Abstract
Mounting evidence suggests that microbiota dysbiosis caused by antibiotic administration is a risk factor for cancer, but few research reports focus on the relationships between antibiotics and chemotherapy efficiency. We evaluated the influence of antibiotic administration on neoadjuvant therapy efficacy in patients with breast cancer (BC) in the present study. BC patients were stratified into two groups: antibiotic-treated and control based on antibiotic administration within 30 days after neoadjuvant therapy initiation. Disease-free survival (DFS) and overall survival (OS) were assessed using the Kaplan–Meier method, and the Cox proportional hazards model was used for multivariate analyses. The pathologic complete response rate of the control group was significantly higher than that of the antibiotic-treated group (29.09% vs. 10.20%, p = 0.017). Further univariate analysis with Kaplan–Meier calculations demonstrated that antibiotic administration was strongly linked with both reduced DFS (p = 0.04) at significant statistical levels and OS (p = 0.088) at borderline statistical levels. Antibiotic administration was identified as a significant independent prognostic factor for DFS [hazard ratio (HR) 3.026, 95%, confidence interval (CI) 1.314–6.969, p = 0.009] and OS (HR 2.836, 95% CI 1.016–7.858, p = 0.047) by Cox proportional hazards model analysis. Antibiotics that initiated reduced efficiency of chemotherapy were more noticeable in the HER2-positive subgroup for both DFS (HR 5.51, 95% CI 1.77–17.2, p = 0.003) and OS (HR 7.0395% CI 1.94–25.53, p = 0.003), as well as in the T3-4 subgroup for both DFS (HR 20.36, 95% CI 2.41–172.07, p = 0.006) and OS (HR 13.45, 95% CI 1.39–130.08, p = 0.025) by stratified analysis. Antibiotic administration might be associated with reduced efficacy of neoadjuvant therapy and poor prognosis in BC patients. As a preliminary study, our research made preparations for further understanding and large-scale analyses of the impact of antibiotics on the efficacy of neoadjuvant therapy.
Similar content being viewed by others
Introduction
The latest cancer data in 2020 indicates that breast cancer is the most prevalent type of cancer worldwide. In China, breast cancer is the most common malignant tumor and the fifth leading cause of female cancer mortality1. The treatment of breast cancer has entered the era of individualized treatment with increasing systemic treatment, especially neoadjuvant therapy—preoperative systemic therapy for breast cancer without distant metastasis. The application of neoadjuvant therapy reduces the tumor burden and increases the likelihood of breast conservation to improve BC patients' quality of life2. It also enables clinicians to obtain individual drug sensitivity information on BC patients to guide further treatment3. The phenomenon of pathological complete response (pCR) was defined as no residual invasive tumor on pathologic assessment after therapy showing an associated survival benefit4.
Antibiotics are required to prevent and treat infectious bacterial diseases, mainly in BC patients with febrile neutropenia (FN), which is a severe adverse effect induced by chemotherapy5. Antibiotics are effective for treating various infections. Nevertheless, their application cannot be located as precisely as targeted drugs, which in turn leads to intestinal dysbacteriosis. The gut microbiota participates in many aspects of the human physiological process, from producing nutrients and vitamins to fighting pathogens and protecting immune system development and epithelial mucosa homeostasis6. Accumulating evidence suggests that gut microbiota has an impact on the efficacy of anti-tumor therapies—including chemotherapy, immunotherapy, radiotherapy, and surgery—used to treat solid tumors (melanoma, lung cancer, and colon cancer) with numerous mechanisms, including xenometabolism, immune microenvironment, and changed microbial community structure6. Antibiotic-treated mice displayed oxaliplatin (OXA) chemoresistance for colon carcinoma and lymphoma compared with specific pathogen-free (SPF) mice, which suggested that antibiotic exposure was associated with reduced chemotherapy efficacy7. Further research is needed in breast cancer to study the relationship between the effect of neoadjuvant therapy and antibiotics.
