Abstract
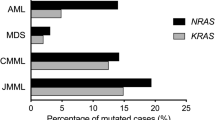
Activating mutations in the RAS oncogenes are among the most common genetic alterations in human cancers, including patients with acute lymphoblastic leukemia (ALL). We sought to define the frequency and spectrum, and possible prognostic importance, of N- and K-RAS mutations in children with ALL treated with contemporary therapy. Leukemic blast DNA from 870 children was analyzed for the presence of activating mutations in the N- or K-RAS oncogenes using a sensitive mutation detection algorithm. RAS mutations were present in the blasts of 131 (15.1%) pediatric ALL patients. The spectrum of mutations included 81 (9.3%) mutations of codons 12/13 of N-RAS, 12 (1.4%) mutations of codon 61 of N-RAS, 39 (4.5%) mutations of codons 12/13 of K-RAS, and 2 (0.2%) mutations of codon 61 of K-RAS. The presence of N- or K-RAS mutations was not associated with white blood cell count at diagnosis, sex, race, extramedullary testicular involvement, central nervous system disease, or NCI/CTEP ALL Risk Group. Patients with an exon 1 K-RAS mutation (codons 12/13) were significantly younger at diagnosis (P=0.001) and less frequently B-lineage phenotype (P=0.01). RAS mutation status did not predict overall survival, event-free survival and disease-free survival. While N- and K-RAS mutations can be identified in 15% of children with newly diagnosed ALL, they do not represent a significant risk factor for outcome using contemporary chemotherapy regimens.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Beaupre DM, Kurzrock R . RAS and leukemia: from basic mechanisms to gene-directed therapy. J Clin Oncol 1999; 17: 1071–1079.
Bos JL . All in the family? New insights and questions regarding interconnectivity of RAS, Rap1 and Ral. EMBO J 1998; 17: 6776–6782.
Barbacid M . RAS oncogenes: their role in neoplasia. Eur J Clin Invest 1990; 20: 225–235.
Jackson JH, Cochrane CG, Bourne JR, Solski PA, Buss JE, Der CJ . Farnesol modification of Kirsten-RAS exon 4B protein is essential for transformation. Proc Natl Acad Sci USA 1990; 87: 3042–3046.
Willumsen BM, Norris K, Papageorge AG, Hubbert NL, Lowy DR . Harvey murine sarcoma virus p21 RAS protein: biological and biochemical significance of the cysteine nearest the carboxy terminus. EMBO J 1984; 3: 2581–2585.
Bos JL . ras oncogenes in human cancer: a review. Cancer Res 1989; 49: 4682–4689.
Lubbert M, Mirro Jr J, Miller CW, Kahan J, Isaac G, Kitchingman G et al. N-RAS gene point mutations in childhood acute lymphocytic leukemia correlate with a poor prognosis. Blood 1990; 75: 1163–1169.
Yokota S, Nakao M, Horiike S, Seriu T, Iwai T, Kaneko H et al. Mutational analysis of the N-RAS gene in acute lymphoblastic leukemia: a study of 125 Japanese pediatric cases. Int J Hematol 1998; 67: 379–387.
Robison LL, Buckley JD, Bunin G . Assessment of environmental and genetic factors in the etiology of childhood cancers: the Childrens Cancer Group epidemiology program. Environ Health Perspect 1995; 103: 111–116.
Linet MS, Hatch EE, Kleinerman RA, Robison LL, Kaune WT, Friedman DR et al. Residential exposure to magnetic fields and acute lymphoblastic leukemia in children (see comments). N Engl J Med 1997; 337: 1–7.
Lubin JH, Linet MS, Boice Jr JD, Buckley J, Conrath SM, Hatch EE et al. Case–control study of childhood acute lymphoblastic leukemia and residential radon exposure. J Natl Cancer Inst 1998; 90: 294–300.
Brondum J, Shu XO, Steinbuch M, Severson RK, Potter JD, Robison LL . Parental cigarette smoking and the risk of acute leukemia in children. Cancer 1999; 85: 1380–1388.
