Abstract
Objective
The objective of this study was to assess the prevalence and trends for neonatal hyperbilirubinemia, and the development of bilirubin neurotoxicity in the USA.
Study Design
We used a de-identified national dataset for the years 2002–2017. The study included all newborn inpatients with postnatal age ≤28 days. Cochran–Armitage trend test was used for trend analyses. Regression analyses were performed and adjusted odds ratios (aOR) were reported.
Results
The study included 57,989,476 infants; of them 53,259,758 (91.8%) were term infants and 4,725,178 (8.2%) were preterm infants. Bilirubin neurotoxicity decreased over the years in term infants (Z = 0.36, p = 0.03) without change in preterm infants (Z = 42.5, p = 0.12). Black neonates were less likely to be diagnosed with hyperbilirubinemia than White neonates (aOR = 0.77, 95% confidence interval (CI): 0.77–0.78, p < 0.001) and more likely to develop bilirubin neurotoxicity than White neonates (aOR = 3.0.5, 95% CI: 2.13–4.36, p < 0.001). Bilirubin neurotoxicity rate in the overall population was 2.4 per 100,000 live births.
Conclusions
Bilirubin neurotoxicity has significantly decreased in term infants and did not change in preterm infants. Despite the less diagnosis of hyperbilirubinemia in Black newborns, they are disproportionately at increased risk of developing bilirubin neurotoxicity when compared to White newborns.
Impact
In this article, we analyzed the National Inpatient Database. This is the largest study of its kind using data on 57,989,476 neonates. The article has multiple novel findings: (1) it demonstrated that utilization of phototherapy has increased significantly over the years, (2) the rate of kernicterus for neonates decreased in term infants and did not change in preterm babies, (3) kernicterus was mostly encountered in infants without isoimmunization jaundice, and (4) there is a clear racial disparity in neonatal jaundice; although Black newborns have less neonatal jaundice, they are at increased risk of developing kernicterus.
Similar content being viewed by others
BACKGROUND
Neonatal jaundice is the most common problem encountered during the first week of the neonatal period.1 It usually resolves without complications, although it has the potential to encompass neurotoxicity in both preterm and term infants.2 The progression of hyperbilirubinemia into bilirubin neurotoxicity is preventable with early detection and treatment.3 Bilirubin screen and risk factor identification are recommended routinely for almost all inpatient neonates in the United States.4 In addition, early screening of all pregnant women at risk of having a newborn with severe hyperbilirubinemia is an essential practice by obstetricians in the United States.5,6 The majority of neonatal jaundice is successfully managed with phototherapy,7 although high-risk infants may require exchange transfusion.8 The crucial challenge is early detection of infants at risk of developing bilirubin neurotoxicity from those who have simple neonatal jaundice;9 this is especially true with the new trend of early neonatal discharge.10
The American Academy of Pediatrics (AAP) issued guidelines for screening, risk factor identification, and management of neonatal jaundice in infants ≥35 weeks of gestation.11 These guidelines have been effectively used since then. However, the impact of these guidelines on the utilization of phototherapy, length of hospital stays, use of exchange transfusion, and, more importantly, occurrence of bilirubin neurotoxicity has not been reported.
Historically, neonatal isoimmunization to maternal blood type and minor blood groups has been the primary attribute to severe neonatal jaundice and the major indication for exchange transfusion.12 Following the risk-stratified guidelines for the management of neonatal jaundice, it is not clear if isoimmunization continues to be the source of bilirubin neurotoxicity. In addition, the systematic and early use of phototherapy is expected to abort the spike of hyperbilirubinemia and decrease the need for exchange transfusion. If true, the experience of caregiver with this procedure may be in jeopardy and instituting simulation programs to practice exchange transfusion procedures would be a requirement.13
This study utilized the National Inpatient Sample (NIS) dataset over 16 years to address neonatal jaundice in the United States. In particular, the study aimed to assess the prevalence and trends for neonatal hyperbilirubinemia, use of phototherapy, length of stay, and development of bilirubin neurotoxicity with and without isoimmunization. Data were reported in two strata: term and preterm. We hypothesized that AAP guidelines led to a higher rate for detection of neonatal hyperbilirubinemia and phototherapy use at the birthing hospital with less occurrence of bilirubin neurotoxicity.
