Abstract
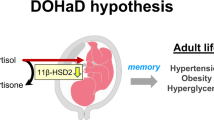
Dietary salt intake increases blood pressure (BP) but the salt sensitivity of BP differs between individuals. The interplay of ageing, genetics and environmental factors, including malnutrition and stress, contributes to BP salt sensitivity. In adults, obesity is often associated with salt-sensitive hypertension. The children of women who experience malnutrition during pregnancy are at increased risk of developing obesity, diabetes and salt-sensitive hypertension as adults. Similarly, the offspring of mice that are fed a low-protein diet during pregnancy develop salt-sensitive hypertension in association with aberrant DNA methylation of the gene encoding type 1A angiotensin II receptor (AT1AR) in the hypothalamus, leading to upregulation of hypothalamic AT1AR and renal sympathetic overactivity. Ageing is also associated with salt-sensitive hypertension. In aged mice, promoter methylation leads to reduced kidney production of the anti-ageing factor Klotho and a decrease in circulating soluble Klotho. In the setting of Klotho deficiency, salt-induced activation of the vascular Wnt5a–RhoA pathway leads to ageing-associated salt-sensitive hypertension, potentially as a result of reduced renal blood flow and increased peripheral resistance. Thus, kidney mechanisms and aberrant DNA methylation of certain genes are involved in the development of salt-sensitive hypertension during fetal development and old age. Three distinct paradigms of epigenetic memory operate on different timescales in prenatal malnutrition, obesity and ageing.
Key points
-
Hyperaldosteronism induced by adipocyte-derived aldosterone-releasing factors, and renal sympathetic overactivity resulting from hypothalamic noradrenergic abnormalities, contribute to the development of obesity-induced salt-sensitive hypertension.
-
The offspring of mothers that experience malnutrition during pregnancy are at increased risk of developing salt-sensitive hypertension in adult life.
-
Aberrant DNA methylation of type 1 angiotensin II receptors in the hypothalamus contributes to prenatal programmed hypertension through salt-induced activation of the hypothalamic renal sympathetic nervous system.
-
An increase in the salt sensitivity of blood pressure with ageing contributes to the high prevalence of hypertension in elderly people.
-
A reduction in circulating Klotho owing to DNA methylation of Klotho in the kidney has a critical role in ageing-associated salt-sensitive hypertension through salt-induced activation of the non-canonical Wnt5a–RhoA pathway in the vasculature and a resulting reduction in renal blood flow.
-
Epigenetic modulation has a role in salt-sensitive hypertension throughout life, including as a result of malnutrition during pregnancy, obesity in adults and ageing.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
World Health Organization. Hypertension. WHO https://www.who.int/news-room/fact-sheets/detail/hypertension (2019).
Zhou, B. et al. Long-term and recent trends in hypertension awareness, treatment, and control in 12 high-income countries: an analysis of 123 nationally representative surveys. Lancet 394, 639–651 (2019).
Bromfield, S. & Muntner, P. High blood pressure: the leading global burden of disease risk factor and the need for worldwide prevention programs. Curr. Hypertens. Rep. 15, 134–136 (2013).
Meneely, G. R. & Dahl, L. K. Electrolytes in hypertension: the effects of sodium chloride: the evidence from animal and human studies. Med. Clin. North. Am. 45, 271–283 (1961).
Intersalt: an international study of electrolyte excretion and blood pressure. Results for 24 hour urinary sodium and potassium excretion. Intersalt Cooperative Research Group. BMJ 297, 319–328 (1988).
Mente, A. et al. Association of urinary sodium and potassium excretion with blood pressure. N. Engl. J. Med. 371, 601–611 (2014).
Luft, F. C. & Weinberger, M. H. Heterogeneous responses to changes in dietary salt intake: the salt-sensitivity paradigm. Am. J. Clin. Nutr. 65, 612S–617S (1997).
Kawasaki, T., Delea, C. S., Bartter, F. C. & Smith, H. The effect of high-sodium and low-sodium intakes on blood pressure and other related variables in human subjects with idiopathic hypertension. Am. J. Med. 64, 193–198 (1978).
Fujita, T., Henry, W. L., Bartter, F. C., Lake, C. R. & Delea, C. S. Factors influencing blood pressure in salt-sensitive patients with hypertension. Am. J. Med. 69, 334–344 (1980).
Guyton, A. C. Kidneys and fluids in pressure regulation. Small volume but large pressure changes. Hypertension 19, I2–I8 (1992).
Hall, J. E., Guyton, A. C. & Brands, M. W. Pressure-volume regulation in hypertension. Kidney Int. Suppl. 55, S35–S41 (1996).
Guyton, A. C. The surprising kidney-fluid mechanism for pressure control — its infinite gain! Hypertension 16, 725–730 (1990).
Hall, J. E. Mechanisms of abnormal renal sodium handling in obesity hypertension. Am. J. Hypertens. 10, 49S–55S (1997).
Hall, J. E., do Carmo, J. M., da Silva, A. A., Wang, Z. & Hall, M. E. Obesity-induced hypertension: interaction of neurohumoral and renal mechanisms. Circ. Res. 116, 991–1006 (2015).
Edwards, A. & McDonough, A. A. Impact of angiotensin II-mediated stimulation of sodium transporters in the nephron assessed by computational modeling. Am. J. Physiol. Renal Physiol. 317, F1656–F1668 (2019).
Zhao, D., Seth, D. M. & Navar, L. G. Enhanced distal nephron sodium reabsorption in chronic angiotensin II-infused mice. Hypertension 54, 120–126 (2009).
Riazi, S., Khan, O., Hu, X. & Ecelbarger, C. A. Aldosterone infusion with high-NaCl diet increases blood pressure in obese but not lean Zucker rats. Am. J. Physiol. Renal Physiol. 291, F597–F605 (2006).
Winternitz, S. R., Katholi, R. E. & Oparil, S. Role of the renal sympathetic nerves in the development and maintenance of hypertension in the spontaneously hypertensive rat. J. Clin. Invest. 66, 971–978 (1980).
