Abstract
Study design:
Experimental animal study.
Objectives:
Stimulus-evoked below-level paw withdrawals in animal models of spinal cord injury (SCI) can be mediated solely by below-level spinal cord reflexes. Interpreting lowered thresholds for such responses as a model for chronic below-level pain after (thoracic contusion) SCI appears not appropriate, which requires reinterpretation of many prior results. However, how to reinterpret the changes in withdrawal thresholds and what can be a better alternative for pain/sensory assessments remains unclear.
Setting:
University of California, San Diego.
Methods:
We introduce a method using supraspinally mediated escape responses to assess pain-like sensitivity thresholds on a continuous/linear scale. To further understand the decrease in hindpaw withdrawal thresholds, we investigated whether they may be interpreted as spasticity.
Results:
The escape response test can be used to assess SCI-induced changes in below-level sensory thresholds. These thresholds were found to increase soon after moderate or severe SCI, while, in parallel, hindpaw withdrawal thresholds decreased. However, the latter did not co-occur with spasticity, suggesting that SCI-induced increased withdrawal responses are probably best interpreted as a form of hyperreflexia with pathophysiological analogies of spasms and/or clonus, or a species-specific phenomenon.
Conclusion:
Decreased below-level withdrawal thresholds do not reflect pain-like hypersensitivity in rodent models of (thoracic contusion) SCI. A large body of previous preclinical SCI pain research needs reinterpretation. We actually found below-level thermal and mechanical hypoesthesia and we also excluded a relation between withdrawal hyperreflexia and spasticity. Withdrawal hyperreflexia might still prove useful to model spasms or clonus, which are, like hypoesthesia, also significant clinical problems after SCI.
Similar content being viewed by others
Introduction
Central neuropathic pain after spinal cord injury (SCI) represents a prevalent, chronic, severe, difficult to treat and poorly understood clinical problem. On the basis of the likelihood of a difference in pain mechanisms, chronic neuropathic pain after SCI can be roughly divided into pain that is projecting either at or below the regions innervated from the spinal level of SCI (at-level or below-level pain).1 Both types are difficult to model in animal models, with below-level pain probably being the most challenging.2, 3 In animal SCI pain models, pain is often investigated by determining hypersensitivity, as is mostly done in dermatomes below the level of injury.4, 5, 6 Changes in below-level responses occur almost immediately after rodent SCI and remain for months.7 Typically, a decreased hindpaw withdrawal threshold to stimuli is observed and considered to reflect a central neuropathic below-level chronic pain state. In contrast, human SCI patients with below-level central neuropathic pain typically present themselves months after injury and with increased below-level sensory thresholds (with, in some cases, the possible exceptions of prodromal hypersensitivity to cold or dynamic mechanical stimuli).8, 9, 10, 11 The question arises whether the below-level withdrawal responses (BL-WRs) in SCI rats can be interpreted as part of a chronic neuropathic pain syndrome. Indeed, a landmark study on SCI pain in thoracic contused rats was able to show that a reduction in the threshold for BL-WRs can present itself without escape/avoidance behavior to those same stimuli.3 Thus, reduced paw withdrawal thresholds do not necessarily implicate an increased aversion to the stimulus. Furthermore, a significantly reduced brain activity, which was evoked by electrical stimulation of skin corresponding to below-level dermatomes, in similar (but anesthetized) SCI rats has been reported, which actually implicates a state of hypoesthesia, not hyperesthesia.12 Thus, BL-WRs are probably due to autonomic spinal hyperreflexia and may not be perceived consciously at all. However, the method used to show the lack of aversion to below-level stimuli in rats as done in the study mentioned above3 is not designed for assessment of pain thresholds on a continuous scale, as aversion to only a single stimulus size can be tested in each session (that is, a dichotomous scale).
