Abstract
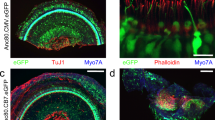
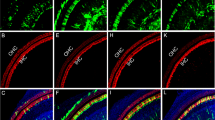
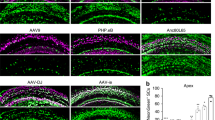
The loss of cochlear hair cells, or the loss of their capacity to transduce acoustic signals, is believed to be the underlying mechanism in many forms of hearing loss. To develop viral vectors that allow for the introduction of genes directly into the cochleae of adult animals, replication-deficient (E1−, E3−) and replication-defective (E1−, E3−, pol−) adenovirus vectors were used to transduce the bacterial β-galactosidase gene into the hair cells of the guinea pig cochlea in vivo. Distortion product otoacoustic emissions, which monitor the functional status of outer hair cells, were measured throughout the viral infection periods to identify hair cell ototoxicity. The results demonstrated that the use of the (E1−, E3−) adenovirus vectors containing CMV-driven LacZ, compromised cochlear function when gradually introduced into scala tympani via an osmotic pump. However, when (E1−, E3−, pol−) adenoviral vectors containing CMV-driven LacZ were used to transduce cochlear hair cells, there was no loss of cochlear function over the frequency regions tested, and β-galactosidase (β-gal) was detected in over 80% of all hair cells. Development of a viral vector that infects cochlear hair cells without virus-induced ototoxic effects is crucial for gene replacement strategies to treat certain forms of inherited deafness and for otoprotective strategies to prevent hair cell losses to treat progressive hearing disorders. Moreover, in vivo (E1−, E3−, pol−) adenovirus mediated gene-transfer techniques applied to adult guinea pig cochleae may be useful in testing several hypotheses concerning what roles specific genes play in normal cochlear function.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Yoshida N et al. Acoustic injury in mice: 129/SvEv is exceptionally resistant to noise-induced hearing loss Hear Res 2000 141: 97–106
Zuo J, Treadaway J, Buckner TW, Fritzsch B . Visualization of alpha 9 acetylcholine receptor expression in hair cells of transgenic mice containing a modified bacterial artificial chromosome Proc Natl Acad Sci USA 1999 96: 14100–14105
Dazert S, Battaglia A, Ryan AF . Transfection of neonatal rat cochlear cells in vitro with an adenovirus vector Int J Dev Neurosci 1997 15: 595–600
Staecker H, Gabaizadeh R, Federoff H, Van De Water TR . Brain-derived neurotrophic factor gene therapy prevents spiral ganglion degeneration after hair cell loss Otolaryngol Head Neck Surg 1998 119: 7–13
Holt JR et al. Functional expression of exogenous proteins in mammalian sensory hair cells infected with adenoviral vectors J Neurophysiol 1999 81: 1881–1888
Van de Water TR, Staecker H, Halterman MW, Federoff HJ . Gene therapy in the inner ear. Mechanisms and clinical implications Ann NY Acad Sci 1999 884: 345–360
Derby ML, Sena-Esteves M, Breakefield XO, Corey DP . Gene transfer into the mammalian inner ear using HSV-1 and vaccinia virus vectors Hear Res 1999 134: 1–8
Hann JJ et al. Transgene expression in the guinea pig cochlea mediated by a lentivirus-derived gene transfer vector Hum Gene Ther 1999 10: 1867–1873
Luebke AE, Peel AL, Muller CD, Foster PK . DPOAE function and transgene expression in guinea pig cochlea using AAV-directed gene-transfer methods Assoc Res Otolaryngol Abstr 1999 22: 81
Raphael Y, Frisancho JC, Roessler BJ . Adenoviral-mediated gene transfer into guinea pig cochlear cells in vivo Acta Otolaryngol 1996 116: 125–131
Luebke AE, Steiger JD, Hodges BL, Amalfitano A . Cochlear function during (E1-) and (E1, E2b-) adenovirus transduction of guinea pig hair cells Mol Ther 2000 1: 580–581
Hu H, Serra D, Amalfitano A . Persistence of an (E1−, polymerase−) adenovirus vector despite transduction of a neoantigen into immune-competent mice Hum Gene Ther 1999 10: 355–364