Shibata, S. et al. Rac1 GTPase in rodent kidneys is essential for salt-sensitive hypertension via a mineralocorticoid receptor-dependent pathway. J. Clin. Invest. 121, 3233–3243 (2011).
Mu, S. et al. Epigenetic modulation of the renal β-adrenergic-WNK4 pathway in salt-sensitive hypertension. Nat. Med. 17, 573–580 (2011).
Coffman, T. M. Under pressure: the search for the essential mechanisms of hypertension. Nat. Med. 17, 1402–1409 (2011).
Fujita, T., Noda, H. & Ando, K. Sodium susceptibility and potassium effects in young patients with borderline hypertension. Circulation 69, 468–476 (1984).
Sullivan, J. M., Prewitt, R. L., Ratts, T. E., Josephs, J. A. & Connor, M. J. Hemodynamic characteristics of sodium-sensitive human subjects. Hypertension 9, 398–406 (1987).
Morris, R. C. Jr., Schmidlin, O., Sebastian, A., Tanaka, M. & Kurtz, T. W. Vasodysfunction that involves renal vasodysfunction, not abnormally increased renal retention of sodium, accounts for the initiation of salt-induced hypertension. Circulation 133, 881–893 (2016).
Redgrave, J., Rabinowe, S., Hollenberg, N. K. & Williams, G. H. Correction of abnormal renal blood flow response to angiotensin II by converting enzyme inhibition in essential hypertensives. J. Clin. Invest. 75, 1285–1290 (1985).
Campese, V. M., Parise, M., Karubian, F. & Bigazzi, R. Abnormal renal hemodynamics in black salt-sensitive patients with hypertension. Hypertension 18, 805–812 (1991).
Fujita, T., Ando, K. & Ogata, E. Systemic and regional hemodynamics in patients with salt-sensitive hypertension. Hypertension 16, 235–244 (1990).
Polichnowski, A. J., Griffin, K. A., Long, J., Williamson, G. A. & Bidani, A. K. Blood pressure-renal blood flow relationships in conscious angiotensin II- and phenylephrine-infused rats. Am. J. Physiol. Renal Physiol. 305, F1074–F1084 (2013).
Oliver, J. A. & Cannon, P. J. The effect of altered sodium balance upon renal vascular reactivity to angiotensin II and norepinephrine in the dog. Mechanism of variation in angiotensin responses. J. Clin. Invest. 61, 610–623 (1978).
Crowley, S. D. et al. Angiotensin II causes hypertension and cardiac hypertrophy through its receptors in the kidney. Proc. Natl Acad. Sci. USA 103, 17985–17990 (2006).
El-Osta, A. et al. Transient high glucose causes persistent epigenetic changes and altered gene expression during subsequent normoglycemia. J. Exp. Med. 205, 2409–2417 (2008).
Kazmi, N. et al. Associations between high blood pressure and DNA methylation. PLoS ONE 15, e0227728 (2020).
Li, Y. et al. The DNA methylome of human peripheral blood mononuclear cells. PLoS Biol. 8, e1000533 (2010).
Friso, S., Carvajal, C. A., Fardella, C. E. & Olivieri, O. Epigenetics and arterial hypertension: the challenge of emerging evidence. Transl Res. 165, 154–165 (2015).
Liu, P. et al. Role of DNA de novo (de)methylation in the kidney in dalt-induced hypertension. Hypertension 72, 1160–1171 (2018).
Dasinger, J. H. et al. Epigenetic modifications in T cells. Hypertension 75, 372–382 (2020).
Garrison, R. J., Kannel, W. B., Stokes, J. & Castelli, W. P. Incidence and precursors of hypertension in young adults: the Framingham offspring study. Prev. Med. 16, 235–251 (1987).
Rocchini, A. P. et al. The effect of weight loss on the sensitivity of blood pressure to sodium in obese adolescents. N. Engl. J. Med. 321, 580–585 (1989).
Chen, J. et al. Metabolic syndrome and salt sensitivity of blood pressure in non-diabetic people in China: a dietary intervention study. Lancet 373, 829–835 (2009).
Tuck, M. L., Sowers, J., Dornfeld, L., Kledzik, G. & Maxwell, M. The effect of weight reduction on blood pressure, plasma renin activity, and plasma aldosterone levels in obese patients. N. Engl. J. Med. 304, 930–933 (1981).
Rocchini, A. P., Katch, V. L., Grekin, R., Moorehead, C. & Anderson, J. Role for aldosterone in blood pressure regulation of obese adolescents. Am. J. Cardiol. 57, 613–618 (1986).
Rossi, G. P. et al. Body mass index predicts plasma aldosterone concentrations in overweight-obese primary hypertensive patients. J. Clin. Endocrinol. Metab. 93, 2566–2571 (2008).
de Paula, R. B., da Silva, A. A. & Hall, J. E. Aldosterone antagonism attenuates obesity-induced hypertension and glomerular hyperfiltration. Hypertension 43, 41–47 (2004).
Engeli, S. et al. Weight loss and the renin-angiotensin-aldosterone system. Hypertension 45, 356–362 (2005).
Kidambi, S. et al. Association of adrenal steroids with hypertension and the metabolic syndrome in blacks. Hypertension 49, 704–711 (2007).
Ehrhart-Bornstein, M. et al. Human adipocytes secrete mineralocorticoid-releasing factors. Proc. Natl Acad. Sci. USA 100, 14211–14216 (2003).
Goodfriend, T. L., Ball, D. L., Egan, B. M., Campbell, W. B. & Nithipatikom, K. Epoxy-keto derivative of linoleic acid stimulates aldosterone secretion. Hypertension 43, 358–363 (2004).
Wong, G. W., Wang, J., Hug, C., Tsao, T. S. & Lodish, H. F. A family of Acrp30/adiponectin structural and functional paralogs. Proc. Natl Acad. Sci. USA 101, 10302–10307 (2004).