Therefore, in the first part of this study we tested a combination of existing paradigms in which sensitivity thresholds for below-level evoked thermal and mechanical stimuli are not assessed with BL-WRs, but with supraspinally mediated above-level escape responses (AL-ERs),3, 13, 14, 15, 16, 17 to see how they relate to the development of BL-WRs. AL-ER thresholds or latencies are defined as force (mechanical) or time (heat) required to elicit (1) escape or struggling behavior or (2) vocalization. The possibility to behaviorally quantify below-level sensitivity not only allows for a better interpretation of (sensory-feedback-dependent) motor tests, but also allows evaluations of therapy efficacy on sensory function, including hypoesthesia and hypersensitivity.
Furthermore, BL-WRs have been postulated to relate to another clinical problem in SCI patients; that is, the spastic syndrome.3 This spastic syndrome does not occur as it does in human patients, in whom often the most evident feature of the spastic syndrome is increased muscle resistance/activity (that is, spasticity). However, spasticity in its most common and narrow definition, is defined as a velocity-dependent increase in muscle activity after tonic passive stretch (stretch hyperreflexia). Thus, such spasticity (and other reflex-like features of the spastic syndrome, such as spasms or clonus) can be easily overlooked in rats, as increases in spastic syndrome related muscle activity only needs to occur, or becomes more evident, after being evoked (for example, by stretch). Therefore, in the present study, we also investigate whether spasticity, defined as stretch/proprioceptive hyperreflexia,18 coincides with the nociceptive hyperreflexia seen with BL-WRs in SCI rats.
Materials and methods
Animals and surgeries
We certify that all applicable institutional and governmental regulations concerning the ethical use of animals were followed during the course of this research. All animal studies were approved by the University of California, San Diego institutional animal care and use committee. Twenty-two 12 weeks old female Sprague–Dawley rats were anesthetized with isoflurane (5% induction, 2% maintenance; room air), placed on a Stereotaxic frame (Stoelting, Cat no. 51600 with Cat no. 51695, Wood Dale, IL, USA) and maintained at a core temperature of 37±0.3 °C using a heating blanket. A Th9 laminectomy was performed using a dental drill. The MASCIS/NYU apparatus (WM Keck Center for Collaborative Neuroscience, Rutgers University, Piscataway, NJ, USA) was used for SCI. The laminectomy site was filled with mineral oil in which the tip of a small thermocouple (Physitemp, Cat no. IT-14, Clifton, NJ, USA) was submerged and used to manually keep the spinal cord at 37±0.3 °C with warmth from surgical lights (Fiber-Lite, Cat no. MI-150 and BGG1823M, Dolan-Jenner, Boxborough, MA, USA). Next, oil and lamps were removed and the rod dropped using a height of 6.25 (mild SCI, n=7), 12.5 (moderate SCI, n=5) or 25 mm (severe SCI, n=5). Five sham-operated animals were included.
Surgeries were performed in a mixed-group fashion. Group designation was not known by the experimenters doing behavioral testing. SvG performed all behavioral testing. ML and SN were furthermore involved in Basso, Beattie and Bresnahan (BBB) scoring.
Post-surgical care
Animals were housed on corn cob bedding, with water and food pellets (Cat no. Teklad 2014, Harlan, KY, USA) ad libitum, in a regular 12/12 h light/dark cycle, with a maximum of three animals per cage and in a mixed-group fashion. Buprenorphine (0.05 mg kg−1, subcutaneously, Reckitt Benckiser, Richmond, VA, USA), 5 ml of Lactated ringer and 10 mg kg−1 of Cefazolin (Novaplus/Sandoz, Holzkirchen, Germany) was given after surgery. Bladders were emptied manually twice daily. Sulfamethoxazole and Trimethoprim USP (200 mg and 40 mg per 250 ml drinking water, Hi-Tech Pharmacal, Amityville, NY, USA) were given for at least 10 days or until autonomic bladder voiding occurred. Any ill-appearing animals received additional days of above-mentioned antibiotic treatments.
