Abstract
The present study describes a novel method for the low energy cyclotron production and radiochemical isolation of no-carrier-added 132/135La3+ from bulk natBa. This separation strategy combines precipitation and single-column extraction chromatography to afford an overall radiochemical yield (92 ± 2%) and apparent molar activity (22 ± 4 Mbq/nmol) suitable for the radiolabeling of DOTA-conjugated vectors. The produced 132/135La3+ has a radiochemical and radionuclidic purity amenable for 132La/135La-based cancer theranostic applications. Longitudinal PET/CT images acquired using the positron-emitting 132La and ex vivo biodistribution data separately corroborated the accumulation of unchelated 132/135La3+ ions in bone and the liver.
Similar content being viewed by others
Introduction
Targeted Radionuclide Therapy (TRT) using electron-emitting radiometals has shown efficacy in the treatment of several malignancies. However, due to the long range of β- emissions, treatments often cause significant toxicities to normal surrounding tissues. Conversely, given the high linear energy transfer (LET) of Auger electrons, isotopes decaying by electron capture have the potential to locally deposit dose in target tissue while sparing normal tissues. In this regard, 135La (t1/2 = 19.93 h, 100% EC) is promising due to its suitable decay characteristics (Fig. 1A) and chemical properties resembling other common therapeutic radiometals (e.g., 177Lu, 90Y or 225Ac). Low energy proton irradiation of natural barium generates a mixture of 132-136La (13xLa) radionuclides including 135La and the positron-emitting 132La (t1/2 = 4.59 h) (Fig. 1B) via natBa(p,x)13xLa reactions. Reported production cross-sections for 13xLa radioisotopes from 12–70 MeV of natBa targets show that differential decay kinetics form 135La with high radionuclidic purity at energies available on most medical cyclotrons1,2,3,4. Furthermore, small quantities of co-produced, positron-emitting 132La enable seamless implementation of a theranostic approach and the noninvasive interrogation of pharmacokinetic profiles by in vivo positron emission tomography imaging and ex vivo biodistribution. To date, relatively few separation strategies have been reported for the radiochemical isolation of no-carrier-added lanthanum from barium, and they have achieved only moderate separation factors and chemical purity3,4,5. Our goal was to develop an optimized production method for 132/135La with a chemical purity suitable for chelation and in vivo PET imaging of radiolabeled, targeted theranostic pharmaceuticals.
Materials and Methods
Barium target material was purchased from Sigma-Aldrich with a purity of 99.9% (trace metal basis) and stored in inert gas atmosphere. Optima grade HNO3 and HCl from Fisher Chemical were used for the radiochemical separation studies. All glassware was washed with Alconox® solution, rinsed with 1 M HNO3, water (18 M Ohm cm−1), and finally dried at 120 °C to ensure the removal of trace metals.
Target preparation and irradiation
13xLa were co-produced by proton irradiation of natBa targets (~450 mg) via natBa(p,n)13xLa reactions using a 16 MeV GE PETtrace cyclotron. To make the targets, metallic barium was pressed (50 kg cm−2) into a niobium crucible with 12.2 mm diameter, 1.2 mm deep pocket. As target preparation was performed in air, the target was immediately installed on the cyclotron and exposed to high vacuum to limit barium oxidation. For imaging studies, irradiations were performed at 10 µA for up to 3 h with direct water cooling to the back of the niobium target holder, and a 0.25 mm Nb foil was used to degrade the incident beam energy from 16 MeV to 11.9 MeV. Irradiations were also performed at nominal 16 MeV beam energy and a maximum current of 25 µA for up to 1.5 h to compare production yields and to investigate additional (p,2n)-type nuclear reactions’ effect on radioisotopic purity.