Materials and methods
Data sources and management
We used de-identified patient data produced by the Healthcare Cost and Utilization Project (HCUP) from the Agency for Healthcare Research and Quality (AHRQ). HCUP contains the largest collection of hospital discharge data in the United States. HCUP produces the NIS dataset annually that includes 20% of the HCUP samples. Each year more than seven million cases are drawn from thousands of hospitals across the United States with various care levels (primary–tertiary), types of insurance (public or private), size of the hospital (small, medium, or large), and many other demographic and clinical characteristics. HCUP used (ICD-9-CM) diagnosis and procedure codes from 2002 to the first 9 months of 2015 and (ICD-10-CM) codes from the last 3 months of 2015–2017.14,15 The NIS is designed as a random sample of all US community hospitals from states that contribute their State Inpatient Databases to the HCUP. Data elements in the NIS are constructed in a uniform format with quality checks in place. NIS data are available from 1988 to 2017, thereby allowing analysis of trends over time. The unweighted data contain >7 million hospital stays each year, whereas weighted data estimates >35 million hospitalizations nationally.16
All comparisons were two-sided. Significance was set at P value <0.05.
Study design and population
The study included all newborn inpatients with postnatal age ≤28 days regardless of their gestational age (GA) or birth weight (BW) from 2002 to 2017. Infants diagnosed with neonatal hyperbilirubinemia with or without isoimmunization were identified. Procedure codes for phototherapy and exchange transfusion were identified. Table S1 (supplement) includes all diagnostic and procedural codes used in this study. Infants with direct hyperbilirubinemia, biliary obstruction, and cholestasis were excluded. Direct hyperbilirubinemia was identified according to ICD-9 and ICD-10 codes as shown in Supplementary Table S1. Infants diagnosed with polycythemia were not included in the calculation for exchange transfusion rate. Overall rates of newborns identified with jaundice and bilirubin neurotoxicity due to isoimmunization or non-isoimmunization were calculated. Rates were then reported by sex, race/ethnicity, premature status, household income, type of insurance, and region. Rates for use of phototherapy and exchange transfusion were calculated. Trend analyses were conducted for the distribution of bilirubin neurotoxicity due to isoimmunization and non-isoimmunization and in term and preterm infants from 2002 to 2017 by using independent samples Cochran–Armitage trend test for ordered alternatives. P value was calculated to decide whether to accept or reject the null hypothesis for each trend. All analyses were performed using SPSS 25 statistical software (SPSS Inc., Chicago, IL). This study used weighted data for all analyses and patient consent was not required.
Results
During the study period 2002–2017, a total of 58,009,098 infants were admitted ≤28 days. A total of 19,622 infants diagnosed with cholestatic diseases were excluded (biliary atresia, neonatal hepatitis, total parental nutrition cholestasis, jaundice due to hepatocellular damage, choledochal cyst, and alpha-1-antitrypsin deficiency) and 57,989,476 were included in this study (Figure S1); of them, 53,259,758 were term infants and 4,725,178 (8.15%) were preterm infants. Neonatal hyperbilirubinemia was diagnosed in 11,371,497 (19.6%) neonates. The vast majority of cases with hyperbilirubinemia were not related to isoimmunization 7,839,052 (76.6%) infants.