Huby, A. C. et al. Adipocyte-derived hormone leptin is a direct regulator of aldosterone secretion, which promotes endothelial dysfunction and cardiac fibrosis. Circulation 132, 2134–2145 (2015).
Nagase, M. et al. Enhanced aldosterone signaling in the early nephropathy of rats with metabolic syndrome: possible contribution of fat-derived factors. J. Am. Soc. Nephrol. 17, 3438–3446 (2006).
Matsui, H. et al. Salt excess causes left ventricular diastolic dysfunction in rats with metabolic disorder. Hypertension 52, 287–294 (2008).
Nagase, M., Matsui, H., Shibata, S., Gotoda, T. & Fujita, T. Salt-induced nephropathy in obese spontaneously hypertensive rats via paradoxical activation of the mineralocorticoid receptor: role of oxidative stress. Hypertension 50, 877–883 (2007).
Kawarazaki, W. et al. Angiotensin II- and salt-induced kidney injury through Rac1-mediated mineralocorticoid receptor activation. J. Am. Soc. Nephrol. 23, 997–1007 (2012).
Kawarazaki, W. & Fujita, T. The role of aldosterone in obesity-related hypertension. Am. J. Hypertens. 29, 415–423 (2016).
Fujita, T. Mechanism of salt-sensitive hypertension: focus on adrenal and sympathetic nervous systems. J. Am. Soc. Nephrol. 25, 1148–1155 (2014).
Briones, A. M. et al. Adipocytes produce aldosterone through calcineurin-dependent signaling pathways: implications in diabetes mellitus-associated obesity and vascular dysfunction. Hypertension 59, 1069–1078 (2012).
Shibata, S. et al. Modification of mineralocorticoid receptor function by Rac1 GTPase: implication in proteinuric kidney disease. Nat. Med. 14, 1370–1376 (2008).
Van Beusecum, J. P. et al. High salt activates CD11c+ antigen-presenting cells via SGK (serum glucocorticoid kinase) 1 to promote renal inflammation and salt-sensitive hypertension. Hypertension 74, 555–563 (2019).
Yoshida, S. et al. Local mineralocorticoid receptor activation and the role of Rac1 in obesity-related diabetic kidney disease. Nephron Exp. Nephrol. 126, 16–24 (2014).
Ando, K. et al. Anti-albuminuric effect of the aldosterone blocker eplerenone in non-diabetic hypertensive patients with albuminuria: a double-blind, randomised, placebo-controlled trial. Lancet Diabetes Endocrinol. 2, 944–953 (2014).
Nishimoto, M. et al. Mineralocorticoid receptor blockade suppresses dietary salt-induced ACEI/ARB-resistant albuminuria in non-diabetic hypertension: a sub-analysis of evaluate study. Hypertens. Res. 42, 514–521 (2019).
Hall, J. E. The kidney, hypertension, and obesity. Hypertension 41, 625–633 (2003).
Hall, J. E., Do Carmo, J. M., Da Silva, A. A., Wang, Z. & Hall, M. E. Obesity, kidney dysfunction and hypertension: mechanistic links. Nat. Rev. Nephrol. 15, 367–385 (2019).
Prior, L. J. et al. Exposure to a high-fat diet alters leptin sensitivity and elevates renal sympathetic nerve activity and arterial pressure in rabbits. Hypertension 55, 862–868 (2010).
Nagae, A. et al. Sympathoexcitation by oxidative stress in the brain mediates arterial pressure elevation in obesity-induced hypertension. Circulation 119, 978–986 (2009).
Grassi, G. et al. Sympathetic activation in obese normotensive subjects. Hypertension 25, 560–563 (1995).
Vaz, M. et al. Regional sympathetic nervous activity and oxygen consumption in obese normotensive human subjects. Circulation 96, 3423–3429 (1997).
Lohmeier, T. E. et al. Systemic and renal-specific sympathoinhibition in obesity hypertension. Hypertension 59, 331–338 (2012).
DiBona, G. F. Physiology in perspective: the wisdom of the body. Neural control of the kidney. Am. J. Physiol. Regul. Integr. Comp. Physiol. 289, R633–R641 (2005).
Hesse, I. F. & Johns, E. J. The role of alpha-adrenoceptors in the regulation of renal tubular sodium reabsorption and renin secretion in the rabbit. Br. J. Pharmacol. 84, 715–724 (1985).
Terker, A. S. et al. Sympathetic stimulation of thiazide-sensitive sodium chloride cotransport in the generation of salt-sensitive hypertension. Hypertension 64, 178–184 (2014).
Castañeda-Bueno, M. et al. Activation of the renal Na+:Cl− cotransporter by angiotensin II is a WNK4-dependent process. Proc. Natl Acad. Sci. USA 109, 7929–7934 (2012).
San-Cristobal, P. et al. Angiotensin II signaling increases activity of the renal Na-Cl cotransporter through a WNK4-SPAK-dependent pathway. Proc. Natl Acad. Sci. USA 106, 4384–4389 (2009).
Davies, M. R. et al. The thiazide-sensitive co-transporter promotes the development of sodium retention in mice with diet-induced obesity. Kidney Blood Press. Res. 40, 509–519 (2015).
Kassab, S. et al. Renal denervation attenuates the sodium retention and hypertension associated with obesity. Hypertension 25, 893–897 (1995).
Henegar, J. R. et al. Catheter-based radiorefrequency renal denervation lowers blood pressure in obese hypertensive dogs. Am. J. Hypertens. 27, 1285–1292 (2014).
Katayama, T. et al. Long-term renal denervation normalizes disrupted blood pressure circadian rhythm and ameliorates cardiovascular injury in a rat model of metabolic syndrome. J. Am. Heart Assoc. 2, e000197 (2013).
Komers, R. et al. Enhanced phosphorylation of Na+-Cl− co-transporter in experimental metabolic syndrome: role of insulin. Clin. Sci. 123, 635–647 (2012).
Nishida, H. et al. Phosphatidylinositol 3-kinase/Akt signaling pathway activates the WNK-OSR1/SPAK-NCC phosphorylation cascade in hyperinsulinemic db/db mice. Hypertension 60, 981–990 (2012).
