 might explain the Ca2+ responses to osmotic shock as a result of modification of the amount of Ca2+ released through the vacuolar calcium channel Yvc1p.
Gdt1p is required for protein glycosylation in yeast
Maintenance of a suitable intraluminal Ca2+ concentration is essential for the activity of many ER- and Golgi-resident enzymes involved in membrane trafficking and protein folding and glycosylation8,9. In this study, we examined whether Gdt1p was required for proper glycosylation of the vacuolar carboxypeptidase Y (CPY)24 and the glucanosyltransferase Gas1p25,26. CPY maturation, which involves N-linked glycosylation, is often used to study the efficiency of the secretory pathway. During its translocation through the ER and Golgi apparatus, CPY undergoes glycosylation at four sites, each glycan accounting for approximatively 2.5 kDa. Afterwards, the glycosylated precursor is delivered to the vacuole and the propeptide segment is proteolytically removed, generating the 61 kDa mature glycosylated form. Gas1p is also often used in glycosylation studies27 and undergoes both N-linked and O-linked glycosylation. A 105 kDa precursor is generated in the ER and is then processed in the Golgi apparatus to the 125 kDa mature glycosylated form. N-deglycosylation results in a 95 kDa protein and complete deglycosylation in a 58 kDa protein.
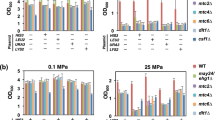
In order to assess the involvement of Gdt1p in protein glycosylation, we used Western blotting to examine glycosylation of CPY and Gas1p in the WT and mutants deleted for GDT1 and/or PMR1 grown either in YD medium alone or in YD medium containing 500 mM Ca2+. As shown in Fig. 3A, both CPY and Gas1p in lysates of the pmr1Δ mutant grown in YD medium migrated more rapidly on SDS gels (lane 3) than those in WT lysates (lane 1), but this difference was not seen when CaCl2 was added to the growth medium (lane 11) (Fig. 3B). This is in good agreement with previous studies on CPY reporting that the size difference results from a glycosylation defect in the Golgi apparatus8 and that addition of Ca2+ bypassed pmr1Δ glycosylation defects28. Interestingly, as shown in Fig. 3A,B, the opposite phenotype was observed for gdt1Δ mutants, as loss of GDT1 did not seem to impair CPY and Gas1p maturation in YD medium (lane 2), but did in Ca2+-supplemented YD medium (lane 10). It is likely that these smaller mature forms reflect defects in glycosylation probably resulting from deregulation of the Ca2+ concentration in the Golgi caused by GDT1 deletion and a high external Ca2+ concentration. As shown in Fig. 3C, this mobility shift was already visible in gdt1Δ cells grown in presence of smaller Ca2+ concentrations (50 mM) and appeared to increase when the Ca2+ concentration is rising. The size differences observed for the double deletant gdt1Δ/pmr1Δ arise from impaired glycosylation in YD medium due to the absence of PMR1 (lane 4) and in Ca2+-containing medium due to the absence of GDT1 (lane 12). Note that the mature form of Gas1p in the gdt1Δ/pmr1Δ mutant is even smaller (lane12), suggesting that both N- and O- glycosylations are impaired.
Glycosylation of CPY and Gas1p is impaired in the gdt1Δ mutant in the presence of 500 mM external Ca2+.
Total membrane protein extracts of the indicated strains were prepared from cultures grown to an OD600 of 1.2 in YD medium (YD) alone (A) or supplemented with 500 mM CaCl2 (Ca2+) (B), increasing Ca2+ concentrations (C), 500 μM MnCl2 (Mn2+) (D) or both 500 mM CaCl2 and 500 μM MnCl2 (Ca2+Mn2+) (E). Where indicated, the proteins were digested with endoglycosidase H (0.5 U/ml). Levels of CPY and Gas1p were then analyzed by SDS-PAGE followed by Western blotting analysis using specific antibodies. The different forms are indicated by arrows. All displayed results are representative of those seen in at least three replicates.
