Abstract
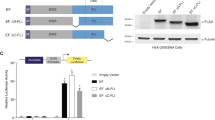
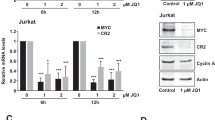
The REL gene is amplified in many human B-cell lymphomas and we have previously shown that expression of REL from a retroviral vector can malignantly transform chicken spleen cells in vitro. To identify REL protein functions necessary for malignant transformation, we have performed deletion analysis on REL sequences encoding residues of two C-terminal subdomains that are involved in transcriptional activation. We find that deletion of both C-terminal transactivation subdomains abolishes the ability of REL to transform chicken spleen cells in vitro. In contrast, deletion of either transactivation subdomain alone, which reduces the transactivation ability of REL, enhances the transforming activity of REL. Transforming REL mutants missing C-terminal sequences can also be selected at a low frequency in vitro. The REL transactivation domain can be functionally replaced in transformation assays by a portion of the VP16 transactivation domain that activates at a level similar to REL-transforming mutants. We also find that deletion of 29 C-terminal amino acids causes the subcellular localization of REL to change from cytoplasmic to nuclear in chicken embryo fibroblasts. In contrast, wild-type REL and all transforming REL mutants are located primarily in the cytoplasm of transformed spleen cells. Nevertheless, treatment of transformed spleen cells with leptomycin B causes wild-type REL and two REL mutants to relocalize to the nucleus, and nuclear extracts from these transformed cells contain REL DNA-binding activity. Taken together, these results suggest the following: (1) that REL must activate transcription to transform cells in vitro; (2) that a reduced level of transactivation enhances the oncogenicity of REL; (3) that REL shuttles from the cytoplasm to the nucleus in transformed chicken spleen cells; and (4) that mutations in REL, in addition to amplifications, could activate its oncogenicity in human lymphomas.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Barkett M, Dooher JE, Lemonnier L, Simmons L, Scarpati JN, Wang Y and Gilmore TD . (2001). Biochim. Biophys. Acta, 1526, 25–36.
Barth TF, Bentz M, Leithäuser F, Stilgenbauer S, Siebert R, Schlotter M, Schlenk RF, Döhner H and Möller P . (2001). Genes Chromosom. Cancer, 31, 316–325.
Barth TFE, Döhner H, Werner CA, Stilgenbauer S, Schlotter M, Pawlita M, Lichter P, Möller P and Bentz M . (1998). Blood, 91, 4321–4330.
Barth TFE, Martín-Subero JI, Joos S, Menz CK, Hasel C, Mechterscheimer, Parwaresch RM, Lichter P, Siebert R and Möller P . (2003). Blood, 101, 3681–3686.
Bull P, Morley KL, Hoekstra MF, Hunter T and Verma IM . (1990). Mol. Cell. Biol., 10, 5473–5485.
Capobianco AJ, Simmons DL and Gilmore TD . (1990). Oncogene, 5, 257–265.
Chen C, Agnès F and Gélinas C . (1999). Mol. Cell. Biol., 19, 307–316.
Donnelly GB, Teruya-Feldstein J, Quin J, Zelenetz AD, Houldsworth J and Chaganti RS . (2001). Blood, 98, 302.
Engelke U, Whittaker L and Lipsick JS . (1995). Virology, 208, 467–477.
Epinat J-C, Kazandjian D, Harkness DD, Petros S, Dave J, White DW and Gilmore TD . (2000). Oncogene, 19, 599–607.
Gilmore TD . (1999). Oncogene, 18, 6925–6937.
Gilmore TD . (2000). Oncogene, 19, 599–607.
Gilmore TD, Cormier C, Jean-Jacques J and Gapuzan M-E . (2001). Oncogene, 20, 7098–7103.
Gilmore TD and Temin HM . (1986). Cell, 44, 791–800.