Radiochemical isolation of 132/135La
The separation of 132/135La from the irradiated natBa targets was carried out by combining a precipitation method with column extraction chromatography using an N,N,N’,N’-tetrakis-2-ethylhexyldiglycolamide functionalized resin (DGA-branched, Eichrom). Between irradiation and the start of the separation, a 2–3 h “cool down” period decayed co-produced 134La (t1/2 = 6.5 min) and 136La (t1/2 = 9.9 min). Afterward, 5 mL of 6 M HNO3 dissolved 132/135La while precipitating bulk Ba as Ba(NO3)2. Following centrifugation, the supernatant was passed through a 1 ml fritted solid phase extraction (SPE) tube filled with ~130 mg of branched DGA resin, trapping 132/135La3+ and eluting Ba2+. Washing with decreasing concentrations of HNO3 (3–0.5 M) eluted trace metal impurities of Cu, Fe and Zn. Finally, no-carrier-added 132/135La3+ was eluted in a small volume of dilute 0.1 M HCl (4 × 300 µL). The loading, rinsing, and elution steps were carried out using a peristaltic pump at a flow rate of 1.6 mL min−1. The activity was quantified using an efficiency calibrated high purity germanium (HPGe) detector (10%, Al-window, 1.9 keV FWHM at 1333 keV).
Radiochemical and chemical analysis
Radiochemical yields were followed by HPGe gamma-ray spectrometry. Trace metal content of the chromatography fractions was measured with an Agilent 4200 microwave plasma-atomic emission spectrometer (MP-AES). Calibration curves were generated using commercially available multielement standards (Sigma-Aldrich). For transition metals, the typical detection limits of this technique are in the ppb range.
Apparent molar activity quantification
The apparent molar activity, an indication of the chemical purity of the produced lanthanum, was measured as described previously6,7. Briefly, the ability of DOTA to complex 13xLa3+ ions was determined by incubating aliquots of 135La3+ (3.7 MBq) with increasing DOTA concentrations (0–100 µg/mL) in 0.5 M NaOAc buffer solution (pH = 4.5) for 30 min at 80 °C. The complexation yield for each 135La3+/DOTA ratio was determined by autoradiographic thin layer chromatography (radio-TLC) using silica-impregnated paper as stationary phase and 1:1 MeOH:10% NH4OAc (w/v) as the mobile phase. Radioactivity distribution was visualized on the TLC plates using a Packard Cyclone phosphor plate reader. To compute the apparent molar activity, 135La3+ activity in MBq was divided by twice the number of moles of DOTA required to complex 50% of the radioactivity, and the value was reported in MBq/nmol (mean ± standard deviation, SD).
Positron-emission tomography (PET) imaging and ex vivo biodistribution
For in vivo distribution studies, “free” 132/135La3+ was prepared for injection by buffering the activity in phosphate buffered saline (PBS) containing 0.05 M sodium acetate. The weakly chelating acetate ion was added to avoid the formation of La radiocolloid at physiological pH (Fig. S1, Supporting Information). Animal experiments were conducted with the approval of the University of Wisconsin Institutional Animal Care and Use Committee (IACUC). All studies were conducted in accordance with the relevant guidelines and regulations. To assess the in vivo biodistribution of “free” La3+ ions, positron emission tomography (PET) imaging was performed in 10-week-old female ICR mice injected intravenously with 0.93 MBq of “free” 132La3+. Longitudinal static PET scans (photon energy window = 350–650 keV; coincidence timing window = 3.432 ns; axial resolution at center of FOV = 1.5 mm) collecting 40 million counts each were acquired of the anesthetized mice (2% isoflurane) at 0.5, 2, 5, and 20 h post-injection (p.i.) using an Inveon micro-PET/micro-CT scanner (Siemens). CT images were employed for anatomical co-registration and attenuation correction (80 kV, 900 μA, resolution 105 μm). Quantification of decay corrected PET/CT images was performed in an Inveon Research Workstation by manually drawing volume-of-interest (VOI) over the heart, muscle, bone, liver, and kidney. Quantitative data was expressed as percent injected activity per gram of tissue (%IA g−1; mean ± SD).
Following the last PET scan, ex vivo tissue distribution studies were performed for comparison with PET results. Mice were sacrificed, and organs were collected, wet-weighed, and counted in a calibrated gamma counter using an energy window from 10 to 100 keV (Wizard 2, PerkinElmer). Tissue radioactivity concentrations were calculated and reported as percent injected activity per gram of tissue (%IA g−1; mean ± SD).