In the present study, we aimed to evaluate the influence of antibiotic administration on neoadjuvant therapy efficacy and prognosis in BC patients. We expect that our findings will provide a basis for future therapeutic concepts in BC patients who require antibiotics during neoadjuvant therapy.
Method
Patients
Patients with BC who received new adjuvant chemotherapy followed by surgery at the Fourth Hospital of Hebei Medical University between January 2013 and September 2015 were enrolled in this retrospective study. All methods were performed in accordance with the relevant guidelines and regulations for human participants and were supervised and approved by the Ethics Committee of the Fourth Hospital of Hebei Medical University (No. 2020KS001). Written informed consent was provided by participants. Neoadjuvant therapy consisted of docetaxel (T), anthracycline (A), cyclophosphamide (C), and Herceptin (H), and the types of neoadjuvant therapy were balanced between the two groups (p = 0.924, Table S2). All the patients in this study received modified radical mastectomies. According to NCCN guidelines, patients received standard postoperative radiotherapy and endocrine therapy based on postoperative pathological results. In total, 120 BC patients were enrolled in this study, all were of Han ethnicity and from Hebei Province (Table 1). The medical records of all patients were reviewed to determine whether any antibiotic administration occurred within 30 days after neoadjuvant therapy initiation. Data on the specific time of antibiotic exposure, antibiotic class, indication, route of administration, and duration were collected (Table S1). Patients who received antibiotics within 30 days after neoadjuvant therapy initiation were assigned to the antibiotic-treated group, while patients who did not receive antibiotics within 30 days after neoadjuvant therapy initiation were placed in the control group. One of the common reasons why patients use antibiotics is febrile neutropenia (FN) caused by the first cycle of neoadjuvant therapy. Data on additional parameters, including age, primary tumor size, regional lymph node, hormone receptor, HER2 status, and Miller-Payne grading criteria (Mp), were also collected. All patients were followed up every three months until death or until the database was closed (2020-10-24). We ordered the postoperative pathological data of the two groups, evaluated the tumor with Mp grade, and evaluated the lymph node status8. We defined pCR as no residual invasive tumor in either the tumor bed or lymph node on pathologic assessment after therapy.
Statistical analysis
Clinical and pathological features, Miller-Payne grading criteria, and pCR rate were compared by using the chi-square test or fisher’s exact test between two groups. The meaning of disease-free survival (DFS) was the time from operation to the date of disease progression or death. Overall survival (OS) was defined as the time from neoadjuvant therapy initiation to the date of death. At the end of follow-up, 3 (2.5%) patients and 9 (7.5%) patients were lost to follow-up, separately in DFS and OS analysis. We used the Kaplan–Meier method to plot survival curves and the log-rank test to evaluate the prognosis of patients. Univariate and multivariate analyses were done using the Cox proportional hazards model to identify the risk factors for survival. We did statistical analyses by using RStudio, version 1.3.1093 (2009–2020 RStudio, PBC) and SPSS software, version 19.0 (IBM Corporation, Armonk, NY), and p < 0.05 was considered statistically significant.
Result
A total of 120 BC patients who received neoadjuvant therapy were enrolled in this study. The distribution of clinicopathologic characteristics was well balanced between the control and antibiotic-treated groups (Table 1). In the antibiotic-treated group, 15 patients had febrile neutropenia, while the control group had none. There were 16 patients in the antibiotic-treated group who received a reduced dose and 19 patients in the control group (p = 0.987). Dose intensity (actual dose received/planned treatment received) and frequency of dose delay (actual delayed number of cycles and planned number of cycles) were not significantly different between the two groups (Fig. 1).
The effect of antibiotic administration on the efficacy of neoadjuvant therapy was evaluated in BC patients. We collected postoperative pathological information estimated using the Miller-Payne grading system. As shown in Fig. 2A, the distribution frequency of Miller-Payne grades was significantly different between these two groups (p = 0.033), with the proportion of Miller-Payne grades 2 and 3 increasing in the antibiotic-treated group and Miller-Payne grades 4 and 5 increasing in the control group (Fig. 2A). The pathological complete response (pCR) rate was also significantly higher in the control group than in the antibiotic-treated group (29.09% vs. 10.20%, p = 0.017) (Fig. 2B). These data demonstrated that antibiotic-induced microbiota dysbiosis might decrease chemotherapy treatment efficiency, referring to the Miller-Payne grading system in BC patients.