Shu XO, Stewart P, Wen WQ, Han D, Potter JD, Buckley JD et al. Parental occupational exposure to hydrocarbons and risk of acute lymphocytic leukemia in offspring. Cancer Epidemiol Biomarkers Prev 1999; 8: 783–791.
Neglia JP, Linet MS, Shu XO, Severson RK, Potter JD, Mertens AC et al. Patterns of infection and day care utilization and risk of childhood acute lymphoblastic leukaemia. Br J Cancer 2000; 82: 234–240.
Hutchinson RJ, Gaynon PS, Sather H, Bertolone SJ, Cooper HA, Tannous R et al. Intensification of therapy for children with lower risk acute lymphoblastic leukemia: long-term follow-up of patients treated on CCG trial 1881. J Clin Oncol 2003; 21: 1790–1797.
Lange BJ, Bostrom BC, Cherlow JM, Sensel MG, La MK, Rackoff W et al. Double delayed intensification improves event-free survival for children with intermediate risk acute lymphoblastic leukemia. Blood 2002; 99: 825–833.
Nachman JB, Sather HN, Sensel MG, Trigg ME, Cherlow JM, Lukens JN et al. Augmented post-induction therapy for children with high risk acute lymphoblastic leukemia. N Engl J Med 1998; 338: 1663–1671.
Heath JA, Steinherz PG, Altman A, Sather H, Jhanwar S, Halpern S et al. Human granulocyte colony-stimulating factor in children with high-risk acute lymphoblastic leukemia. J Clin Oncol 2003; 21: 1612–1617.
Boyle EB, Steinbuch M, Tekautz T, Gutman JR, Robison LL, Perentesis JP . Accuracy of DNA amplification from archival hematological slides for use in genetic biomarker studies. Cancer Epidemiol Biomarkers Prev 1998; 7: 1127–1131.
Kaplan EL, Meier P . Nonparametric estimation from incomplete observations. J Am Stat Assoc 1958; 53: 457–481.
Tarone RE, Ware J . On distribution-free tests for equality of survival distribution. Biometrika 1977; 64: 156–160.
Cox DR . Regression models and life-tables. J Statist Soc B 1972; 34: 187–220.
Kawamura M, Ohnishi H, Guo SX, Sheng XM, Minegishi M, Hanada R et al. Alterations of the p53, p21, p16, p15 and RAS genes in childhood T-cell acute lymphoblastic leukemia. Leuk Res 1999; 23: 115–126.
Nakao M, Janssen JW, Seriu T, Bartram CR . Rapid and reliable detection of N-RAS mutations in acute lymphoblastic leukemia by melting curve analysis using LightCycler technology. Leukemia 2000; 14: 312–315.
Rodenhuis S, Bos JL, Slater RM, Behrendt H, van't Veer M, Smets LA . Absence of oncogene amplifications and occasional activation of N-RAS in lymphoblastic leukemia of childhood. Blood 1986; 67: 1698–1704.
Terada N, Miyoshi J, Kawa-Ha K, Sasai H, Orita S, Yumura-Yagi K et al. Alteration of N-RAS gene mutation after relapse in acute lymphoblastic leukemia. Blood 1990; 75: 453–457.
Senn HP, Tran-Thang C, Wodnar-Filipowicz A, Jiricny J, Fopp M, Gratwohl A et al. Mutation analysis of the N-RAS proto-oncogene in active and remission phase of human acute leukemias. Int J Cancer 1988; 41: 59–64.
Neri A, Knowles DM, Greco A, McCormick F, Dalla-Favera R . Analysis of RAS oncogene mutations in human lymphoid malignancies. Proc Natl Acad Sci USA 1988; 85: 9268–9272.
Browett PJ, Norton JD . Analysis of RAS gene mutations and methylation state in human leukemias. Oncogene 1989; 4: 1029–1036.
Ahuja HG, Foti A, Bar-Eli M, Cline MJ . The pattern of mutational involvement of RAS genes in human hematologic malignancies determined by DNA amplification and direct sequencing. Blood 1990; 75: 1684–1690.