To analyze whether these mobility shifts were due to alterations in the N-glycosylation, the proteins were treated with endoglycosidase H (Endo H) before Western blotting. As shown in Fig. 3A,B, enzymatic removal of N-glycans from CPY led to one unique smaller size product within all four strains in both YD medium (lanes 5–8) and YD medium with added Ca2+ (lanes 13–16). These results confirm that N-linked glycosylation of CPY was impaired in pmr1Δ grown in YD medium and highlight the requirement for Gdt1p in order for N-glycosylation to take place in the presence of high calcium. As shown in Fig. 3A,B, slightly different results were obtained for Gas1p, which, after treatment with Endo H, showed a higher mobility in the pmr1Δ mutant than in the WT and gdt1Δ mutant in YD medium (lane 7) and in the gdt1Δ mutant than in the other two strains in Ca2+-supplemented YD medium (lane 14). Note that, depending on the growth medium, after Endo H digestion, the gdt1Δ/pmr1Δ strain showed a phenotype comparable to either the pmr1Δ strain (lane 8) or the gdt1Δ strain (lane 16). As Gas1p is subjected to both N-linked and O-linked glycosylation25 and Endo H exclusively cleaves N-linked glycans, we propose that these changes in Gas1p mobility reflect impaired O-linked glycosylation. The role of Pmr1p in O-glycosylation was already known9, but the involvement of Gdt1p in O-glycosylation in the presence of Ca2+ is a new observation.
Thus, our results demonstrate, for the first time, that Gdt1p is required for both N-linked and O-linked protein glycosylation at a high external calcium concentration and are consistent with the fact that mutation in the human ortholog, TMEM165, is linked to a genetic disease (Congenital Disorders of Glycosylation) that is caused by defects in glycosylation16.
Mn2+ restores the glycosylation defect observed in gdt1Δ mutant
Mn2+ is an important cation required as cofactor for many glycosyltransferases and glycosidases involved in glycosylation29,30. We analyzed the glycosylation pattern of CPY and Gas1p in presence of 500 μM Mn2+ alone (Mn2+) or combined with 500 mM Ca2+ (Ca2+Mn2+). A similar glycosylation profile to that found in cells grown in YD medium was observed for CPY and Gas1p in presence of Mn2+ (Fig. 3D). Addition of Mn2+ in the growth medium did not affect the glycosylation in the wild type (lane 17) and gdt1Δ strain (lane 18) and did not restore N-linked and O-linked glycosylation defects in pmr1Δ (lane 19) and gdt1Δ/pmr1Δ (lane 20) since changes in mobility were still observable. Note that the mobility shift observed for CPY seems less important in presence of Mn2+ compared to YD suggesting a possible partial restoration of the N-glycosylation. Strikingly no glycosylation defect could be observed in Ca2+Mn2+ medium (Fig. 3E), highlighting that the addition of Mn2+ to Ca2+-containing medium restores the glycosylation defects of gdt1Δ (lane 26) and gdt1Δ/pmr1Δ (lane 28) strains. Interestingly, no mobility shift could be observed after removal of N-glycans by Endo H treatment (lanes 29–32), confirming that the addition of Mn2+ to Ca2+-containing medium restores both N- and O-glycosylation pathways for Gas1p in the gdt1Δ mutants.
Discussion
We recently suggested the existence of a novel Golgi-localized Ca2+ transport system involving members of the highly-conserved UPF0016 family and showed that the yeast protein Gdt1 and its human ortholog, TMEM165, are functionally related and involved in Ca2+ and pH homeostasis13. Mutation in TMEM165 is known to cause a subtype of CDG, a group of rare diseases characterized by defects in glycosylation16. In this study, we provided direct evidence that Gdt1p mediates Ca2+ transport across membranes. When direct transport assays were carried out in L. lactis, Gdt1p-dependent Ca2+ influx through the plasma membrane was observed upon addition of calcium to the external medium (Fig. 1B,C). In addition, we also highlighted the pH-dependency of this Ca2+ transport, as calcium influx increased with an increase in the external pH (Fig. 1D). These results indicate that Gdt1p is a Ca2+ transporter and suggest that Gdt1p could act as a Ca2+/H+ exchanger. In these experiments, it was assumed that the cytosolic pH of L. lactis was not modified by the external pH. Indeed, the Kd of Fura-2 for Ca2+ depends on the pH and directly affects Ca2+ concentration determination. It is generally accepted that cytosolic pH may vary from 0.1 unit per unit of external pH variation43. Nevertheless the intracellular pH dependency should be formally measured in L. lactis cells incubated at pH7.0 and 8.0.