The impact of antibiotic administration on the clinical outcome and efficacy of BC patients. (A) Miller-Payne grade in BC patients. (B) Analysis of the pathological complete response (pCR) rate in BC patients. (C) The Kaplan–Meier curve of progression-free survival (DFS). (D) The Kaplan–Meier curve of overall survival (OS).
Further univariate analysis with Kaplan–Meier calculations demonstrated a significant difference in DFS (p = 0.04) and a borderline significant difference in OS (p = 0.088) between antibiotic-treated and control BC patients (Fig. 2C,D).
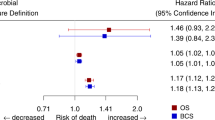
The potential outcome predictors were analyzed using the Cox proportional hazards model for multivariate analysis. As shown in Tables 2 and 3, antibiotic administration was identified as a significant independent prognostic factor for DFS (HR 3.026, 95% CI 1.314–6.969, p = 0.009) and OS (HR 2.836, 95% CI 1.016–7.858, p = 0.047). Furthermore, HER2 status was also identified as a significant independent prognostic factor for DFS (HR 2.946, 95% CI 1.306–6.645 p = 0.009) and OS (HR 6.320, 95% CI 2.235–17.872, p < 0.001). These data demonstrated that antibiotic-induced microbiota dysbiosis might modify BC patient outcomes by decreasing chemotherapy treatment efficiency.
The influence of antibiotic administration on DFS and OS was further investigated within individual subgroups of BC patients by stratified analysis, and the antibiotic-treated group displayed a trend of reduced DFS (Fig. 3A) and OS (Fig. 3B) within most subgroups. Antibiotics that initiated reduced efficiency of chemotherapy were more noticeable in the HER2-positive subgroup for both DFS (HR 5.51, 95% CI 1.77–17.2, p = 0.003) and OS (HR 7.03, 95% CI 1.94–25.53, p = 0.003), as well as in the T3-4 subgroup for both DFS (HR 20.36, 95% CI 2.41–172.07, p = 0.006) and OS (HR 13.45, 95% CI 1.39–130.08, p = 0.025) by stratified analysis. The p-values for interaction were less than 0.05 in both the HER2 subgroup and T3-4 subgroup, indicating that antibiotic administration might change the outcome in HER2-positive BC patients and those whose primary tumor was larger than 5 cm.
Discussion
We estimated the effect of antibiotic administration on neoadjuvant therapy and prognosis in BC patients. We found that patients who administrated antibiotics might have a decreased treatment efficiency of neoadjuvant therapy referring to the Miller-Payne system and ORR. It is known that the composition of the intestinal flora of cancer patients is different from that of individuals without cancer. The intestinal flora plays an important role in the human immune system9,10. Antibiotics induce dysbacteriosis by changing the intestinal flora environment involved in tumorigenesis, causing inflammation and disorders of the immune system11,12. The gastrointestinal microbiota could influence cancer immunotherapy on both the prognostic and therapeutic sides13, and the application of antibiotics could reduce the clinical benefit of immune checkpoint inhibitors (ICIs) in renal cell carcinoma (RCC) and non-small-cell lung cancer (NSCLC)14. A previous article also found that antibiotics could affect the efficiency of chemotherapy in esophageal cancer15. Consistent with a previous report, we discovered that antibiotic-induced dysbacteriosis might also modified neoadjuvant therapy efficiency in BC patients.
Changes in the microbial community structure may affect the efficacy of anti-tumor therapy, including chemotherapy. Intestinal dysbacteriosis reduces regulatory T cells (Tregs) and increases Th1 and Th17 cells to modulate the immune microenvironment of tumors, resulting in cyclophosphamide (CTX) chemoresistance7. Similar results were observed with oxaliplatin (OXA) treatment7. In addition to immune modulations, bacterial translocation can also directly modulate chemotherapy efficacy. In colorectal cell lines (HCT116 and HT29), autophagy of Fusobacterium nucleatum was associated with chemoresistance to 5-fluorouracil (5-FU) and oxaliplatin (OXA)16.