Gilmore TD, White DW, Sarkar S and Sif S . (1995). The DNA Provirus: Howard Temin's Scientific Legacy Cooper GM, Greenberg Temin R and Sugden B (eds). American Society for Microbiology Press: Washington, DC, pp 109–128.
Goff LK, Neat MJ, Crawley CR, Jones L, Jones E, Lister TA and Gupta RK . (2000). Br. J. Haematol., 111, 618–625.
Grigoriadis G, Zhan Y, Grumont RJ, Metcalf D, Handman E, Cheers C and Gerondakis S . (1996). EMBO J., 15, 7099–7107.
Houldsworth J, Mathew S, Rao PH, Dyomina K, Louie DC, Parsa N, Offit K and Chaganti RSK . (1996). Blood, 87, 25–29.
Hrdlicková R, Nehyba J and Humphries EH . (1994). J. Virol., 68, 2371–2382.
Hrdlicková R, Nehyba J, Roy A, Humphries EH and Bose Jr HR . (1995). J. Virol., 69, 403–413.
Joos S, Menz CK, Wrobel G, Siebert R, Gesk S, Ohl S, Mechtersheimer G, Trümper L, Möller P, Lichter P and Barth TFE . (2002). Blood, 99, 1381–1387.
Joos S, Otaño-Joos MI, Ziegler S, Brüderlein S, du Manoir S, Bentz M, Möller P and Lichter P . (1996). Blood, 87, 1571–1578.
Kabrun N, Bumstead N, Hayman MJ and Enrietto PJ . (1990). Mol. Cell. Biol., 10, 4788–4794.
Kalaitzidis D, Davis RE, Rosenwald A, Staudt LM and Gilmore TD . (2002). Oncogene, 21, 8759–8768.
Kalaitzidis D and Gilmore TD . (2002). Genes Chromosom. Cancer, 34, 129–135.
Kamens J, Richardson P, Mosialos G, Brent R and Gilmore T . (1990). Mol. Cell. Biol., 10, 2840–2847.
Köntgen F, Grumont RJ, Strasser A, Metcalf D, Li R, Tarlinton D and Gerondakis S . (1995). Genes Dev., 9, 1965–1977.
Kralova J, Liss AS, Bargmann W, Pendleton C, Varadarajan J, Ulug E and Bose Jr HR . (2002). J. Virol., 76, 11960–11970.
Lu D, Thompson JD, Gorski GK, Rice NR, Mayer MG and Yunis JJ . (1991). Oncogene, 6, 1235–1241.
Martin AG, San-Antonio B and Fresno M . (2001). J. Biol. Chem., 276, 15840–15849.
Martín-Subero JI, Gesk S, Harder L, Sonoki T, Tucker PW, Schlegelberger B, Grote W, Novo FJ, Calasanz MJ, Hansmann ML, Dyer MJS and Siebert R . (2002). Blood, 99, 1474–1477.
Molinari E, Gilman M and Natesan S . (1999). EMBO J., 18, 6439–6447.
Mosialos G, Hamer P, Capobianco AJ, Laursen RA and Gilmore TD . (1991). Mol. Cell. Biol., 11, 5867–5877.
Nagy M, Balazs, Adam Z, Petko Z, Timar B, Szereday Z, Laszlo T, Warnke TA and Matolcsy A . (2000). Leukemia, 14, 2142–2148.
Neat MJ, Foot N, Jenner M, Goff L, Ashcroft K, Burford D, Dunham A, Norton A, Lister TA and Fitzgibbon J . (2001). Genes Chromosom. Cancer, 32, 236–243.
Nehyba J, Hrdlicková R and Humphries EH . (1994). J. Virol., 68, 2039–2050.
Palanisamy N, Abou-Elella AA, Chaganti SR, Houldsworth J, Offit K, Louie DC, Terayu-Feldstein J, Cigudosa JC, Rao PH, Sanger WG, Weisenburger DD and Chaganti RS . (2002). Genes Chromosom. Cancer, 33, 114–122.