Gdt1p therefore constitutes a novel Ca2+ system in the yeast Golgi apparatus distinct from the well-studied Ca2+/Mn2+-ATPase, Pmr1p, the major calcium pump under normal growth conditions. Pmr1p plays a dual function in the cell. Firstly, given its localization, it is required to provide a suitable Ca2+ (and Mn2+) concentration in the organelles involved in the secretory pathway. Secondly, it is responsible for the detoxification of the cytosol when the external Ca2+ concentration rises. The pmr1Δ mutant shows a growth defect on medium containing a low (~ 3 μM) or high (~400 mM) Ca2+ concentration reflecting, respectively, Ca2+ starvation of the secretory pathway organelles or toxic accumulation of Ca2+ in the cytosol9,11. At the cellular level, PMR1 deletion induces depletion of the secretory Ca2+ pools. Then the cell integrity-related mitogen-activated protein kinase Slt2p becomes activated and stimulates Ca2+ influx by activating the high-affinity Ca2+ uptake system (HACS) in order to refill Ca2+ stores in organelles involved in the secretory pathway3. Although the rate of Ca2+ influx is increased in a pmr1Δ mutant, the rate of Ca2+ efflux is unaffected, leading to an elevated [Ca2+]cyt3,21 and activation of the Ca2+/calcineurin-dependent pathway3,20. Calcineurin inhibits Ca2+ uptake via the HACS channel by a negative feedback mechanism involving direct dephosphorylation of Cch1p31, but induces PMC1 expression via Crz1p22. Ca2+ uptake into the vacuole via Pmc1p is then increased, which results in higher cellular calcium stores21. Our data for the pmr1Δ mutant are consistent with those described in the literature. In the absence of PMR1, we observed an increase in the resting [Ca2+]cyt (Fig. 2A), PMC1 expression (Fig. 2B) and cellular calcium stores (Fig. 2C). We also found that the pmr1Δ mutant showed a higher Ca2+ response after saline or osmotic stress than the wild type. This may be due to the higher Ca2+ stores observed in this strain, which could be responsible for greater release of Ca2+ through the Yvc1p channel. Calcineurin activation is observed by the overexpression of PMC1 in pmr1Δ, pmr1Δ/gdt1Δ and pmr1Δ + GDT1 strains. Moreover FK506 clearly increases steady-state [Ca2+]cyt in those strains probably because of the absence of PMC1 overexpression.
We also investigated the involvement of Gdt1p in Ca2+ homeostasis. We demonstrated that Gdt1p has an impact on the Ca2+ response following saline stress (Fig. 2A) or osmotic stress (Fig. S2) and on the internal Ca2+ stocks (Fig. 2C). When the Golgi Ca2+-ATPase Pmr1p was absent, overexpression of GDT1 induced an increase of the Ca2+ response intensity and total Ca2+ store, while the opposite results were seen in the double deletant gdt1Δ/pmr1Δ. These results could be interpreted as either a direct or indirect role of Gdt1p. The simplest interpretation would be that Gdt1p transports Ca2+ out of the Golgi and directly participates in the Ca2+ response. However, the Ca2+ response results from the integration of different parameters, each of which is capable of modifying the shape of the curve. We tested the effect of two of these parameters, namely the Ca2+ stores and the abundance of other transporters. Our results showed that the Ca2+ response correlated with the total Ca2+ store level, which potentially influences the amount of Ca2+ released through Yvc1p and that this correlation was dependent on Gdt1p. However, the mechanism by which GDT1 overexpression increased the Ca2+ stores is still unknown. One possibility is that Gdt1p transports Ca2+ from the cytosol to the Golgi lumen and the Ca2+ then travels through the cell by vesicular trafficking and accumulates in the vacuole. In addition, we observed that Pmr1p, Pmc1p and Yvc1p levels were not altered in the gdt1Δ mutant compared to the WT, whereas Vcx1p levels were modified in several strains tested (pmr1Δ, pmr1Δ/gdt1Δ and pmr1Δ + GDT1) and an inverse correlation was found between Vcx1p levels and the Ca2+ response. This observation is compatible with the proposed role of Vcx1p in Ca2+ reabsorption after a transient increase in its cytosolic concentration4. However, Vcx1p activity is difficult to evaluate, since, in the pmr1Δ strains, the calcineurin pathway is activated and Vcx1p should be inhibited6.
Together, our data clearly demonstrate that Gdt1p plays an important role in Ca2+ homeostasis. One important question that requires answering is the direction of Ca2+ transport. From a thermodynamic point of view, both directions can be considered. In the hypothesis of a Ca2+/H+ exchange and applying the Gibbs equation to the concentration values reported in literature, the transport of Ca2+ against its gradient (from the cytosol to the Golgi apparatus) would be thermodynamically feasible in exchange for 3 H+. On the other hand, Gdt1p could acidify the Golgi apparatus by transporting H+ against its gradient in a stoichiometry 1:1 (1 H+ for 1 Ca2+) or 2:1 (2 H+ for 1 Ca2+). To date, our current data do not provide the answer, but this point could be resolved, for instance, if an adapted Golgi-localized luminal calcium sensor could be engineered. Note that we cannot exclude the possibility that Gdt1p transport would be reversible, adapting the direction of transport according to conditions.