Open-field locomotion testing
Locomotion recovery after SCI was monitored using a rating scale based on the BBB open-field locomotor rating scale (0 to 21), as described by Basso et al.19, 20
Sensory testing
Hindpaw withdrawal response thresholds (dorsally applied von Frey filaments; see below, this section) and supraspinally mediated AL-ER thresholds were assessed. An AL-ER is defined as an escape-attempt of the animal from the investigator’s loose, but fully supportive, grip (Figure 1) and has to include the elaborate use of its front paws or a vocalization. The applied stimuli were either a gradually increasing force to the hindpaws (using the Analgesy-Meter; Cat no. 37215, Ugo-Basile, Collegeville, PA, USA), or a constant heat stimulus (intensity 17, cutoff at 30 s) to the hindpaws (using a constant infrared heat source; Cat no. 37360, Ugo-Basile, Collegeville, PA, USA). The hindpaw tested was gently restrained by the investigator to prevent reflex-related paw withdrawal (Figure 1).
Schematic of above-level escape response (AL-ER) testing upon below-level stimulation. Shown is the way in which the animals were held for the AL-ER testing upon hindpaw stimulation with a mechanical (black arrow, dorsal) or a thermal (gray arrow, plantar) stimulus. The animals were loosely held so that animals could escape from the investigator’s grip. An AL-ER refers to escape behavior elicited by structures innervated above the level of injury used. Here, we used a thoracic injury; hence, AL-ERs were defined as the transition of a calm state to a state of fleeing/distress, as defined by activation of frontlimbs, shoulder/neck/head area and/or vocalization. Per time point, the hindpaws were tested in an alternating manner at 1–2 h intervals for up to a total of four times per paw for each stimulus.
For the AL-ER tests, both hindpaws were tested four times, alternately, for each test, with a testing interval of 1–2 h and maximally four measurements per day. Maximum cutoff values for the stimuli were chosen at approximately two times the response threshold of uninjured animals (to prevent tissue damage). Before (7 days) and during the entire experimental period (5 days a week), the animals were extensively habituated to the experimenter in such a way that the animals could be (loosely) held upright during all sensory assessments. Habituation consisted of picking up the animal and holding/handling it twice daily for 3–5 min. During habituation, the animals were also presented to the test setup, as if they were tested, but without the application of mechanical or thermal stimuli (the testing devices were switched on and operated during habituation, to acclimatize the animals to any noise of each device when operated).
To determine the hindpaw withdrawal thresholds (von Frey monofilaments 0.4, 1, 2, 4, 8, 15, 26, 60, 100 g, up-down method15), the animals were also held loosely and in an upright position, to circumvent the requirement of weight support.4, 14
Assessment of stretch-induced muscle resistance
Peripheral muscle resistance to stretch was measured using a previously described spasticity meter.18 Briefly, animals were placed in a restrainer and a hindpaw was taped to a metal plate driven by a computer-controlled stepper motor. The resistance of the ankle to dorsiflexion was measured during stepper motor-driven ankle flexion (40°), and always in conjunction with the electromyography (EMG) of the ipsilateral m.gastrocnemius (described below, this section). Resistance was measured in awake animals using medium, fast and slow ankle rotation speeds (that is, 40° in 0.5, 0.25 and 1 s, respectively). Consecutively, the muscle resistance was measured in anesthetized animals (at medium speed), to identify the mechanical (vs the neurogenic, isoflurane-sensitive) component of measured ankle resistance. Hence, active muscle resistance could be computed (resistance when awake minus resistance when anesthetized). Also, spastic components of muscle resistance could be detected, as spasticity is velocity dependent. To record the EMG activity, a pair of tungsten electrodes was inserted percutaneously into the gastrocnemius muscle 1 cm apart. EMG signals were bandpass filtered (100 Hz to 10 kHz) and recorded before, during and after ankle dorsiflexion. For the detection of spasticity (i.e. rotation-induced EMG amplitude increases) only EMG data from the fast ankle rotation measurements were used. Average EMG amplitudes were calculated for the 2 s before ankle rotation, and a period of 0.20 s in which the maximal ankle rotation was achieved. Each recorded value was the average of three repetitions.