Table 1 provides the characteristics of the study population with and without hyperbilirubinemia. The rate of hyperbilirubinemia in term infants after birth and before discharge was 16.6%, and in preterm infants, it was 53.4%. Neonatal hyperbilirubinemia was higher in preterm infants when compared to term infants; 2.6 vs 1.8% for hyperbilirubinemia due to isoimmunization and 50.7 vs 14.7% for hyperbilirubinemia without isoimmunization. The frequency of hyperbilirubinemia was greater in males and less frequent in Black infants. Table 2 presents the treatment modalities received in term and preterm infants with jaundice. Phototherapy and exchange transfusion were used more frequently in preterm infants. Table 3 shows the result of bilirubin neurotoxicity due to isoimmunization and not due to isoimmunization during the 16 years study. A total of 1437 infants had bilirubin neurotoxicity. Exchange transfusion was not used for all cases that developed bilirubin neurotoxicity; only 30.7% of infants with bilirubin neurotoxicity with isoimmunization and 69.2% of infants with non-isoimmunization bilirubin neurotoxicity received exchange transfusion (Table 3). When limiting the analysis to infants who did not leave the birthing hospital, and excluding infants readmitted for severe jaundice, the percentages of infants who received exchange transfusion were 62 and 74% in infants diagnosed with kernicterus with and without isoimmunization, respectively. Logistic regression analyses for bilirubin neurotoxicity due to isoimmunization and without isoimmunization are presented in Table 4. Bilirubin neurotoxicity was significantly associated with male sex, Black race, private insurance, and larger hospital size. Compared to control infants, length of stay, and hospital cost were significantly increased in infants with hyperbilirubinemia and further increased in those with bilirubin neurotoxicity (Supplementary Table S2).
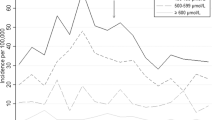
Trend analyses (Fig. 1) showed increased diagnosis for neonatal hyperbilirubinemia over the years (Z = 3.068, p = 0.002); while the rate of bilirubin neurotoxicity decreased in term infants, it did not change in preterm infants (Z = 0.36, p = 0.03 and Z = 42.5, p = 0.12, respectively).
The upper panel presents the trend for neonatal hyperbilirubinemia in percentages during the years 2002–2017. There is a significant rising trend for the diagnosis of hyperbilirubinemia (Z = 3.068, p = 0.002). The middle panel presents trends for bilirubin neurotoxicity per 100,000 neonates. The solid line represents bilirubin neurotoxicity in term neonates and the dotted line represents bilirubin neurotoxicity in preterm neonates (p = 0.03 and p = 0.12, respectively). The frequency trends decreased over the years in term (Z = 0.36, p = 0.03), but did not change in preterm neonates (Z = 42.5, p = 0.12). The lower panel presents trends for bilirubin neurotoxicity per 100,000 neonates with or without isoimmunization. The solid line represents bilirubin neurotoxicity without isoimmunization and the dotted line represents bilirubin neurotoxicity due to isoimmunization. The frequency trends did not change over the years in both types (p = 0.115 and p = 0.891, respectively). Trend analyses were performed using independent samples Cochran–Armitage trend test.
Discussion
This study reported neonatal hyperbilirubinemia to be more frequently diagnosed in males and in preterm infants, whereas it was less commonly diagnosed in the Black population. Exchange transfusion was used more frequently in preterm infants and in infants with non-isoimmunization jaundice. Bilirubin neurotoxicity was more frequently encountered in male preterm infants with non-isomerization jaundice. This study showed a significant increase in the diagnosis of neonatal hyperbilirubinemia over the 16 years of the study. The bilirubin neurotoxicity trend decreased in term and remained unchanged in preterm babies. Although the less diagnosis of jaundice, bilirubin neurotoxicity is disproportionately increased in Black newborns.