Using a complementation assay on Ca2+-containing medium, we previously demonstrated that the human ortholog TMEM165 is able to restore the growth defect observed in gdt1Δ on Ca2+-containing medium13, indicating that the function is conserved through evolution. Recently, Foulquier et al.16 reported that mutations of TMEM165 are involved in a subtype of CDG, inborn metabolic diseases linked to defects in the glycosylation pathway. Using MALDI-TOF analysis, they observed a slight defect in sialylation and galactosylation of N-glycans in TMEM165-deficient patients. In the present study, we showed that, in the presence of a high external Ca2+ concentration, Gdt1p was required for N-linked and O-linked protein glycosylation in yeast. Interestingly, Gdt1p and Pmr1p affected glycosylation in different ways, as we showed that CPY and Gas1p glycosylation defects occurred in the pmr1Δ strain in YD medium and were overcome by addition of Ca2+, whereas impaired glycosylation in the gdt1Δ strain was only observed in the presence of added Ca2+ (Fig. 3). Glycosylation requires a suitable concentration of both Ca2+ and Mn2+ in the ER and Golgi9. Mn2+ is needed as cofactor for various enzymes involved in the addition of carbohydrates to proteins undergoing N- and O-glycosylation32, while Ca2+ is important for membrane protein trafficking through the secretory pathway33. In this context, it was recently reported that addition of CaCl2 overcomes the glycosylation defect in the pmr1Δ mutant by stimulating intra-organelle redistribution through intracellular vesicle trafficking of Mn2+ imported into the ER via Spf1p and into the trans-Golgi apparatus via Smf2p28. In our study, we confirmed the important role of Ca2+ in Mn2+ redistribution as we showed that the addition of Mn2+ alone in the growth medium did not restore the glycosylation defects observed in pmr1Δ cells. In contrast to the results for the pmr1Δ strain, lack of Gdt1p only altered glycosylation in the presence of Ca2+, suggesting that the Ca2+ concentration in the Golgi lumen was increased. Based on these observations, Gdt1p would extrude Ca2+ from the Golgi to the cytosol. However, this is currently only a hypothesis as the direction of transport by Gdt1p is not yet known. In this context, restoration of glycosylation defects by Mn2+ in gdt1Δ (and gdt1Δ/pmr1Δ) may be explained by two ways. First, if we consider that Ca2+ concentration is too high in gdt1Δ mutant, Mn2+ could compete with Ca2+ to enter into the Golgi apparatus via the Mn2+/Ca2+-ATPase Pmr1p and therefore reduce the total intraluminal Ca2+ content. Alternatively, Ca2+ could compete with Mn2+ ions within the ER/Golgi lumen and alter glycosylation process. In this case, adding back more Mn2+ could restore the defect. Measuring the ions concentration in the organelle lumen would help to answer this question.
In conclusion, we have demonstrated that Gdt1p is a calcium transporter localized in the Golgi apparatus and plays a crucial role in calcium homeostasis and protein glycosylation. Our results provide new insights into the molecular causes of the defect in glycosylation described in TMEM165-deficient patients.
Experimental procedures
Strains, culture media and growth conditions
The Saccharomyces cerevisiae strains used are listed in Table 1. The BY4741 or BY4742 background strains were purchased from the Euroscarf systematic deletion library (kanamycin deletion cassette). The double-deletant created in this study was obtained by crossing the two single deletants. Non-transformed yeast cells were routinely cultured at 28 °C in YD medium (2% yeast extract KAT, 2% glucose). Cells transformed with plasmids were grown in SD minimal medium [0.7% yeast nitrogen base without amino acids (Difco), 2% glucose, supplemented with all amino acids except those used as selection markers for plasmid maintenance]. Solid media were produced by addition of 2% agar to the mixture. Where indicated, calcium chloride was added at the required concentration; the required amount of calcium chloride dissolved in 50 ml of distilled water was autoclaved and added to the autoclaved medium to avoid precipitation. Lactococcus lactis NZ9000 wild type strain and its derivative, the evolved DML1 strain, were kindly provided by B. Poolman (Groningen, Holland)19; strains transformed with pNZ8048-10His-strep-TEV-Δ23GDT1 (See below) were grown in M17 medium (Merck) supplemented with 1% glucose and 10 μg/ml of chloramphenicol at 28 °C without agitation. After preliminary trials to determine the optimal nisin concentration and induction time for the highest Gdt1p expression (Fig. S1), expression was induced under the control of the nisA promoter by adding nisin at a final concentration of 2.5 μg/L to cultures in the log phase (OD600 ~0.4–0.5) and harvesting the cells 3 hours later.