Results and Discussion
Irradiation and production yield
The irradiation of the target was performed at a proton energy of 11.9 MeV and a beam current of 10 µA for 1.5–3 h. Under these irradiation conditions, 135La was produced with an end-of-bombardment (EOB) physical yield of 5.6 ± 1.1 MBq μAh−1 (n = 3). Due to the natural isotopic composition of the target, short-lived 134La and 136La were co-produced but at the end of chemistry (EOC) (ca. 5 h post-EOB), had decayed below the detection limit3. Relatively longer-lived 132La was also co-produced with a yield of 0.26 ± 0.05 MBq μAh−1 (~5% relative to 135La activity at EOB). Irradiations carried out at 16 MeV resulted in a marked increase in 135La production yields (16.4 ± 1.1 MBq μAh−1) but resulted in a reduction in the radionuclidic purity due to the co-production of the positron-emitter 133La (t1/2 = 3.91 h). Production yields of 132/133/135La at the two different proton irradiation energies are summarized in Table S1 (Supporting Information).
Separation of 13xLa from natBa targets
Following irradiation and target “cool down”, 5 mL of 6 M HNO3 was added to dissolve the produced 132/135La while precipitating bulk Ba as Ba(NO3)2 (s). Ba(NO3)2 is a soluble salt, but in the presence of concentrated HNO3 the solubility significantly decreases with increasing acid concentration8. In 6 M HNO3 the solubility of Ba(NO3)2 is about 2.4 mg ml−1, allowing the precipitation of the bulk Ba (~99%) target material. After centrifugation, the supernatant was passed through a branched DGA resin which retained 132/135La3+ but not the remaining Ba2+ ions. Two subsequent rinses of the precipitate with 5 mL of 3 M HNO3 were loaded onto the column to ensure the maximum recovery of lanthanum.
The loaded column was then rinsed with decreasing concentrations of HNO3 (3–0.5 M), eluting the trace metal impurities of Cu, Zn and Fe. Efficiently removing these metal contaminants is essential to obtain elevated radiolabeling yields, especially when non-specific chelators such as DOTA are employed. The quantitative removal of metal impurities was possible given their low affinity constant (Kd < 2) over a wide range of HNO3 concentrations for the branched DGA resin9,10. A final 0.5 M HNO3 rinse was performed to decrease the acidity of the column bed while preventing 132/135La3+elution from the column.
Due to the low affinity of the DGA resin for La3+ in 0.1 M HCl, 132/135La3+ was eluted in a small volume (<600 µL), with a final recovery efficiency of 92 ± 2% (n = 6) and a Ba/La separation factor of 106. This separation factor is four orders of magnitude higher than those reported in previous separation strategies3. Figure 2 summarizes the separation strategy and the elution profile from the branched DGA column.
Radionuclidic and chemical impurities
The isotopic composition of the natural Barium target (0.11% 130Ba; 0.10% 132Ba; 2.42% 134Ba; 6.59% 135Ba; 7.85% 136Ba; 11.23% 137Ba; and 71.70% 138Ba) bears weight on the radionuclidic composition of the final isolate. As measured by γ-ray spectrometry (Fig. 3) at EOC (ca. 5 h post-EOB), besides 135La, the other detectable lanthanum isotope was the positron-emitting 132La (2.47%). As discussed in section 3.5, the co-produced positron-emitting 132La was leveraged to describe the biodistribution of the therapeutic 135La3+ in vivo. In future therapeutic applications requiring higher 135La production yields, the irradiations can be performed at higher energies (i.e. 16 MeV) using enriched target material which will avoid the co-production of radionuclidic impurities. Nevertheless, for initial preclinical evaluations, our 135La production method from natBa and its associated radionuclidic purity is entirely feasible.