The prevalence of hyperbilirubinemia diagnosis has increased significantly during the study period 2002–2017. This is possibly related to the categorical use of guidelines to diagnose and treat neonatal hyperbilirubinemia. The American Academy of Pediatrics updated its guidelines for neonatal hyperbilirubinemia and use of phototherapy in hospitalized infants ≥35 weeks gestation 2004.11 In addition, the availability of noninvasive devices that enable the estimation of bilirubin concentrations transcutaneously provides a powerful screening tool for neonatal jaundice. However, the rate of bilirubin neurotoxicity did not change over the years for preterm babies, despite the above-mentioned tools and the more frequent utilization of phototherapy. The data from this study identified that the primary source of bilirubin neurotoxicity was not related to isoimmunization. Therefore, the focus on maternal–fetal isoimmunization may, in fact, be deceiving with false reassurance toward infants without isoimmunization setup. Although universal newborn metabolic testing screens for hemoglobinopathies commonly cause non-isoimmunization hyperbilirubinemia, the reports of these testing are not available at the time of newborn discharge from the hospital. Therefore, this study urges for the need to have a short reporting time for hemoglobinopathies and to have clear guidelines to immediately screen for hyperbilirubinemia in infants diagnosed with these diseases. Of note, the prevalence of bilirubin neurotoxicity in term infants has decreased over the years. It is understandable that there is a current shift in nomenclature from kernicterus to bilirubin neurotoxicity; however, the decrease in prevalence is a true finding as both terms are included in the same category in the ICD-9 and ICD-10 codes.
The interaction of race, hyperbilirubinemia and bilirubin neurotoxicity is interesting. Neonatal jaundice was less frequently diagnosed in Black newborns across the nation. There is not a clear biological explanation for this phenomenon. It could be the dark skin that makes it harder to clinically diagnose jaundice. However, a previous study has shown Black newborns to have a less frequent incidence of serum bilirubin levels >20 mg/dl when compared to Whites and more frequent bilirubin concentrations >30 mg/day.17 The increased bilirubin neurotoxicity in Black infants could be partially explained by the increased prevalence of glucose-6-phosphate dehydrogenase (G6PD) and other hemoglobinopathies in the Black population. However, bilirubin neurotoxicity due to isoimmunization is increased in Black compared to White by threefolds (adjusted odds ratio (aOR) 3.05, 95% confidence interval (CI): 2.13–4.36, p < 0.001). We are not aware of a plausible biological explanation of such a finding. Of note, the current AAP guidelines do not differentiate between races. We speculate that Black infants with borderline bilirubin concentrations might benefit from follow-up for a longer period than currently recommended.
The study demonstrated male vulnerability to neonatal hyperbilirubinemia. It is understood that non-isoimmunization hyperbilirubinemia would have a male preference as G6PD is known to be inherited via sex chromosome. However, the male vulnerability to hyperbilirubinemia in the absence of G6PD cannot be explained. Multiple studies have shown male infants to be more vulnerable to fetal distress, birth asphyxia, intraventricular hemorrhage of prematurity, and neonatal mortality.18 Apparently, neonatal jaundice is yet another condition to add to this list. It is suggested that the “Y chromosome” does no harm; however, there is an added protection related to the presence of X chromosomes.19
Exchange transfusion and phototherapy were used more frequently in preterm with neonatal hyperbilirubinemia. Despite the fact that having an isoimmunization setup is considered a risk factor for increased bilirubin concentration to a dangerously high level, bilirubin neurotoxicity occurred more frequently in infants without isoimmunization setup. Interestingly, many bilirubin neurotoxicity infants did not receive exchange transfusion. Only 69% of infants diagnosed with bilirubin neurotoxicity without isoimmunization and 31% of infants who developed bilirubin neurotoxicity with isoimmunization received exchange transfusion. There are multiple possibilities for the lack of treatment in these infants. It could be simply a reflection of the caregiver in compliance with current guidelines. However, it is more likely to be related to a lack of guidelines to follow up bilirubin after discharge from birth hospitals. Many infants may have had their bilirubin spiked after 3 days of life at the time infants were at home.20
This study has the strength of being the largest reported in the literature with a sample that exceeds 58 million infants that represents 47 states, thereby eliminating the significant variation in practice that may be observed in smaller size studies. The national administrative database depends on ICD-9 and ICD-10 coding for diagnoses and procedures; therefore, errors in coding could compromise the accuracy of the data. However, the NIS has developed its validation methods to ensure the integrity of the data.