Statistical analyses
Results were analyzed using analysis of variance (ANOVA; one-way, or two-way group × time repeated measures, using a fixed-effect model), with a Bonferroni post hoc test for multiple comparisons (GraphPad Prism, La Jolla, CA, USA). Unequal variances were not observed. Results are analyzed as two-tailed and expressed with s.e.m., unless specified otherwise. A P-value of 0.05 was considered significant.
Results
Behavioral description and confirmation of differences in injury severities
SCIs induced with the validated MASCIS/NYU apparatus, using 6.25 mm, 12.5 mm and 25 mm impact/drop height settings, resulted in significantly different injury severities as assessed with the widely used BBB locomotor score (variation between any two experimental groups; Bonferroni; P⩽0.04; Figure 2a). Trends in differences between BBB scores are similar to the ones reported originally, indicating a similar extent of the injuries.19, 20 Weight support (that is, BBB score ⩾9) was absent in moderate SCI animals before the 3 weeks assessment and remained absent in most severe SCI animals.
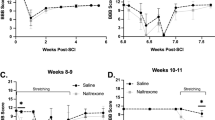
Differences in spinal and supraspinal pain-like responses and spasticity after thoracic SCI. (a) Classical open-field Basso, Beattie and Bresnahan (BBB) locomotor scores of sham, mild, moderate and severe SCI groups. (b) Using the up-down method to the dorsal aspect of the hindpaws decreases in below-level withdrawal responses were observed in SCI animals. Compared with sham-operated animals, only moderate and severe SCI animals showed a significant decrease in hindpaw withdrawal thresholds (repeated measures analysis of variance; P<0.001). (c, d) In contrast to below-level withdrawal responses, pain perception/above-level escape responses thresholds to below-level mechanical (c) and thermal (d) stimuli increased in animals with a moderate or severe SCI (repeated measures analysis of variance; P<0.001). (e) The occurrence of below-level paw withdrawal hyperreflexia did not coincide with stretch-evoked muscle activity, as no increases in (active) muscle resistance were noted in any SCI group up to the end of the study (that is, 6 weeks post injury).
Below-level dorsal hindpaw withdrawal thresholds decrease quickly after moderate and severe SCI
Graded SCI resulted in a decreased hindpaw withdrawal threshold in the moderate and severely injured groups (that is, 12.5 and 25 mm weight drop groups), when compared with the sham-operated group (repeated measures ANOVA; P<0.0001; Figure 2b). However, mild injury (6.25 mm) did not give significant threshold changes in comparison with sham-operated animals (P=0.21). No significant differences were present between moderate and severe SCI.
Thresholds for below-level evoked AL-ERs increase after moderate and severe SCI
Using supraspinally mediated AL-ERs, the below-level sensitivity thresholds were assessed. AL-ER thresholds for mechanical stimuli changed only for moderate and severe SCI, when compared with sham-operated animals (repeated measures ANOVA; P<0.001; Figure 2c). Testing was somewhat difficult owing to pronounced bilateral and complex hind limb motor activity (that is, jumping/kicking-like behavior), but AL-ER thresholds after moderate SCI were noted to increase for thermal stimuli (P<0.001; Figure 2d); yet, it was impossible to confidently assess thermal AL-ER thresholds in the severe SCI rats. This complex motor response to stimuli was present even in the acute SCI phase, in which stepping was absent during open-field testing.
SCI-induced decrease of hindpaw withdrawal thresholds occurs independent of stretch hyperreflexia
Analysis of active muscle resistance in the gastrocnemius muscle upon ankle rotations to assess stretch hyperreflexia, a measure of spasticity, showed no difference between experimental groups at any rotation speed (One-way ANOVAs; P⩾0.63) nor at any time point measured (that is, at 1, 3 and 6 weeks after injury; P=0.94, P=0.92 and P=0.88, respectively; Figure 2e shows active muscle resistance at six weeks). Also, ankle rotations m.gastrocnemius EMG amplitudes did not increase in any of the experimental groups, when compared with their resting stages (relative values; fastest ankle rotation speed: sham, mild, moderate and severe SCI groups; Student’s t-tests; P=0.75, P=0.82, P=0.48 and P=0.25, respectively).