Brands, M. W. & Hall, J. E. Insulin resistance, hyperinsulinemia, and obesity-associated hypertension. J. Am. Soc. Nephrol. 3, 1064–1077 (1992).
Hall, J. E. et al. Hemodynamic and renal responses to chronic hyperinsulinemia in obese, insulin-resistant dogs. Hypertension 25, 994–1002 (1995).
Koepke, J. P. & DiBona, G. F. High sodium intake enhances renal nerve and antinatriuretic responses to stress in spontaneously hypertensive rats. Hypertension 7, 357–363 (1985).
Fujita, T. & Sato, Y. Changes in renal and central noradrenergic activity with potassium in DOCA-salt rats. Am. J. Physiol. 246, F670–F675 (1984).
Fujita, T. & Sato, Y. Hypotensive effect of taurine. Possible involvement of the sympathetic nervous system and endogenous opiates. J. Clin. Invest. 82, 993–997 (1988).
Sato, Y., Ando, K., Ogata, E. & Fujita, T. High potassium intake attenuates antinatriuretic response to air stress in DOCA-salt rats. Am. J. Physiol. 260, R941–R945 (1991).
Bell, B. B. & Rahmouni, K. Leptin as a mediator of obesity-induced hypertension. Curr. Obes. Rep. 5, 397–404 (2016).
Morgan, D. A., Thedens, D. R., Weiss, R. & Rahmouni, K. Mechanisms mediating renal sympathetic activation to leptin in obesity. Am. J. Physiol. Regul. Integr. Comp. Physiol. 295, R1730–R1736 (2008).
Purkayastha, S., Zhang, G. & Cai, D. Uncoupling the mechanisms of obesity and hypertension by targeting hypothalamic IKK-β and NF-κB. Nat. Med. 17, 883–887 (2011).
do Carmo, J. M. et al. Control of blood pressure, appetite, and glucose by leptin in mice lacking leptin receptors in proopiomelanocortin neurons. Hypertension 57, 918–926 (2011).
Xue, B. et al. Leptin mediates high-fat diet sensitization of angiotensin II-elicited hypertension by upregulating the brain renin–angiotensin system and inflammation. Hypertension 67, 970–976 (2016).
Xue, B. et al. Central renin-angiotensin system activation and inflammation induced by high-fat diet sensitize angiotensin II-elicited hypertension. Hypertension 67, 163–170 (2016).
De Kloet, A. D. et al. Neuroimmune communication in hypertension and obesity: a new therapeutic angle? Pharmacol. Ther. 138, 428–440 (2013).
De Kloet, A. D. et al. Angiotensin type 1a receptors in the paraventricular nucleus of the hypothalamus protect against diet-induced obesity. J. Neurosci. 33, 4825–4833 (2013).
Fujita, M., Ando, K., Nagae, A. & Fujita, T. Sympathoexcitation by oxidative stress in the brain mediates arterial pressure elevation in salt-sensitive hypertension. Hypertension 50, 360–367 (2007).
Choi, J. et al. Role of the histone deacetylase inhibitor valproic acid in high-fat diet-induced hypertension via inhibition of HDAC1/angiotensin II axis. Int. J. Obes. 41, 1702–1709 (2017).
Kawakami-Mori, F. et al. Aberrant DNA methylation of hypothalamic angiotensin receptor in prenatal programmed hypertension. JCI Insight 3, e95625 (2018).
Boström, A. E. et al. Longitudinal genome-wide methylation study of Roux-en-Y gastric bypass patients reveals novel CpG sites associated with essential hypertension. BMC Med. Genomics 9, 20 (2016).
Barker, D. J., Osmond, C., Golding, J., Kuh, D. & Wadsworth, M. E. Growth in utero, blood pressure in childhood and adult life, and mortality from cardiovascular disease. BMJ 298, 564–567 (1989).
Gluckman, P. D. Living with the past: evolution, development, and patterns of disease. Science 305, 1733–1736 (2004).
Bateson, P. et al. Developmental plasticity and human health. Nature 430, 419–421 (2004).
Hanson, M. A. & Gluckman, P. D. Early developmental conditioning of later health and disease: physiology or pathophysiology? Physiol. Rev. 94, 1027–1076 (2014).
Vehaskari, V. M. & Woods, L. L. Prenatal programming of hypertension: lessons from experimental models. J. Am. Soc. Nephrol. 16, 2545–2556 (2005).
Seckl, J. R. Glucocorticoids, feto-placental 11 beta-hydroxysteroid dehydrogenase type 2, and the early life origins of adult disease. Steroids 62, 89–94 (1997).
Ortiz, L. A., Quan, A., Zarzar, F., Weinberg, A. & Baum, M. Prenatal dexamethasone programs hypertension and renal injury in the rat. Hypertension 41, 328–334 (2003).
Sheen, J.-M. et al. Prenatal dexamethasone-induced programmed hypertension and renal programming. Life Sci. 132, 41–48 (2015).
Manning, J. & Vehaskari, V. M. Postnatal modulation of prenatally programmed hypertension by dietary Na and ACE inhibition. Am. J. Physiol. Regul. Integr. Comp. Physiol. 288, R80–R84 (2005).
Tang, J. I., Kenyon, C. J., Seckl, J. R. & Nyirenda, M. J. Prenatal overexposure to glucocorticoids programs renal 11β-hydroxysteroid dehydrogenase type 2 expression and salt-sensitive hypertension in the rat. J. Hypertens. 29, 282–289 (2011).
Baum, M. Role of renal sympathetic nerve activity in prenatal programming of hypertension. Pediatr. Nephrol. 33, 409–419 (2018).
Dagan, A., Kwon, H. M., Dwarakanath, V. & Baum, M. Effect of renal denervation on prenatal programming of hypertension and renal tubular transporter abundance. Am. J. Physiol. Renal Physiol. 295, F29–F34 (2008).