MP-AES determination of Ba and other transition metals influencing the 132/135La3+ apparent molar activity, including Cu, Zn and Fe was 638 ± 148 ppb, 64 ± 40 ppb, 138 ± 97 ppb, and <50 ppb, respectively (n = 3). The efficient removal of the target material and other metallic impurities (e.g., Cu, Zn, and Fe) played a decisive role in the obtained radiolabeling yields, as carboxylate chelating agents typically show high thermodynamic stability constants with these ubiquitous transition metals. The ppb levels of all these metals found in the final eluate did not preclude a high 132/135La3+ apparent molar activity with common chelators such as DOTA.
Apparent molar activity using the DOTA chelator
The use of chelating agents like DOTA is a common method to probe the reactivity of produced radiometals6,7,11. The non-specific character of DOTA allows for the effective formation of stable complexes with many metal ions12, providing practical information about the chemical purity of the produced radiometal. The 135La3+ apparent molar activity obtained by the titration of 135LaCl3 solutions with DOTA was 22 ± 4 MBq (135La) nmol−1 (n = 3), sufficient for labeling biological targeting vectors with 132/135La3+ for potential theranostic applications.
In vivo and ex vivo biodistribution
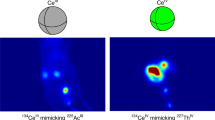
For the first time, the evaluation of the in vivo biodistribution of 132/135La3+ was performed in normal ICR mice (n = 3) by following the 132La positron emissions via longitudinal PET/CT. Figure 4A shows representative coronal maximum intensity projection (MIP) PET images at 0.5, 2, 5 and 20 h after intravenous injection of the unchelated 132/135La3+. Quantitative VOI analysis was performed to quantify the radionuclide accumulation values in all major organs/tissues. In all four time points, liver and bone showed persistent 132/135La3+ uptake, higher than that seen in other normal tissue including heart/blood, kidneys, and muscle. As seen in Fig. 4B (Table S2, Supporting Information), liver uptake peaked at 29.28 ± 2.32 %IA g−1 at 2.5 h post-injection and bone showed highest activity accumulation (5.23 ± 0.33 %IA g−1) at the earliest measured timepoint 0.5 h after injection.
Ex vivo biodistribution studies were carried out at 20 h p.i. to validate PET data and obtain a more detailed distribution profile of 132/135La3+. Corroborating the results of the PET imaging, a high accumulation of the radiometal in liver and bone (30.45 ± 2.62 %IA g−1 and 6.65 ± 0.42 %IA g−1; n = 3) was observed (Fig. 5). The results of both PET imaging and biodistribution studies show good agreement in trend and magnitude with previous reports showing a preferential in vivo distribution of La3+ ions to the liver and the skeleton13.
These findings confirmed the feasibility of using the positron-emitting 132La to track longitudinal in vivo biodistribution of free La3+ and La compounds noninvasively with PET imaging. Additionally, this work also suggests the use of 132La as a diagnostic congener of therapeutic radionuclides currently of intense interest, such as 135La and 225Ac, given the chemical similarities between these radiometals14.
Conclusion
The methods described above achieve the highest chemical purity, apparent molar activity, and Ba/La separation factor yet reported for the production of 135La using natBa targets. More importantly, to the best of our knowledge, this is the first study that employs the co-produced 132La to monitor the in vivo biodistribution of the therapeutic Auger-emitting 135La using PET imaging. Further studies exploring isotopically enriched targets for 135La-based targeted radionuclide therapy are warranted.
References
Prescher, K. et al. Thin-target cross sections of proton-induced reactions on barium and solar cosmic ray production rates of xenon-isotopes in lunar surface materials. Nucl. Instruments Methods Phys. Res. Sect. B Beam Interact. with Mater. Atoms 53, 105–121 (1991).
Tárkányi, F. et al. Study of activation cross sections of proton induced reactions on barium: Production of 131Ba→131Cs. Appl. Radiat. Isot. 68, 1869–1877 (2010).
Fonslet, J. et al. 135 La as an Auger-electron emitter for targeted internal radiotherapy. Phys. Med. Biol. 63, 15026 (2018).
Abel, E. P., Clause, H. K., Fonslet, J., Nickles, R. J. & Severin, G. W. Half-lives of la 132 and la 135. Phys. Rev. C, https://doi.org/10.1103/PhysRevC.97.034312 (2018).