Discussion
In this study, we show that thresholds for below-level sensitivity increase after moderate and severe SCI (assessed with AL-ER thresholds for both thermal and mechanical stimuli) during the first 6 weeks after SCI. This makes the AL-ER test a promising test for SCI therapy evaluations, as it is in line with a largely understudied clinical problem after SCI (chronic hypoesthesia) and allows detection of changes in sensory function on a continuous/linear scale (for example, improvements of hypoesthesia or an onset of hypersensitivity).
In sharp contrast to the AL-ERs, the BL-WR thresholds decreased, indicating a limited correlation of BL-WR with pain perception. This has important implications for the interpretation of most preclinical rodent SCI pain studies, as pain actually was not studied.4, 5, 6 Furthermore, the AL-ER test revealed forceful bilateral hindpaw motor ability (kicking/jumping) upon modest sensory stimulation of a single hindpaw, even when SCI animals were still unable to support their bodyweight during locomotion. The latter is in line with observations that complex BL-WR can also be evoked in complete spinal cord-transected animals, even within hours after injury.21 Hence, one explanation for the origin of increased withdrawal reflexes might be that the moderate and severe injuries uncouple an intrinsic spinal autonomous sensorimotor circuit from its supraspinal descending inhibition/regulation. This circuit might be a part of what is known as the central pattern generator, which is located in the lumbar rat spinal cord. The latter may then explain why mostly thoracic injuries are used to study BL-WRs.
Importantly, however, it remains an unanswered question to what extent AL-ERs help represent a clinical problem (that is, hypoesthesia or hypersensitivity). It is unclear whether improvements in AL-ERs represent a consciously perceivable improvement. Although AL-ERs are supraspinally mediated (strictly speaking they are above-level mediated), they should be carefully interpreted as AL-ERs reflect supraspinal responses, which do not necessarily require cortical processing and could thus merely be brainstem mediated reflexes and remain unrelated to the experience of pain.22 In this context, it also needs to be stressed that cortical/supraspinal processes by themselves can be important modulators of the pain experience.
The observed nociception-evoked hyperreflexia is different from the clinical reflex-like problem spasticity, as it did not coincide with increased (stretch evoked) muscle activity. Nonetheless, both the spastic syndrome in humans and the decreased BL-WR thresholds in SCI rats respond to baclofen (a gamma-aminobutyric acid type B receptor agonist) treatment, while central neuropathic pain in humans hardly responds to this treatment, if at all.23 The spastic syndrome, however, consists of an array of only partly overlapping ‘positive signs’ of the upper motor neuron syndrome, which includes not only spasticity, but also spasms, pathological reflexes, clonus, hypertonia and dystonia (whereas its ‘negative signs’ relate to the loss of motor control). The hindpaw hyperreflexia as seen in the current study/model (that is, complex hindpaw motor activity upon sensory stimulation in paraplegic rats) could still correspond well to the spastic syndrome, as observed in human SCI patients, as it shows similarities to, for instance, spasms or clonus. The latter has similarities with the withdrawal responses in terms of the rhythmicity and a fading ending, although clonus is classically elicited through stretch receptors not nociception.
When compared with humans,24 it is remarkable that rats with moderate (or even severe) SCI show a high degree of motor function as well as complex below-level paw withdrawal behavior, but still have poor below-level perception. In addition, rat SCI results in vastly fewer cases of spasticity and less pronounced spinal shock phases.25, 26 Apparently, interspecies differences in the degree of spinal autonomy (supraspinal involvement) for locomotion function do exist. A strong complex autonomic sensorimotor function seems to be present in the rodent spinal cord, which could be the central pattern generator. This poses difficulties not only for interpreting sensory function, but also for the evaluation and translation of conscious and voluntary motor and locomotor function in rodent/quadruped SCI models (also see our other manuscript).27, 28
In summary, nociception-evoked hindpaw hyperreflexia as observed in rat (thoracic contusion) SCI has, on one hand, a debatable clinical relevance as it does not correlate with hypersensitivity or spasticity and could be solely due to species-specific properties of spinal function, but, on the other hand, resembles (and can probably be best interpreted as) spasms and/or clonus, as also seen in human SCI patients. These findings suggest that many conclusions that were based on the study of below-level paw withdrawal hyperreflexia in SCI rats require careful reevaluation.