Mangues R, Pellicer A . RAS activation in experimental carcinogenesis. Semin Cancer Biol 1992; 3: 229–239.
Kelly MJ, Johnson BE . Molecular biology of lung cancer. In: Mendelsohn J, Howeley PM, Israel MA, Liotta LA (eds). Molecular Basis of Cancer, 2nd edn. Philadelphia PA: WB Saunders, 2001, pp 260–287.
Slebos RJ, Hruban RH, Dalesio O, Mooi WJ, Offerhaus GJ, Rodenhuis S . Relationship between K-RAS oncogene activation and smoking in adenocarcinoma of the human lung. J Natl Cancer Inst 1991; 83: 1024–1027.
Feng Z, Hu W, Chen JX, Pao A, Li H, Rom W et al. Preferential DNA damage and poor repair determine ras gene mutational hotspot in human cancer. J Natl Cancer Inst 2002; 94: 1527–1536.
Lemoine NR, Mayall ES, Williams ED, Thurston V, Wynford-Thomas D . Agent-specific RAS oncogene activation in rat thyroid tumours. Oncogene 1988; 3: 541–544.
Lemoine NR, Mayall ES, Wyllie FS, Farr CJ, Hughes D, Padua RA et al. Activated RAS oncogenes in human thyroid cancers. Cancer Res 1988; 48: 4459–4463.
van't Veer LJ, Burgering BM, Versteeg R, Boot AJ, Ruiter DJ, Osanto S et al. N-ras mutations in human cutaneous melanoma from sun-exposed body sites. Mol Cell Biol 1989; 9: 3114–3116.
Greenblatt MS, Bennett WP, Hollstein M, Harris CC . Mutations in the p53 tumor suppressor gene: clues to cancer etiology and molecular pathogenesis. Cancer Res 1994; 54: 4855–4878.
LeMaistre A, Lee MS, Talpaz M, Kantarjian HM, Freireich EJ, Deisseroth AB et al. RAS oncogene mutations are rare late stage events in chronic myelogenous leukemia. Blood 1989; 73: 889–891.
Perentesis JP, Siever EL . Targeted therapies for high risk acute myeloid leukemia. Hematol Oncol Clin N Am 2001; 15: 677–701, viii–ix.
Bollag G, Clapp DW, Shih S, Adler F, Zhang YY, Thompson P et al. Loss of NF1 results in activation of the RAS signaling pathway and leads to aberrant growth in haematopoietic cells. Nat Genet 1996; 12: 144–148.
Side LE, Emanuel PD, Taylor B, Franklin J, Thompson P, Castleberry RP et al. Mutations of the NF1 gene in children with juvenile myelomonocytic leukemia without clinical evidence of neurofibromatosis, type 1. Blood 1998; 92: 267–272.
Sawyers CL, Denny CT . Chronic myelomonocytic leukemia: Tel-a-kinase what Ets all about. Cell 1994; 77: 171–173.
Acknowledgements
This work was supported by National Institutes of Health award R01 ES07819 to LLR and by support to the University of Minnesota from the Children's Cancer Research Fund. Samples were obtained with the assistance of the Cooperative Human Tissue Network. Contributing Children's Oncology Group investigators, institutions and grant numbers are provided in the appendix.