Mansel, A. & Franke, K. Production of no-carrier-added 135La at an 18 MeV cyclotron and its purification for investigations at a concentration range down to 10–15 mol/L. Radiochimica Acta 103, 759 (2015).
Yoo, J. et al. Preparation of high specific activity 86Y using a small biomedical cyclotron. Nucl. Med. Biol. 32, 891–897 (2005).
Aluicio-Sarduy, E. et al. Simplified and automatable radiochemical separation strategy for the production of radiopharmaceutical quality 86Y using single column extraction chromatography. Appl. Radiat. Isot. 142, 28–31 (2018).
Greene, C. H. The Solubility of Barium Nitrate in Concentrated Nitric Acid. J. Am. Chem. Soc. 59, 1186–1188 (1937).
Horwitz, E. P., McAlister, D. R., Bond, A. H. & Barrans, R. E. Novel Extraction of Chromatographic Resins Based on Tetraalkyldiglycolamides: Characterization and Potential Applications. Solvent Extr. Ion Exch. 23, 319–344 (2005).
Pourmand, A. & Dauphas, N. Distribution coefficients of 60 elements on TODGA resin: Application to Ca, Lu, Hf, U and Th isotope geochemistry. Talanta 81, 741–753 (2010).
Avila-Rodriguez, M. A., Nye, J. A. & Nickles, R. J. Production and separation of non-carrier-added 86Y from enriched 86Sr targets. Appl. Radiat. Isot. 66, 9–13 (2008).
Byegård, J., Skarnemark, G. & Skålberg, M. The stability of some metal EDTA, DTPA and DOTA complexes: Application as tracers in groundwater studies. J. Radioanal. Nucl. Chem. 241, 281–290 (1999).
Laszlo, D., Ekstein, D. M., Lewin, R. & Stern, K. G. Biological Studies on Stable and Radioactive Rare Earth Compounds. I. On the Distribution of Lanthanum in the Mammalian Organism2. JNCI J. Natl. Cancer Inst. 13, 559–573 (1952).
Thiele, N. A. et al. An Eighteen-Membered Macrocyclic Ligand for Actinium-225 Targeted Alpha Therapy. Angew. Chemie Int. Ed. 56, 14712–14717 (2017).
Khazov, Y., Rodionov, A. A., Sakharov, S. & Singh, B. Nuclear Data Sheets for A = 132. Nucl. Data Sheets 104, 497–790 (2005).
Singh, B., Rodionov, A. A. & Khazov, Y. L. Nuclear Data Sheets for A = 135. Nucl. Data Sheets 109, 517–698 (2008).
Author information
Authors and Affiliations
Contributions
E.A. performed preparatory experimentation and method development, collected and analyzed in vivo, and ex vivo data, and prepared the manuscript. R.H. contributed to experimental design and assisted with PET image collection and ex vivo biodistribution studies. A.P.O., P.A.E. and T.E.B. developed radioisotope production tools, assisted with tracer quality assurance, and contributed to experimental design. W.C. assisted with interpretation of data, animal protocol development, and manuscript revision. J.W.E. supervised project execution and assisted with interpretation of data and manuscript preparation. All authors discussed the results and implications and commented on the manuscript at all stages.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Aluicio-Sarduy, E., Hernandez, R., Olson, A.P. et al. Production and in vivo PET/CT imaging of the theranostic pair 132/135La. Sci Rep 9, 10658 (2019). https://doi.org/10.1038/s41598-019-47137-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-019-47137-0
This article is cited by
-
Production of the PET radionuclide 61Cu via the 62Ni(p,2n)61Cu nuclear reaction
EJNMMI Radiopharmacy and Chemistry (2024)
-
Cutting edge rare earth radiometals: prospects for cancer theranostics
EJNMMI Radiopharmacy and Chemistry (2022)
-
High yield cyclotron production of a novel 133/135La theranostic pair for nuclear medicine
Scientific Reports (2020)
-
Guest Edited Collection: Radioisotopes and radiochemistry in health science
Scientific Reports (2020)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.