Vector construction
Yeast and bacterial plasmids were obtained following standard molecular biology protocols and the authenticity of all genetic constructs was validated by sequencing. pRS416-pTPI-GDT1, the yeast plasmid overexpressing GDT1, was obtained previously and has been described by Demaegd et al.13. Yeast transformation was performed following the method of Gietz et al.34. For the heterologous expression of Gdt1p in L. lactis, we used the pNZ8048 plasmid expressing a tagged version of GDT1 lacking the 23 first amino acids corresponding to the predicted signal peptide (pNZ8048-10His-strep-TEV-Δ23GDT1) under the control of the nisin-inducible promoter. This plasmid was constructed as follows. The yeast pRS416 vector containing the sequence coding for the 10His-strep-TEV tagged Δ23GDT1 was used as the DNA template for PCR amplification, then the amplified PCR products were digested with Pst1/SacI and inserted into the pNZ8048 vector carrying the nisin-inducible promoter. Bacterial transformations were performed by electrotransformation (Bio-Rad Laboratories) as described previously by Holo et al.35 and transformants selected by chloramphenicol resistance.
Preparation of the total membrane fraction from L. lactis
Recombinant Gdt1p proteins were expressed in L. lactis as described above, then the cells were harvested (1,700 g for 12 min at 4 °C) and washed once with washing buffer (50 mM Tris/HCl pH 7.6, 500 mM NaCl and 10% glycerol) and centrifuged as above, then the pellet was resuspended in one volume of ice-cold lysis buffer [50 mM Tris/HCl pH 7.6, 500 mM NaCl, 10% glycerol, 1 mM Tris(2-carboxyethyl) phosphine (TCEP), 1 mM PMSF, 2 mg/ml lysozyme and protease inhibitor cocktail (PIC, 4 μg/ml of leupeptin, aprotinin, antipain, pepstatin and chymostatin). After 30 min incubation at 28 °C, the cells were lysed with glass beads (cell pellet/ice-cold lysis buffer/glass beads at a weight ratio of 1:1:1) using a Precellys cell disrupter (Bertin Technologies) at 5,000 RPM for 5 × 30 sec. Cells debris were removed by centrifugation at 1,700 g for 12 min at 4 °C and the supernatant centrifuged at 112,000 g for 60 min at 4 °C to pellet the total membrane fraction, which was resuspended in ice-cold resuspension buffer (50 mM Tris/HCl pH 7.6, 500 mM NaCl, 10% glycerol, 1 mM TCEP, 1 mM PMSF and PIC) and stored at −80 °C.
Yeast crude membrane extracts
Yeast total membrane extracts were prepared from 100 ml of culture at an OD600 of 1.2 as described previously by Morsomme et al.36, except that dithiothreitol was not added at any step of the protocol and the last centrifugation was performed at a higher speed (100,000 g). The protein concentration was determined by the method of Smith et al.37.
Antibodies and Western blotting
Routinely, 15–20 μg of membrane proteins was mixed with 4× concentrated non-reducing sample loading buffer (0.32 M Tris-HCl pH 6.8, 8% SDS, 40% glycerol, 0.02% bromophenol blue). Yeast samples were used as such, while L. lactis samples were incubated at 37 °C for 30 min. Membrane proteins were separated on a 10% SDS/PAGE gel and Western blotting performed as described previously13. Primary rabbit polyclonal antibodies against Pmc1p (1:125 dilution), Pmr1p (1:125 dilution), Yvc1p (1:500 dilution), or Vcx1p (1:500 dilution) were produced for this study by Perbio Science and were raised against a synthetic peptide designed specifically for each protein (Pmc1p, residues 1,155–1,173; Pmr1p, residues 932–950; Yvc1p, residues 657–675; Vcx1p, residues 13–26 + 399–411). The other primary antibodies used were rabbit anti-Gdt1p [1:333; produced previously in our laboratory13], rabbit anti-Gas1p (1:2,000; gift from H. Riezman, Geneva, Switzerland) and rabbit anti-CPY (1:2,000; gift from H. Riezman, Geneva, Switzerland). Horseradish peroxidase-coupled anti-rabbit IgG antibodies (1:10,000 dilution) were purchased from IMEX.
Endoglycosidase H digestion
Samples (17 μg) of total membrane protein were precipitated using chloroform/methanol as described by Wessel et al.38, then solubilized by boiling for 10 min in 16 μl of denaturation buffer [50 mM sodium citrate, pH 5.5 (HCl), 0.5% (w/v) SDS, 0.1 M β-mercaptoethanol], followed by addition of 1 mM PMSF, PIC and 23 μl of citrate buffer [50 mM sodium citrate, pH 5.5 (HCl)] either alone or containing 0.5 unit/ml of endoglycosidase H (Roche). After incubation for 30 min at 30 °C, the reaction was stopped by addition of 15 μl of 4× sample loading buffer.