Data Archiving
There were no data to deposit.
References
Siddall PJ, Loeser JD . Pain following spinal cord injury. Spinal Cord 2001; 39: 63–73.
Hulsebosch CE, Xu GY, Perez-Polo JR, Westlund KN, Taylor CP, McAdoo DJ . Rodent model of chronic central pain after spinal cord contusion injury and effects of gabapentin. J Neurotrauma 2000; 17: 1205–1217.
Baastrup C, Maersk-Moller CC, Nyengaard JR, Jensen TS, Finnerup NB . Spinal-, brainstem- and cerebrally mediated responses at- and below-level of a spinal cord contusion in rats: evaluation of pain-like behavior. Pain 2010; 151: 670–679.
Detloff MR, Clark LM, Hutchinson KJ, Kloos AD, Fisher LC, Basso DM . Validity of acute and chronic tactile sensory testing after spinal cord injury in rats. Exp Neurol 2010; 225: 366–376.
Gwak YS, Hulsebosch CE . Remote astrocytic and microglial activation modulates neuronal hyperexcitability and below-level neuropathic pain after spinal injury in rat. Neuroscience 2009; 161: 895–903.
Hama A, Sagen J . Combinations of intrathecal gamma-amino-butyrate receptor agonists and N-methyl-d-aspartate receptor antagonists in rats with neuropathic spinal cord injury pain. Eur J Pharmacol 2012; 683: 101–108.
Jung JI, Kim J, Hong SK, Yoon YW . Long-term follow-up of cutaneous hypersensitivity in rats with a spinal cord contusion. Korean J Physiol Pharmacol 2008; 12: 299–306.
Zeilig G, Enosh S, Rubin-Asher D, Lehr B, Defrin R . The nature and course of sensory changes following spinal cord injury: predictive properties and implications on the mechanism of central pain. Brain 2012; 135 (Pt 2): 418–430.
Finnerup NB, Johannesen IL, Fuglsang-Frederiksen A, Bach FW, Jensen TS . Sensory function in spinal cord injury patients with and without central pain. Brain 2003; 126 (Pt 1): 57–70.
Celik EC, Erhan B, Lakse E . The clinical characteristics of neuropathic pain in patients with spinal cord injury. Spinal Cord 2012; 50: 585–589.
Finnerup NB, Norrbrink C, Trok K, Piehl F, Johannesen IL, Sorensen JC et al. Phenotypes and predictors of pain following traumatic spinal cord injury: a prospective study. J Pain 2013; 15: 40–48.
Hofstetter CP, Schweinhardt P, Klason T, Olson L, Spenger C . Numb rats walk—a behavioural and fMRI comparison of mild and moderate spinal cord injury. Eur J Neurosci 2003; 18: 3061–3068.
Vierck CJ Jr., Lee CL, Willcockson HH, Kitzmiller A, Bullitt E, Light AR . Effects of anterolateral spinal lesions on escape responses of rats to hindpaw stimulation. Somatosens Mot Res 1995; 12: 163–174.
Detloff MR, Fisher LC, McGaughy V, Longbrake EE, Popovich PG, Basso DM . Remote activation of microglia and pro-inflammatory cytokines predict the onset and severity of below-level neuropathic pain after spinal cord injury in rats. Exp Neurol 2008; 212: 337–347.
Chaplan SR, Bach FW, Pogrel JW, Chung JM, Yaksh TL . Quantitative assessment of tactile allodynia in the rat paw. J Neurosci Methods 1994; 53: 55–63.