Mizuno, M., Lozano, G., Siddique, K., Baum, M. & Smith, S. A. Enalapril attenuates the exaggerated sympathetic response to physical stress in prenatally programmed hypertensive rats. Hypertension 63, 324–329 (2014).
Mizuno, M., Siddique, K., Baum, M. & Smith, S. A. Prenatal programming of hypertension induces sympathetic overactivity in response to physical stress. Hypertension 61, 180–186 (2013).
Pladys, P. et al. Role of brain and peripheral angiotensin II in hypertension and altered arterial baroreflex programmed during fetal life in rat. Pediatr. Res. 55, 1042–1049 (2004).
Li, P. et al. Role of paraventricular angiotensin AT1 receptors in salt-sensitive hypertension in mRen-2 transgenic rats. Am. J. Physiol. 270, R1178–R1181 (1996).
Senanayake, P. D. et al. Increased expression of angiotensin peptides in the brain of transgenic hypertensive rats. Peptides 15, 919–926 (1994).
De Kloet, A. D. et al. A unique “angiotensin-sensitive” neuronal population coordinates neuroendocrine, cardiovascular, and behavioral responses to stress. J. Neurosci. 37, 3478–3490 (2017).
Aguilera, G., Kiss, A. & Luo, X. Increased expression of type 1 angiotensin II receptors in the hypothalamic paraventricular nucleus following stress and glucocorticoid administration. J. Neuroendocrinol. 7, 775–783 (1995).
Treviño, L. S. et al. Epigenome environment interactions accelerate epigenomic aging and unlock metabolically restricted epigenetic reprogramming in adulthood. Nat. Commun. 11, 2316 (2020).
Jones, P. A. Functions of DNA methylation: islands, start sites, gene bodies and beyond. Nat. Rev. Genet. 13, 484–492 (2012).
Nenke, M. A. et al. Differential effects of estrogen on corticosteroid-binding globulin forms suggests reduced cleavage in pregnancy. J. Endocr. Soc. 1, 202–210 (2017).
Stern, J. E. et al. Astrocytes contribute to angiotensin II stimulation of hypothalamic neuronal activity and sympathetic outflow. Hypertension 68, 1483–1493 (2016).
Liu, G. et al. Angiotensin II induces human astrocyte senescence through reactive oxygen species production. Hypertens. Res. 34, 479–483 (2011).
Cruz, J. C., Flôr, A. F., França-Silva, M. S., Balarini, C. M. & Braga, V. A. Reactive oxygen species in the paraventricular nucleus of the hypothalamus alter sympathetic activity during metabolic syndrome. Front. Physiol. 6, 384 (2015).
Rodríguez-Rodríguez, P. et al. Fetal undernutrition is associated with perinatal sex-dependent alterations in oxidative status. J. Nutr. Biochem. 26, 1650–1659 (2015).
De Sousa, S. M. et al. Oxidative injuries induced by maternal low-protein diet in female brainstem. Nutr. Neurosci. 21, 580–588 (2017).
de Brito Alves, J. L. et al. Maternal protein restriction induced-hypertension is associated to oxidative disruption at transcriptional and functional levels in the medulla oblongata. Clin. Exp. Pharmacol. Physiol. 43, 1177–1184 (2016).
Ojeda, N. B., Grigore, D., Robertson, E. B. & Alexander, B. T. Estrogen protects against increased blood pressure in postpubertal female growth restricted offspring. Hypertension 50, 679–685 (2007).
Dasinger, J. H., Davis, G. K., Newsome, A. D. & Alexander, B. T. Developmental programming of hypertension: physiological mechanisms. Hypertension 68, 826–831 (2016).
He, F. et al. Association between DNA methylation in obesity-related genes and body mass index percentile in adolescents. Sci. Rep. 9, 2079 (2019).
Kohno, D. et al. Dnmt3a in Sim1 neurons is necessary for normal energy homeostasis. J. Neurosci. 34, 15288–15296 (2014).
Forrester, S. J., Kikuchi, D. S., Hernandes, M. S., Xu, Q. & Griendling, K. K. Reactive oxygen species in metabolic and inflammatory signaling. Circ. Res. 122, 877–902 (2018).
Guarino, D., Nannipieri, M., Iervasi, G., Taddei, S. & Bruno, R. M. The role of the autonomic nervous system in the pathophysiology of obesity. Front. Physiol. 8, 665 (2017).
Mahbouli, S. et al. Leptin induces ROS via NOX5 in healthy and neoplastic mammary epithelial cells. Oncol. Rep. 38, 3254–3264 (2017).
Wolf-Maier, K. et al. Hypertension prevalence and blood pressure levels in 6 European countries, Canada, and the United States. JAMA 289, 2363–2369 (2003).
Lim, S. S. et al. A comparative risk assessment of burden of disease and injury attributable to 67 risk factors and risk factor clusters in 21 regions, 1990–2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet 380, 2224–2260 (2012).
Beckett, N. S. et al. Treatment of hypertension in patients 80 years of age or older. N. Engl. J. Med. 358, 1887–1898 (2008).
Luft, F. C., Grim, C. E., Fineberg, N. & Weinberger, M. C. Effects of volume expansion and contraction in normotensive whites, blacks, and subjects of different ages. Circulation 59, 643–650 (1979).
Weinberger, M. H. & Fineberg, N. S. Sodium and volume sensitivity of blood pressure. Age and pressure change over time. Hypertension 18, 67–71 (1991).
Elijovich, F. et al. Salt sensitivity of blood pressure: a scientific statement from the American Heart Association. Hypertension 68, e7–e46 (2016).
Oliver, W. J., Cohen, E. L. & Neel, J. V. Blood pressure, sodium intake, and sodium related hormones in the Yanomamo Indians, a “no-salt” culture. Circulation 52, 146–151 (1975).
Fliser, D. et al. Renal function in the elderly: impact of hypertension and cardiac function. Kidney Int. 51, 1196–1204 (1997).
Knox, F. G., Mertz, J. I., Burnett, J. C. Jr. & Haramati, A. Role of hydrostatic and oncotic pressures in renal sodium reabsorption. Circ. Res. 52, 491–500 (1983).