In vivo Ca2+ transport assay in L. lactis cells
Intracellular Ca2+ concentrations in L. lactis were measured using the fluorescent calcium dye Fura-2/AM, following the method previously described by Chang et al.39, with minor modifications: these were a longer incubation time of cells in the presence of EDTA (30 min at 30 °C without shaking), addition of 1.7 mM probenecid to solution A in the Fura-2 loading step in order to limit its leakage40 and addition of 0.1 mM EGTA to solution A prior to measurement. To assess the pH dependency of calcium transport, the Fura-2/AM-loaded L. lactis cells were resuspended in solution A containing a final concentration of 0.1 mM EGTA and 50 mM Tris-HCl (final pH 7.0 or 8.0) and fluorescence measurements performed on 2 ml aliquots at 25 °C with constant stirring. The intracellular Ca2+ concentration was monitored as the change in the ratio of the fluorescence intensities (510 nm) at the excitation wavelengths of 340 nm and 380 nm using a JASCO FP8500 fluorimeter controlled by Spectra Manager softwareTM. The baseline fluorescence was routinely recorded every 10 sec for 2 min before addition of the indicated concentration of Ca2+. The fluorescence intensity ratio was converted into the Ca2+ concentration using the equation described by Liao et al. (Kd = 315 nM)41.
Aequorin assay
Aequorin-based experiments were performed as described by Demaegd et al.13. In this case, we applied osmotic stress to a culture at an OD600 of 1.2 by adding a final concentration of 1.33 M NaCl or 2.66 M. sorbitol.
Measurement of whole-cell Ca2+ content
Yeast cultures were grown in 50 ml of synthetic medium at 28 °C to a final OD600 of 3, then were collected by vacuum filtration using membrane filters (Millipore, 0.45 μm pore size) and washed successively with 1 mM ethylene glycol tetraacetic acid disodium salt solution and water. The cells were then resuspended in 10 ml of water and dried in an oven at 70 °C for 24 h, then in a desiccator for 24 h. The dried matter was weighted and mineralized by heating at 500 °C overnight, then the ashed sample was dissolved in 65% HNO3 and used for inductively couple plasma atomic emission spectroscopy (ICP-AES) analysis. Ca2+ measurements were performed using a ICAP 6500 spectrometer (Thermo Scientific) and the cellular Ca2+ concentration calculated based on the dry weight of the samples and the dilution factor.
Additional Information
How to cite this article: Colinet, A.-S. et al. Yeast Gdt1 is a Golgi-localized calcium transporter required for stress-induced calcium signaling and protein glycosylation. Sci. Rep. 6, 24282; doi: 10.1038/srep24282 (2016).
References
Cunningham, K. W. Acidic calcium stores of Saccharomyces cerevisiae. Cell Calcium 50, 129–38 (2011).
Muller, E. M., Locke, E. G. & Cunningham, K. W. Differential regulation of two Ca(2+) influx systems by pheromone signaling in Saccharomyces cerevisiae. Genetics 159, 1527–38 (2001).
Locke, E. G., Bonilla, M., Liang, L., Takita, Y. & Cunningham, K. W. A homolog of voltage-gated Ca(2+) channels stimulated by depletion of secretory Ca(2+) in yeast. Mol Cell Biol 20, 6686–94 (2000).
Denis, V. & Cyert, M. S. Internal Ca(2+) release in yeast is triggered by hypertonic shock and mediated by a TRP channel homologue. J Cell Biol 156, 29–34 (2002).
Cui, J., Kaandorp, J. A., Sloot, P. M., Lloyd, C. M. & Filatov, M. V. Calcium homeostasis and signaling in yeast cells and cardiac myocytes. FEMS Yeast Res 9, 1137–47 (2009).
Cunningham, K. W. & Fink, G. R. Calcineurin inhibits VCX1-dependent H+/Ca2+ exchange and induces Ca2+ ATPases in Saccharomyces cerevisiae. Mol Cell Biol 16, 2226–37 (1996).
Stathopoulos, A. M. & Cyert, M. S. Calcineurin acts through the CRZ1/TCN1-encoded transcription factor to regulate gene expression in yeast. Genes Dev 11, 3432–44 (1997).
Antebi, A. & Fink, G. R. The yeast Ca(2+)-ATPase homologue, PMR1, is required for normal Golgi function and localizes in a novel Golgi-like distribution. Mol Biol Cell 3, 633–54 (1992).
Durr, G. et al. The medial-Golgi ion pump Pmr1 supplies the yeast secretory pathway with Ca2+ and Mn2+ required for glycosylation, sorting and endoplasmic reticulum-associated protein degradation. Mol Biol Cell 9, 1149–62 (1998).