Acosta-Rua AJ, Cannon RL, Yezierski RP, Vierck CJ . Sex differences in effects of excitotoxic spinal injury on below-level pain sensitivity. Brain Res 2011; 1419: 85–96.
Yezierski RP, Green M, Murphy K, Vierck CJ . Effects of gabapentin on thermal sensitivity following spinal nerve ligation or spinal cord compression. Behavioural Pharmacol 2013; 24: 598–609.
Marsala M, Hefferan MP, Kakinohana O, Nakamura S, Marsala J, Tomori Z . Measurement of peripheral muscle resistance in rats with chronic ischemia-induced paraplegia or morphine-induced rigidity using a semi-automated computer-controlled muscle resistance meter. J Neurotrauma 2005; 22: 1348–1361.
Basso DM, Beattie MS, Bresnahan JC . A sensitive and reliable locomotor rating scale for open field testing in rats. J Neurotrauma 1995; 12: 1–21.
Basso DM, Beattie MS, Bresnahan JC . Graded histological and locomotor outcomes after spinal cord contusion using the NYU weight-drop device versus transection. Exp Neurol 1996; 139: 244–256.
Schouenborg J, Holmberg H, Weng HR . Functional organization of the nociceptive withdrawal reflexes. II. Changes of excitability and receptive fields after spinalization in the rat. Exp Brain Res 1992; 90: 469–478.
Vierck CJ . Animal models of pain. In: McMahon S, Koltzenburg M, eds.. Wall and Melzack’s Textbook of Pain. Elsevier Churchill Livingstone: Philadelphia, 175–186 2006.
Teasell RW, Mehta S, Aubut JL, Ashe MC, Sequeira K, Macaluso S et al. A systematic review of the therapeutic interventions for heterotopic ossification after spinal cord injury. Spinal Cord 2010; 48: 512–521.
Jacobs SR, Yeaney NK, Herbison GJ, Ditunno JF Jr . Future ambulation prognosis as predicted by somatosensory evoked potentials in motor complete and incomplete quadriplegia. Arch Phys Med Rehabil 1995; 76: 635–641.
Bennett DJ, Gorassini M, Fouad K, Sanelli L, Han Y, Cheng J . Spasticity in rats with sacral spinal cord injury. J Neurotrauma. 1999; 16: 69–84.
Akhtar AZ, Pippin JJ, Sandusky CB . Animal models in spinal cord injury: a review. Rev Neurosci 2008; 19: 47–60.
Barriere G, Leblond H, Provencher J, Rossignol S . Prominent role of the spinal central pattern generator in the recovery of locomotion after partial spinal cord injuries. J Neurosci 2008; 28: 3976–3987.
Magnuson DS, Lovett R, Coffee C, Gray R, Han Y, Zhang YP et al. Functional consequences of lumbar spinal cord contusion injuries in the adult rat. J Neurotrauma 2005; 22: 529–543.
Acknowledgements
We would like to thank Dr O Kakinohana and C Santucci for their help and advice, which was invaluable for the successful completion of this study. No funding sources were provided.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
van Gorp, S., Deumens, R., Leerink, M. et al. Translation of the rat thoracic contusion model; part 1—supraspinally versus spinally mediated pain-like responses and spasticity. Spinal Cord 52, 524–528 (2014). https://doi.org/10.1038/sc.2014.72
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sc.2014.72
This article is cited by
-
Electromyographic patterns of the rat hindlimb in response to muscle stretch after spinal cord injury
Spinal Cord (2018)
-
Neuropathic Pain After Spinal Cord Injury: Challenges and Research Perspectives
Neurotherapeutics (2018)
-
Neuropathic pain and spasticity: intricate consequences of spinal cord injury
Spinal Cord (2017)
-
Preclinical models of muscle spasticity: valuable tools in the development of novel treatment for neurological diseases and conditions
Naunyn-Schmiedeberg's Archives of Pharmacology (2016)