Hodges BL et al. Multiply deleted (E1, polymerase−, and pTP−) adenovirus vector persists despite deletion of the preterminal protein J Gene Med 2000 2: 250–259
Salt AN, Inamura N, Thalmann R, Vora AR . Evaluation of procedures to reduce fluid flow in the fistulized guinea-pig cochlea Acta Otolaryngol 1991 111: 899–907
Yagi M et al. Hair cell protection from aminoglycoside ototoxicity by adenovirus-mediated overexpression of glial cell line-derived neurotrophic factor Hum Gene Ther 1999 10: 813–823
Stover T, Yagi M, Raphael Y . Cochlear gene transfer: round window versus cochleostomy inoculation Hear Res 1999 136: 124–130
Mittereder N, March KL, Trapnell BC . Evaluation of the concentration and bioactivity of adenovirus vectors for gene therapy J Virol 1996 70: 7498–7509
Stover T, Yagi M, Raphael Y . Transduction of the contralateral ear after adenovirus-mediated cochlear gene transfer Gene Therapy 2000 7: 377–383
Dallos P . Membrane potential and response changes in mammalian cochlear hair cells during intracellular recording J Neurosci 1985 5: 1609–1615
Preyer S et al. Frequency response of mature guinea-pig outer hair cells to stereociliary displacement Hear Res 1994 77: 116–124
Cheatham MA, Dallos P . The dynamic range of inner hair cell and organ of Corti responses J Acoust Soc Am 2000 107: 1508–1520
Martin GK et al. Locus of generation for the 2f1-f2 vs 2f2-f1 distortion-product otoacoustic emissions in normal-hearing humans revealed by suppression tuning, onset latencies, and amplitudes correlations J Acoust Soc Am 1998 103: 1957–1971
He TC et al. A simplified system for generating recombinant adenoviruses Proc Natl Acad Sci USA 1998 95: 2509–2514
Amalfitano A et al. Production and characterization of improved adenovirus vectors with the E1, E2b, and E3 genes deleted J Virol 1998 72: 926–933
Amalfitano A, Chamberlain JS . Isolation and characterization of packaging cell lines that coexpress the adenovirus E1, DNA polymerase, and preterminal proteins: implications for gene therapy Gene Therapy 1997 4: 258–263
Prieskorn DM, Miller JM . Technical report: chronic and acute intracochlear infusion in rodents Hear Res 2000 140: 212–215
Couffinhal T et al. Histochemical staining following LacZ gene transfer underestimates transfection efficiency Hum Gene Ther 1997 8: 929–934
Bohne BA . Location of small cochlear lesions by phase contrast microscopy prior to thin sectioning Laryngoscope 1972 82: 1–16
Acknowledgements
This work was supported by grants from the Public Health Service DC03086 (AEL), DK52925 (AA), Muscular Dystrophy Association, USA (AA), and funds from the University of Miami's Stanley Glaser Research Foundation and Chandler Chair (AEL). We would like to thank Dr Ken Muller for his assistance with plastic sectioning.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Luebke, A., Steiger, J., Hodges, B. et al. A modified adenovirus can transfect cochlear hair cells in vivo without compromising cochlear function. Gene Ther 8, 789–794 (2001). https://doi.org/10.1038/sj.gt.3301445
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.gt.3301445
Keywords
This article is cited by
-
Gene transfer in inner ear cells: a challenging race
Gene Therapy (2013)
-
Adeno-associated virus-mediated gene delivery into the scala media of the normal and deafened adult mouse ear
Gene Therapy (2011)
-
Effects of Localized Neurotrophin Gene Expression on Spiral Ganglion Neuron Resprouting in the Deafened Cochlea
Molecular Therapy (2010)
-
Coxsackie adenovirus receptor and ανβ3/ανβ5 integrins in adenovirus gene transfer of rat cochlea
Gene Therapy (2007)
-
An in vitro model system to study gene therapy in the human inner ear
Gene Therapy (2007)