Shimamoto, H. & Shimamoto, Y. Time course of hemodynamic responses to sodium in elderly hypertensive patients. Hypertension 16, 387–397 (1990).
Hill, C., Lateef, A. M., Engels, K., Samsell, L. & Baylis, C. Basal and stimulated nitric oxide in control of kidney function in the aging rat. Am. J. Physiol. 272, R1747–R1753 (1997).
De Nicola, L., Blantz, R. C. & Gabbai, F. B. Nitric oxide and angiotensin II. Glomerular and tubular interaction in the rat. J. Clin. Invest. 89, 1248–1256 (1992).
Zhou, X. J., Vaziri, N. D., Zhang, J., Wang, H. W. & Wang, X. Q. Association of renal injury with nitric oxide deficiency in aged SHR: prevention by hypertension control with AT1 blockade. Kidney Int. 62, 914–921 (2002).
Brown, J. M. et al. Aldosterone dysregulation with aging predicts renal vascular function and cardiovascular risk. Hypertension 63, 1205–1211 (2014).
Nanba, K. et al. Age-related autonomous aldosteronism. Circulation 136, 347–355 (2017).
Arnold, A. C. et al. Mineralocorticoid receptor activation contributes to the supine hypertension of autonomic failure. Hypertension 67, 424–429 (2016).
Ayuzawa, N. et al. Rac1-mediated activation of mineralocorticoid receptor in pressure overload-induced cardiac injury. Hypertension 67, 99–106 (2016).
Bauersachs, J., Jaisser, F. & Toto, R. Mineralocorticoid receptor activation and mineralocorticoid receptor antagonist treatment in cardiac and renal diseases. Hypertension 65, 257–263 (2015).
Bender, S. B. et al. Mineralocorticoid receptor antagonism treats obesity-associated cardiac diastolic dysfunction. Hypertension 65, 1082–1088 (2015).
Jia, G. et al. Endothelial mineralocorticoid receptor deletion prevents diet-induced cardiac diastolic dysfunction in females. Hypertension 66, 1159–1167 (2015).
McCurley, A. et al. Direct regulation of blood pressure by smooth muscle cell mineralocorticoid receptors. Nat. Med. 18, 1429–1433 (2012).
Kim, S. K. et al. Smooth muscle cell–mineralocorticoid receptor as a mediator of cardiovascular stiffness with aging. Hypertension 71, 609–621 (2018).
DuPont, J. J. et al. Vascular mineralocorticoid receptor regulates microRNA-155 to promote vasoconstriction and rising blood pressure with aging. JCI Insight 1, e88942 (2016).
Köttgen, A. et al. Multiple loci associated with indices of renal function and chronic kidney disease. Nat. Genet. 41, 712–717 (2009).
Mutig, K. et al. Activation of the bumetanide-sensitive Na+,K+,2Cl− cotransporter (NKCC2) is facilitated by Tamm-Horsfall protein in a chloride-sensitive manner. J. Biol. Chem. 286, 30200–30210 (2011).
Trudu, M. et al. Common noncoding UMOD gene variants induce salt-sensitive hypertension and kidney damage by increasing uromodulin expression. Nat. Med. 19, 1655–1660 (2013).
Guzik, T. J. & Touyz, R. M. Oxidative stress, inflammation, and vascular aging in hypertension. Hypertension 70, 660–667 (2017).
Wirth, A. et al. Age-dependent blood pressure elevation is due to increased vascular smooth muscle tone mediated by G-protein signalling. Cardiovasc. Res. 109, 131–140 (2016).
Dominguez, L. J. & Barbagallo, M. The biology of the metabolic syndrome and aging. Curr. Opin. Clin. Nutr. Metab. Care 19, 5–11 (2016).
Bonomini, F., Rodella, L. F. & Rezzani, R. Metabolic syndrome, aging and involvement of oxidative stress. Aging Dis. 6, 109 (2015).
Ferrucci, L. & Fabbri, E. Inflammageing: chronic inflammation in ageing, cardiovascular disease, and frailty. Nat. Rev. Cardiol. 15, 505–522 (2018).
Sato, Y. & Yanagita, M. Immunology of the ageing kidney. Nat. Rev. Nephrol. 15, 625–640 (2019).
Barnes, J. N. et al. Aging enhances autonomic support of blood pressure in women. Hypertension 63, 303–308 (2014).
Zubcevic, J. et al. Altered inflammatory response is associated with an impaired autonomic input to the bone marrow in the spontaneously hypertensive rat. Hypertension 63, 542–550 (2014).
López-Andrés, N. et al. Absence of cardiotrophin 1 is associated with decreased age-dependent arterial stiffness and increased longevity in mice. Hypertension 61, 120–129 (2013).
Seals, D. R., Jablonski, K. L. & Donato, A. J. Aging and vascular endothelial function in humans. Clin. Sci. 120, 357–375 (2011).
Cao, W. et al. Sympathetic overactivity in CKD disrupts buffering of neurotransmission by endothelium-derived hyperpolarizing factor and enhances vasoconstriction. J. Am. Soc. Nephrol. 31, 2312–2325 (2020).
El Assar, M. et al. Mechanisms involved in the aging-induced vascular dysfunction. Front. Physiol. 3, 132 (2012).
Thompson, R. F., Fazzari, M. J. & Greally, J. M. Experimental approaches to the study of epigenomic dysregulation in ageing. Exp. Gerontol. 45, 255–268 (2010).
Shi, Y. et al. Histone demethylation mediated by the nuclear amine oxidase homolog LSD1. Cell 119, 941–953 (2004).
Williams, J. S. et al. Lysine-specific demethylase 1: an epigenetic regulator of salt-sensitive hypertension. Am. J. Hypertens. 25, 812–817 (2012).
Krug, A. W. et al. Lysine-specific demethylase-1 modifies the age effect on blood pressure sensitivity to dietary salt intake. Age 35, 1809–1820 (2013).