Rao PH, Houldsworth J, Dyomina K, Parsa NZ, Cigudosa JC, Louie DC, Popplewell L, Offit K, Jhanwar SC and Chaganti RSK . (1998). Blood, 92, 234–240.
Richardson PM and Gilmore TD . (1991). J. Virol., 65, 3122–3130.
Rosenwald A, Wright G, Chan WC, Connors JM, Campo E, Fisher RI, Gascoyne RD, Muller-Hermelink HK, Smeland EB, Giltnane JM, Hurt EM, Zhao H, Averett L, Yang L, Wilson WH, Jaffe ES, Simon R, Klausner RD, Powell J, Duffey PL, Longo DL, Greiner TC, Weisenburger DD, Sanger WG, Dave BJ, Lynch JC, Vose J, Armitage JO, Montserrat E, Lopez-Guillermo A, Grogan TM, Miller TP, LeBlanc M, Ott G, Kvaloy S, Delabie J, Holte H, Krajci P, Stokke T and Staudt LM . (2002). New Engl. J. Med., 346, 1937–1947.
Sachdev S, Diehl JA, McKinsey TA, Hans A and Hannink M . (1997). Oncogene, 14, 2585–2594.
Sachdev S and Hannink M . (1998). Mol. Cell. Biol., 18, 5445–5456.
Sambrook J, Fritsch EF and Maniatis T . (1989). Molecular Cloning: A Laboratory Manual. Cold Spring Harbor Press: Cold Spring Harbor, NY.
Sarkar S and Gilmore TD . (1993). Oncogene, 8, 2245–2252.
Schreiber E, Matthias P, Muller MM and Schaffner W . (1989). Nucleic Acids Res., 17, 6419.
Sif S, Capobianco AJ and Gilmore TD . (1993). Oncogene, 8, 2501–2509.
Smardova J, Walker A, Morrison LE, Kabrun N and Enrietto PJ . (1995). Oncogene, 10, 2017–2026.
Verma IM, Stevenson JK, Schwarz EM, Van Antwerp D and Miyamoto S . (1995). Genes Dev., 9, 2723–2735.
Wang Y and Gilmore TD . (2001). Biochim. Biophys. Acta, 1538, 260–272.
White DW and Gilmore TD . (1996). Oncogene, 13, 891–899.
White DW, Pitoc GA and Gilmore TD . (1996). Mol. Cell. Biol., 16, 1169–1178.
Acknowledgements
We thank Nancy Rice for anti-REL antiserum and Joseph Lipsick for the GAL4 site luciferase reporter plasmid. We also thank Jims Jean-Jacques for excellent technical assistance, and Demetrios Kalaitzidis and Mei-Chih Liang for helpful discussions. This work was supported by NIH grant CA47763 (to TDG). DS was partially supported by a Pre-doctoral Fellowship from the Natural Sciences & Engineering Research Council of Canada.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Starczynowski, D., Reynolds, J. & Gilmore, T. Deletion of either C-terminal transactivation subdomain enhances the in vitro transforming activity of human transcription factor REL in chicken spleen cells. Oncogene 22, 6928–6936 (2003). https://doi.org/10.1038/sj.onc.1206801
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.onc.1206801
Keywords
This article is cited by
-
The NF-κB subunit c-Rel regulates Bach2 tumour suppressor expression in B-cell lymphoma
Oncogene (2016)
-
Overexpression of an activated REL mutant enhances the transformed state of the human B-lymphoma BJAB cell line and alters its gene expression profile
Oncogene (2009)
-
Deletion analysis and alternative splicing define a transactivation inhibitory domain in human oncoprotein REL
Oncogene (2008)
-
An optimal range of transcription potency is necessary for efficient cell transformation by c-Rel to ensure optimal nuclear localization and gene-specific activation
Oncogene (2007)
-
Mutation of an IKK phosphorylation site within the transactivation domain of REL in two patients with B-cell lymphoma enhances REL's in vitro transforming activity
Oncogene (2007)