Author information
Authors and Affiliations
Corresponding author
Appendix
Appendix
The following lists the principal investigators from the Children's Cancer Group, their institutions, and the grants that supported them in this work: W Archie Bleyer, Anita Khayat, Harland Sather, Mark Krailo, Jonathan Buckley, Daniel Stram, and Richard Sposto, Group Operations Center, Grant CA13539; Raymond Hutchinson, University of Michigan Medical Center, Ann Arbor, CA02971; Katherine Matthay, University of California Medical Center, San Francisco, CA17829; Paul Gaynon, University of Wisconsin Hospital, Madison, CA05436; Ronald Chard, Children's Hospital and Medical Center, Seattle, WA, CA10382; Susan Shurin, Rainbow Babies and Children's Hospital, Cleveland, OH, CA20320, Gregory Reaman, Children's National Medical Center, Washington, DC, CA03888; Edward Baum, Children's Memorial Hospital, Chicago, IL, CA07431; Jorge Ortega, Children's Hospital of Los Angeles, CA, CA02649; Frederick Ruymann, Children's Hospital of Columbus, OH, CA03750; Sergio Piomelli, Columbia Presbyterian College of Physicians and Surgeons, New York, NY, CA03526; Joseph Mirro, Children's Hospital of Pittsburgh, PA, CA36015; John Lukens, Vanderbilt University School of Medicine, Nashville, TN, CA26270; Lawrence Wolff, Doernbecher Memorial Hospital for Children, Portland, OR, CA26044; William Woods, University of Minnesota Health Sciences Center, Minneapolis, CA07306; Thomas Williams, University of Texas Health Sciences Center, San Antonio, CA36004; Anna Meadows, Children's Hospital of Philadelphia, PA, CA11796; Peter Steinherz, Memorial Sloan-Kettering Cancer Center, New York, NY, cA42764; Philip Breitfeld, James Whitcomb Riley Hospital for Children, Indianapolis, IN, CA13809; Mark Greenerg, Hospital for Sick Children, Toronto, Ont., Canada; Richard O'Brien, University of Utah Medical Center, Salt Lake City, CA10198; Harvey Cohen, Strong Memorial Hospital, Rochester, NY, CA11174; Cristopher Fryer, University of British Columbia, Vancouver, Canada, CA29013; Robert Wells, Children's Hospital Medical Center, Cincinnati, OH, CA26126; Jerry Finklestein, Harbor/UCLA and Miller Children's Medical Center, Torrance/Long Beach, CA, CA14560; Stephen Feig, University of California Medical Center, Los Angeles, CA27678; Raymond Tannous, University of Iowa Hospitals and Clinics, Iowa City, CA29314; Lorrie Odom, Children's Hospital of Denver, CO, CA28851; Gerald Gilchrist, Mayo Clinic and Foundation, Rochester, MN, CA28882; Dorothy Barnard, Izaak Walton Killam Hospital for Children, Halifax, Nova Scotia, Canada; Joseph Wiley, University of North Carolina, Chapel Hill; Milton Donaldson, University of Medicine and Dentistry of New Jersey, Camden; Maxine Hetherington, Children's Mercy Hospital Kansas City, MO; Peter Coccia, University of NebRASka Medical Center, Omaha; Donald Norris, Cleveland Clinic Foundation, OH; F Leonard Johnson, Wyler Children's Hospital, Chicago, IL; R Beverly Raney, MD Anderson Cancer Center, Houston, TX; David Baker, Princess Margaret Hospital, Perth, Western Australia; Jean Sanders, Fred Hutchinson Cancer Research Center, Seattle, WA; Aaron Rausen, New York University Medical Center, NY; Mitchell Cairo, Children's Hospital of Orange County, CA.
Rights and permissions
About this article
Cite this article
Perentesis, J., Bhatia, S., Boyle, E. et al. RAS oncogene mutations and outcome of therapy for childhood acute lymphoblastic leukemia. Leukemia 18, 685–692 (2004). https://doi.org/10.1038/sj.leu.2403272
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.leu.2403272
Keywords
This article is cited by
-
Spectrum and clinical features of gene mutations in Chinese pediatric acute lymphoblastic leukemia
BMC Pediatrics (2023)
-
Validation of a small molecule inhibitor of PDE6D-RAS interaction with favorable anti-leukemic effects
Blood Cancer Journal (2022)
-
Synthetic modeling reveals HOXB genes are critical for the initiation and maintenance of human leukemia
Nature Communications (2019)
-
Anti-leukemic effects of simvastatin on NRASG12D mutant acute myeloid leukemia cells
Molecular Biology Reports (2019)
-
RAS pathway mutations as a predictive biomarker for treatment adaptation in pediatric B-cell precursor acute lymphoblastic leukemia
Leukemia (2018)