Treesaranuwattana, T. et al. Lysine-specific demethylase-1 deficiency increases agonist signaling via the mineralocorticoid receptor. Hypertension 75, 1045–1053 (2020).
Kuro-o, M. et al. Mutation of the mouse klotho gene leads to a syndrome resembling ageing. Nature 390, 45–51 (1997).
Kurosu, H. et al. Suppression of aging in mice by the hormone Klotho. Science 309, 1829–1833 (2005).
Zhou, X., Chen, K., Lei, H. & Sun, Z. Klotho gene deficiency causes salt-sensitive hypertension via monocyte chemotactic protein-1/CC chemokine receptor 2-mediated inflammation. J. Am. Soc. Nephrol. 26, 121–132 (2015).
Liu, Y. et al. CD8+ T cells stimulate Na-Cl co-transporter NCC in distal convoluted tubules leading to salt-sensitive hypertension. Nat. Commun. 8, 14037 (2017).
Koyama, D. et al. Soluble alpha Klotho as a candidate for the biomarker of aging. Biochem. Biophys. Res. Commun. 467, 1019–1025 (2015).
Xiao, N. M., Zhang, Y. M., Zheng, Q. & Gu, J. Klotho is a serum factor related to human aging. Chin. Med. J. 117, 742–747 (2004).
Citterio, L. et al. Klotho gene in human salt-sensitive hypertension. Clin. J. Am. Soc. Nephrol. 15, 375–383 (2020).
Kawarazaki, W. et al. Salt causes aging-associated hypertension via vascular Wnt5a under Klotho deficiency. J. Clin. Invest. 130, 4152–4166 (2020).
Foulquier, S. et al. WNT signaling in cardiac and vascular disease. Pharmacol. Rev. 70, 68–141 (2018).
Liu, H. et al. Augmented Wnt signaling in a mammalian model of accelerated aging. Science 317, 803–806 (2007).
Niehrs, C. The complex world of WNT receptor signalling. Nat. Rev. Mol. Cell Biol. 13, 767–779 (2012).
Nusse, R. Wnt signaling and stem cell control. Cell Res. 18, 523–527 (2008).
Mikels, A., Minami, Y. & Nusse, R. Ror2 receptor requires tyrosine kinase activity to mediate Wnt5A signaling. J. Biol. Chem. 284, 30167–30176 (2009).
Florian, M. C. et al. A canonical to non-canonical Wnt signalling switch in haematopoietic stem-cell ageing. Nature 503, 392–396 (2013).
Naito, A. T. et al. Complement C1q activates canonical Wnt signaling and promotes aging-related phenotypes. Cell 149, 1298–1313 (2012).
Sumida, T. et al. Complement C1q-induced activation of beta-catenin signalling causes hypertensive arterial remodelling. Nat. Commun. 6, 6241 (2015).
Franceschi, C., Garagnani, P., Parini, P., Giuliani, C. & Santoro, A. Inflammaging: a new immune–metabolic viewpoint for age-related diseases. Nat. Rev. Endocrinol. 14, 576–590 (2018).
Wilck, N. et al. Salt-responsive gut commensal modulates TH17 axis and disease. Nature 551, 585–589 (2017).
Park, S. Y., Kang, M. J. & Han, J. S. Interleukin-1 beta promotes neuronal differentiation through the Wnt5a/RhoA/JNK pathway in cortical neural precursor cells. Mol. Brain 11, 39 (2018).
Laffer, C. L., Scott, R. C. 3rd, Titze, J. M., Luft, F. C. & Elijovich, F. Hemodynamics and salt-and-water balance link sodium storage and vascular dysfunction in salt-sensitive subjects. Hypertension 68, 195–203 (2016).
Ando, K. et al. Effect of aging on salt sensitivity of blood pressure in patients with essential hypertension. Clin. Exp. Nephrol. 1, 18–22 (1999).
Kopp, C. et al. 23Na magnetic resonance imaging-determined tissue sodium in healthy subjects and hypertensive patients. Hypertension 61, 635–640 (2013).
Machnik, A. et al. Macrophages regulate salt-dependent volume and blood pressure by a vascular endothelial growth factor-C-dependent buffering mechanism. Nat. Med. 15, 545–552 (2009).
Shao, Y. et al. Biological functions of macrophage-derived Wnt5a, and its roles in human diseases. Oncotarget 7, 67674–67684 (2016).
Fuster, J. J. et al. Noncanonical Wnt signaling promotes obesity-induced adipose tissue inflammation and metabolic dysfunction independent of adipose tissue expansion. Diabetes 64, 1235–1248 (2015).
Akoumianakis, I. et al. Adipose tissue-derived WNT5A regulates vascular redox signaling obesity via USP17/RAC1-mediated activation of NADPH oxidases. Sci. Transl Med. 11, eaav5055 (2019).
Urakawa, I. et al. Klotho converts canonical FGF receptor into a specific receptor for FGF23. Nature 444, 770–774 (2006).
Dalton, G. et al. Soluble klotho binds monosialoganglioside to regulate membrane microdomains and growth factor signaling. Proc. Natl Acad. Sci. USA 114, 752–757 (2017).
Fakhar, M., Najumuddin, Gul, M. & Rashid, S. Antagonistic role of Klotho-derived peptides dynamics in the pancreatic cancer treatment through obstructing WNT-1 and Frizzled binding. Biophys. Chem. 240, 107–117 (2018).
Chu, Y. et al. Glutathione peroxidase-1 overexpression reduces oxidative stress, and improves pathology and proteome remodeling in the kidneys of old mice. Aging Cell 19, e13154 (2020).
Carracedo, J. et al. Klotho modulates the stress response in human senescent endothelial cells. Mech. Ageing Dev. 133, 647–654 (2012).
Jiang, W. et al. Klotho inhibits PKCα/p66SHC-mediated podocyte injury in diabetic nephropathy. Mol. Cell. Endocrinol. 494, 110490 (2019).
Funato, Y., Michiue, T., Asashima, M. & Miki, H. The thioredoxin-related redox-regulating protein nucleoredoxin inhibits Wnt-beta-catenin signalling through Dishevelled. Nat. Cell Biol. 8, 501–508 (2006).