Miseta, A., Kellermayer, R., Aiello, D. P., Fu, L. & Bedwell, D. M. The vacuolar Ca2+/H+ exchanger Vcx1p/Hum1p tightly controls cytosolic Ca2+ levels in S. cerevisiae. FEBS Lett 451, 132–6 (1999).
Szigeti, R., Miseta, A. & Kellermayer, R. Calcium and magnesium competitively influence the growth of a PMR1 deficient Saccharomyces cerevisiae strain. FEMS Microbiol Lett 251, 333–9 (2005).
Cui, J. et al. Simulating calcium influx and free calcium concentrations in yeast. Cell Calcium 45, 123–32 (2009).
Demaegd, D. et al. Newly characterized Golgi-localized family of proteins is involved in calcium and pH homeostasis in yeast and human cells. Proc Natl Acad Sci USA 110, 6859–64 (2013).
Cai, X. & Lytton, J. The cation/Ca(2+) exchanger superfamily: phylogenetic analysis and structural implications. Mol Biol Evol 21, 1692–703 (2004).
Demaegd, D., Colinet, A. S., Deschamps, A. & Morsomme, P. Molecular evolution of a novel family of putative calcium transporters. PLos One 9, e100851 (2014).
Foulquier, F. et al. TMEM165 deficiency causes a congenital disorder of glycosylation. Am J Hum Genet 91, 15–26 (2012).
van de Guchte, M., Kok, J. & Venema, G. Gene expression in Lactococcus lactis. FEMS Microbiol Rev 8, 73–92 (1992).
Mierau, I. & Kleerebezem, M. 10 years of the nisin-controlled gene expression system (NICE) in Lactococcus lactis. Appl Microbiol Biotechnol 68, 705–17 (2005).
Linares, D. M., Geertsma, E. R. & Poolman, B. Evolved Lactococcus lactis strains for enhanced expression of recombinant membrane proteins. J Mol Biol 401, 45–55 (2010).
Matsumoto, T. K. et al. An osmotically induced cytosolic Ca2+ transient activates calcineurin signaling to mediate ion homeostasis and salt tolerance of Saccharomyces cerevisiae. J Biol Chem 277, 33075–80 (2002).
Halachmi, D. & Eilam, Y. Elevated cytosolic free Ca2+ concentrations and massive Ca2+ accumulation within vacuoles, in yeast mutant lacking PMR1, a homolog of Ca2+-ATPase. FEBS Lett 392, 194–200 (1996).
Marchi, V., Sorin, A., Wei, Y. & Rao, R. Induction of vacuolar Ca2+-ATPase and H+/Ca2+ exchange activity in yeast mutants lacking Pmr1, the Golgi Ca2+-ATPase. FEBS Lett 454, 181–6 (1999).
Dunn, T., Gable, K. & Beeler, T. Regulation of cellular Ca2+ by yeast vacuoles. J Biol Chem 269, 7273–8 (1994).
Klionsky, D. J., Herman, P. K. & Emr, S. D. The fungal vacuole: composition, function and biogenesis. Microbiol Rev 54, 266–92 (1990).
Fankhauser, C. & Conzelmann, A. Purification, biosynthesis and cellular localization of a major 125-kDa glycophosphatidylinositol-anchored membrane glycoprotein of Saccharomyces cerevisiae. Eur J Biochem 195, 439–48 (1991).
Gatti, E., Popolo, L., Vai, M., Rota, N. & Alberghina, L. O-linked oligosaccharides in yeast glycosyl phosphatidylinositol-anchored protein gp115 are clustered in a serine-rich region not essential for its function. J Biol Chem 269, 19695–700 (1994).
Gentzsch, M. & Tanner, W. Protein-O-glycosylation in yeast: protein-specific mannosyltransferases. Glycobiology 7, 481–6 (1997).
Garcia-Rodriguez, N. et al. Manganese Redistribution by Calcium-stimulated Vesicle Trafficking Bypasses the Need for P-type ATPase Function. J Biol Chem 290, 9335–47 (2015).
Pedersen, L. C. et al. Heparan/chondroitin sulfate biosynthesis. Structure and mechanism of human glucuronyltransferase I. J Biol Chem 275, 34580–5 (2000).
Charnock, S. J. & Davies, G. J. Structure of the nucleotide-diphospho-sugar transferase, SpsA from Bacillus subtilis, in native and nucleotide-complexed forms. Biochemistry 38, 6380–5 (1999).
Bonilla, M., Nastase, K. K. & Cunningham, K. W. Essential role of calcineurin in response to endoplasmic reticulum stress. EMBO J 21, 2343–53 (2002).