A recent study showed that gut microbiota was directly involved in the immune-mediated trastuzumab anti-tumor efficacy in 24 BC patients and mice17. Researchers used antibiotic administration or fecal microbiota transplantation from antibiotic-treated donors to change the community structure of gut microbiota to influence treatment efficiency17. Fecal microbiota transplantation provided an opportunity to correct antibiotic-induced chemotherapy failure. Our study was retrospective and lacked information on the intestinal flora of patients at the beginning of neoadjuvant therapy. It is vital to identify the gut microbiota responsible for dysbacteriosis-related treatment inefficiency in BC patients. The discovery of targeted bacteria capable of rescuing antibiotic-associated dysbiosis made it feasible to promote anti-tumor efficacy by modulating microbiome diversity, especially for HER2-positive BC patients.
In our study, the pathological complete response (pCR) rate was significantly higher in the control group than in the antibiotic-treated group (29.09% vs. 10.20%, p = 0.017). However, the pCR rate was not a significant independent prognostic factor for DFS and OS. The pCR rate is an effective indicator of short-term treatment in neoadjuvant therapy4. However, most studies show that the pCR rate is higher in hormone receptor (HR)-negative breast cancer patients after neoadjuvant therapy and improves DFS and OS outcomes compared patients with less than a pCR. However, there was no advantage in overall survival compared with HR-positive patients18. As this study was small and there were 82 (68.33%) HR-positive patients in our study, the pCR rate was not a significant factor for determining DFS or OS.
HER2 status was a significant independent prognostic factor of breast cancer, and we obtained the same conclusion in our study. In the subgroup analysis, the use of antibiotics dramatically decreased the treatment efficiency in HER2-positive and T3-4 subgroups of BC patients. Cancer patients may be treated with antibiotics because of conditions such as chemotherapy-related agranulocytosis, malnutrition, and cachexia19. We hope that our research draws clinicians’ attention to optimizing management of cancer patients during chemotherapy to reduce the incidence of chemotherapy-related side effects. As this study was small and retrospective, no conclusion for clinical practice is possible. More trials are necessary to assess the benefits and risks of antibiotics in breast cancer patients receiving neoadjuvant therapy.
Conclusion
Antibiotic administration might be associated with reduced chemotherapy efficacy and poor prognosis in BC patients, especially in HER2-positive BC patients and patients whose primary tumor was larger than 5 cm. As a preliminary study, our research made preparations for further understanding and large-scale analyses of the impact of antibiotics on the efficacy of neoadjuvant therapy.
Data availability
All data generated or analyzed during this study are included in this published article (for information, please contact the corresponding author). Ethics Approval and Informed Consent was obtained.
References
GLOBOCAN 2020 Graph production, https://gco.iarc.fr/today/home. (2020).
Cortazar, P. & Kluetz, P. G. Neoadjuvant breast cancer therapy and drug development. Clin. Adv. Hematol. Oncol. 13, 755–761 (2015).
Guerrero-Zotano, A. L. & Arteaga, C. L. Neoadjuvant trials in ER+ breast cancer: A tool for acceleration of drug development and discovery. Cancer Discov. 7, 561–574. https://doi.org/10.1158/2159-8290.Cd-17-0228 (2017).
Cortazar, P. & Geyer, C. E. Jr. Pathological complete response in neoadjuvant treatment of breast cancer. Ann. Surg. Oncol. 22, 1441–1446. https://doi.org/10.1245/s10434-015-4404-8 (2015).
Hashiguchi, Y. et al. Chemotherapy-induced neutropenia and febrile neutropenia in patients with gynecologic malignancy. Anticancer Drugs 26, 1054–1060. https://doi.org/10.1097/CAD.0000000000000279 (2015).