Camacho Londoño, J. E. et al. Angiotensin-II-Evoked Ca2+ entry in murine cardiac fibroblasts does not depend on TRPC channels. Cells 9, 322 (2020).
Guilluy, C. et al. The Rho exchange factor Arhgef1 mediates the effects of angiotensin II on vascular tone and blood pressure. Nat. Med. 16, 183–190 (2010).
Sen, P., Shah, P. P., Nativio, R. & Berger, S. L. Epigenetic mechanisms of longevity and aging. Cell 166, 822–839 (2016).
Liang, M. Epigenetic mechanisms and hypertension. Hypertension 72, 1244–1254 (2018).
Shchukina, I. et al. Enhanced epigenetic profiling of classical human monocytes reveals a specific signature of healthy aging in the DNA methylome. Nat. Aging 1, 124–141 (2020).
Chen, K. & Sun, Z. Activation of DNA demethylases attenuates aging-associated arterial stiffening and hypertension. Aging Cell 17, e12762 (2018).
Sahu, A. et al. Age-related declines in α-Klotho drive progenitor cell mitochondrial dysfunction and impaired muscle regeneration. Nat. Commun. 9, 4859 (2018).
Han, K. et al. Sarcopenia as a determinant of blood pressure in older Koreans: findings from the Korea National Health and Nutrition Examination Surveys (KNHANES) 2008-2010. PLoS ONE 9, e86902 (2014).
Nzietchueng, R. et al. Klotho KL-VS genotype is involved in blood pressure regulation. Clin. Chim. Acta 412, 1773–1777 (2011).
Semba, R. D. et al. Klotho in the cerebrospinal fluid of adults with and without Alzheimer’s disease. Neurosci. Lett. 558, 37–40 (2014).
Almeida, O. P. et al. Longevity Klotho gene polymorphism and the risk of dementia in older men. Maturitas 101, 1–5 (2017).
Ninomiya, T. et al. Midlife and late-life blood pressure and dementia in Japanese elderly. Hypertension 58, 22–28 (2011).
Morimoto, A. et al. Sodium sensitivity and cardiovascular events in patients with essential hypertension. Lancet 350, 1734–1737 (1997).
Acknowledgements
This review article is based on T.F.’s 2019 ASN Homer W. Smith Award lecture, ASN Kidney Week, Washington DC, USA. T.F. acknowledges the excellent support and contributions of K. Ando, T. Shimosawa, M. Nagase, M. Isshiki, T. Marumo, S. Oba, R. Mizuno, Y. Shibagaki, M. Fujita, F. Kawakami-Mori, S. Shibata, H. Kawarazaki, S. Mu, W. Kawarazaki, M. Nishimoto, N. Ayuzawa, D. Hirohama and K. Ueda to his research into salt-sensitive hypertension. The authors’ research was supported by JSPS KAKENHI (Grant nos 15H05788, 18K08028 and 20K21596). The authors are members of the Division of Clinical Epigenetics, University of Tokyo, which is supported by an unrestricted grant from EA Pharma, MSD K.K., Asahi Group Holdings, Astellas Pharma, Omron Healthcare, Shionogi, Mochida Pharmaceutical, Chugai Pharmaceutical, Mitsubishi Tanabe Pharma, Toray Industries, Nippon Boehringer Ingelheim, Fukuda Denshi, Kyowa Kirin, Novartis Pharma, Pfizer and Takeda Pharmaceutical.
Author information
Authors and Affiliations
Contributions
W.K. and T.F. researched the data for the article; discussed its content; and wrote, reviewed and edited the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information
Nature Reviews Nephrology thanks Thu Lee, who co-reviewed with Joseph Gigliotti, and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Glossary
- Air-jet stress
-
An air jet is an environmental stimulus that induces a stress reaction in rats, including increased renal sympathetic nerve activity and decreased urinary sodium excretion.
- Hyperphagia
-
An abnormally strong sensation of hunger or desire to eat, often leading to or accompanied by overeating. Hyperphagia does not subside after eating and often leads to the intake of excessive quantities of food.
- Optogenetic stimulation
-
Optogenetics is a technology that enables optical modulation of cells that have been genetically engineered to express proteins that contain a photoreceptive domain coupled to biological function. Optogenetic stimulation of specific neuron populations enables them to be activated or inhibited using light.
- L-type calcium channels
-
These channels have long been considered the primary route of Ca2+ entry into vascular smooth muscle cells (VSMCs). Ca2+ influx through L-type calcium channels is the principal mediator of the myogenic response, which is the intrinsic ability of VSMCs to contract and relax in response to changes in intraluminal pressure.
- Sarcopenia
-
A common condition among older adults that is characterized by age-dependent loss of muscle mass and function. Sarcopenia is associated with several adverse health outcomes, including frailty.
- Vascular dementia
-
A common type of dementia that is caused by reduced blood flow to the brain.
Rights and permissions
About this article
Cite this article
Kawarazaki, W., Fujita, T. Kidney and epigenetic mechanisms of salt-sensitive hypertension. Nat Rev Nephrol 17, 350–363 (2021). https://doi.org/10.1038/s41581-021-00399-2
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41581-021-00399-2
This article is cited by
-
Adding salt to foods and hazards of microvascular, cerebrovascular and cardiovascular diseases
European Journal of Clinical Nutrition (2024)
-
Associations between metabolic profiles and incident CKD in the Chinese population aged 45–85 years
International Urology and Nephrology (2024)
-
Relationship between changes in blood pressure from summer to winter and estimated 24-hour salt excretion using spot urine: the Niigata Wellness Study
Hypertension Research (2023)
-
Precision Nutrition and Cardiovascular Disease Risk Reduction: the Promise of High-Density Lipoproteins
Current Atherosclerosis Reports (2023)
-
Development of a short-form Chinese health literacy scale for low salt consumption (CHLSalt-22) and its validation among hypertensive patients
BMC Nutrition (2022)