Petrova, P., Koca, J. & Imberty, A. Molecular dynamics simulations of solvated UDP-glucose in interaction with Mg2+ cations. Eur J Biochem 268, 5365–74 (2001).
Carnell, L. & Moore, H. P. Transport via the regulated secretory pathway in semi-intact PC12 cells: role of intra-cisternal calcium and pH in the transport and sorting of secretogranin II. J Cell Biol 127, 693–705 (1994).
Gietz, D., St Jean, A., Woods, R. A. & Schiestl, R. H. Improved method for high efficiency transformation of intact yeast cells. Nucleic Acids Res 20, 1425 (1992).
Holo, H. & Nes, I. F. High-Frequency Transformation, by Electroporation, of Lactococcus lactis subsp. cremoris Grown with Glycine in Osmotically Stabilized Media. Appl Environ Microbiol 55, 3119–23 (1989).
Morsomme, P. et al. Characterization of a hyperthermophilic P-type ATPase from Methanococcus jannaschii expressed in yeast. J Biol Chem 277, 29608–16 (2002).
Smith, P. K. et al. Measurement of protein using bicinchoninic acid. Anal Biochem 150, 76–85 (1985).
Wessel, D. & Flugge, U. I. A method for the quantitative recovery of protein in dilute solution in the presence of detergents and lipids. Anal Biochem 138, 141–3 (1984).
Chang, Y. et al. Structural basis for a pH-sensitive calcium leak across membranes. Science 344, 1131–5 (2014).
Di Virgilio, F., Steinberg, T. H. & Silverstein, S. C. Inhibition of Fura-2 sequestration and secretion with organic anion transport blockers. Cell Calcium 11, 57–62 (1990).
Liao, J. et al. Structural insight into the ion-exchange mechanism of the sodium/calcium exchanger. Science 335, 686–90 (2012).
Allen, D. G., Blinks, J. R. & Prendergast, F. G. Aequorin luminescence: relation of light emission to calcium concentration–a calcium-independent component. Science 195, 996–8 (1977).
Slonczewski, J. L., Fujisawa, M., Dopson, M. & Krulwich, T. A. Cytoplasmic pH measurement and homeostasis in bacteria and archaea. Adv. Microb. Physiol. 55, 1–79 (2009).
Acknowledgements
The authors thank M. Ghislain and R. Rao for providing plasmids, H. Riezman for providing anti-Gas1p and anti-CPY antibodies, B. Poolman for providing the DML1 strain, A. Iserentant for technical assistance with the calcium store measurements in yeast, E. Peiter for helpful advice with the aequorin assay, G. Matthijs and E. Van Schaftingen for discussions. The work was supported by grants from the Fonds National de la Recherche Scientifique (FNRS) (to P.M., P.H. and P.S.) and the Communauté française de Belgique–Actions de Recherches Concertées (to P.M., P.H. and A.D.). A.-S.C., L.T., M.-C.D. and D.D. are research fellows at the ‘Fonds pour le Formation à la Recherche dans l’Industrie et dans l’Agriculture’.
Author information
Authors and Affiliations
Contributions
A.-S.C., P.S., A.D., D.D., M.-C.D., L.T., M.L.C., F.F., P.H. and P.M. designed research; A.-S.C., P.S., D.D., A.D., M.-C.D., L.T. and M.L.C. performed research; A.-S.C., P.S., D.D., A.D., M.-C.D., L.T., M.L.C., P.H. and P.M. analyzed data; and A.-S.C., P.S. and P.M. wrote the paper.
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Electronic supplementary material
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Colinet, AS., Sengottaiyan, P., Deschamps, A. et al. Yeast Gdt1 is a Golgi-localized calcium transporter required for stress-induced calcium signaling and protein glycosylation. Sci Rep 6, 24282 (2016). https://doi.org/10.1038/srep24282
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep24282
This article is cited by
-
Calmodulin kinase 2 genetically interacts with Rch1p to negatively regulate calcium import into Saccharomyces cerevisiae after extracellular calcium pulse
Archives of Microbiology (2022)
-
Bruchid beetle ovipositioning mediated defense responses in black gram pods
BMC Plant Biology (2021)
-
A new pH sensor localized in the Golgi apparatus of Saccharomyces cerevisiae reveals unexpected roles of Vph1p and Stv1p isoforms
Scientific Reports (2020)
-
Original association of ion transporters mediates the ECM-induced breast cancer cell survival: Kv10.1-Orai1-SPCA2 partnership
Scientific Reports (2019)
-
CaGdt1 plays a compensatory role for the calcium pump CaPmr1 in the regulation of calcium signaling and cell wall integrity signaling in Candida albicans
Cell Communication and Signaling (2018)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.