Villéger, R. et al. Intestinal microbiota: A novel target to improve anti-tumor treatment?. Int. J. Mol. Sci. 20, 4584. https://doi.org/10.3390/ijms20184584 (2019).
Iida, N. et al. Commensal bacteria control cancer response to therapy by modulating the tumor microenvironment. Science 342, 967–970. https://doi.org/10.1126/science.1240527 (2013).
Ogston, K. N. et al. A new histological grading system to assess response of breast cancers to primary chemotherapy: Prognostic significance and survival. Breast 12, 320–327. https://doi.org/10.1016/s0960-9776(03)00106-1 (2003).
Fernández, M. F. et al. Breast cancer and its relationship with the microbiota. Int. J. Environ. Res. Public Health 15, 1747. https://doi.org/10.3390/ijerph15081747 (2018).
Mikó, E. et al. Microbiome-microbial metabolome-cancer cell interactions in breast cancer-familiar, but unexplored. Cells https://doi.org/10.3390/cells8040293 (2019).
Chen, J. et al. The microbiome and breast cancer: A review. Breast Cancer Res. Treat. 178, 493–496. https://doi.org/10.1007/s10549-019-05407-5 (2019).
Ferreira, R. M. et al. Gastric microbial community profiling reveals a dysbiotic cancer-associated microbiota. Gut 67, 226–236. https://doi.org/10.1136/gutjnl-2017-314205 (2018).
Guven, D. C., Aktas, B. Y., Simsek, C. & Aksoy, S. Gut microbiota and cancer immunotherapy: Prognostic and therapeutic implications. Future Oncol. 16, 497–506. https://doi.org/10.2217/fon-2019-0783 (2020).
Derosa, L. et al. Negative association of antibiotics on clinical activity of immune checkpoint inhibitors in patients with advanced renal cell and non-small-cell lung cancer. Ann. Oncol. 29, 1437–1444. https://doi.org/10.1093/annonc/mdy103 (2018).
Wu, C. et al. Antibiotics modulate chemotherapy efficacy in patients with esophageal cancer. Cancer Manag. Res. 12, 4991–4997. https://doi.org/10.2147/cmar.S248130 (2020).
Yu, T. et al. Fusobacterium nucleatum promotes chemoresistance to colorectal cancer by modulating autophagy. Cell 170, 548-563.e516. https://doi.org/10.1016/j.cell.2017.07.008 (2017).
Di Modica, M. et al. Gut microbiota condition the therapeutic efficacy of trastuzumab in HER2-positive breast cancer. Cancer Res. 81, 2195–2206. https://doi.org/10.1158/0008-5472.Can-20-1659 (2021).
Mittendorf, E. A. et al. The neo-bioscore update for staging breast cancer treated with neoadjuvant chemotherapy: Incorporation of prognostic biologic factors into staging after treatment. JAMA Oncol 2, 929–936. https://doi.org/10.1001/jamaoncol.2015.6478 (2016).
Haeseker, M. B. et al. Trends in antibiotic prescribing in adults in Dutch general practice. PLoS ONE 7, e51860. https://doi.org/10.1371/journal.pone.0051860 (2012).
Author information
Authors and Affiliations
Contributions
X.Z. and L.Y. wrote the main manuscript text and prepared figures. C.G. and Z.G. made substantial contributions to conception and design. The rest of the authors took part in drafting the article or revising it critically for important intellectual content. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Zhang, X., Yu, L., Shi, J. et al. Antibiotics modulate neoadjuvant therapy efficiency in patients with breast cancer: a pilot analysis. Sci Rep 11, 14024 (2021). https://doi.org/10.1038/s41598-021-93428-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-021-93428-w
This article is cited by
-
Characterization of immunomodulating agents from Staphylococcus aureus for priming immunotherapy in triple-negative breast cancers
Scientific Reports (2024)
-
Breast cancers as ecosystems: a metabolic perspective
Cellular and Molecular Life Sciences (2023)
-
The involvement of oncobiosis and bacterial metabolite signaling in metastasis formation in breast cancer
Cancer and Metastasis Reviews (2021)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.