One of the limitations of this study is the lack of detailed information on clinical presentation and other risk factors that could impact the outcome of these infants. Furthermore, the analysis of this study offers associations and trends and cannot suggest causation. In addition, this study does not provide long-term outcomes as the study is limited to the time of discharge from the hospital. The study showed that infants with bilirubin neurotoxicity did not consistently receive exchange transfusion. This was observed more frequently in infants who were discharged and readmitted from home. Although it is plausible that this observation is factual and infants who did not receive the recommended preventive therapy developed the adverse consequence, the study could not absolutely exclude the possibility of misclassification.
Conclusions
In summary, the rate of bilirubin neurotoxicity has significantly decreased in term infants since the release of AAP guidelines for the management of jaundice in infants ≥35 weeks of gestation. However, the rate of bilirubin neurotoxicity has not changed in preterm infants. Despite the less diagnosis of jaundice in Black newborns, they disproportionately are at increased risk of developing bilirubin neurotoxicity. Both exchange transfusion and bilirubin neurotoxicity were more frequently encountered in preterm infants and in infants with non-isoimmunization jaundice. The report calls for new AAP guidelines for the management of jaundice in infants <35 weeks of gestation.
References
Ullah, S., Rahman, K. & Hedayati, M. Hyperbilirubinemia in neonates: types, causes, clinical examinations, preventive measures and treatments: a narrative review article. Iran. J. Public Health 45, 558–68 (2016).
Okumura, A. et al. Kernicterus in preterm infants. Pediatrics. 123(6):e1052–8 (2009).
Van Der Geest, B. A. M. et al. Screening and treatment to reduce severe hyperbilirubinaemia in infants in primary care (STARSHIP): a factorial stepped-wedge cluster randomised controlled trial protocol. BMJ Open 9, 1–10 (2019).
Aziz, Khalid, Farine, Dan & Barrington, Keith Canadian Paediatric Society. Guidelines for detection, management and prevention of hyperbilirubinemia in term and late preterm newborn infants (35 or more weeks’ gestation) – summary. Paediatr. Child Health 12, 401–7 (2007).
Yu, T. C. et al. Prevalence and burden of illness of treated hemolytic neonatal hyperbilirubinemia in a privately insured population in the United States. BMC Pediatr. 19, 1–15 (2019).
Kalakheti, B. K., Singh, R., Bhatta, N. K., Karki, A. & Baral, N. Mothers: a prospective cohort study. Kathmandu Univ. Med. J. 7, 11–5 (2009).
Kuzniewicz, M. W. et al. Incidence, etiology, and outcomes of hazardous hyperbilirubinemia in newborns. Pediatrics 134, 504–9 (2014).
Bujandric, N. & Grujic, J. Exchange transfusion for severe neonatal hyperbilirubinemia: 17 years’ experience from Vojvodina, Serbia. Indian J. Hematol. Blood Transfus. 32, 208–14 (2016).
Subhabrata Mitra, J. R. Neonatal jaundice: aetiology, diagnosis and treatment. J. Pediatr. 90, 699–704 (2017).
Woodgate, P. & Jardine, L. A. Neonatal jaundice: phototherapy neonatal jaundice is the most common problem encountered during the first week of neonatal period. BMJ Clin. Evid. 2015, 1–21 (2015).
Jeffrey Maisels, M. et al. Pediatrics AA of AAP weeks of gestation background. Pediatrics 114, 297–316 (2004).
Goodwin, R. & Heard, D. H. B. Haemolytic disease of the newborn. J. Hyg. 54, 153–71 (1956).
Newman, ThomasB., Vittinghoff, Eric, Charles, E. & McCulloch, P. Efficacy of phototherapy for newborns with hyperbilirubinemia: a cautionary example of an instrumental variable analysis. Med. Decis. Making 32, 83–92 (2012).
Barrett, M., Coffey, R. & Levit, K. Population Denominator Data for Use with the HCUP Databases (Updated with 2016 Population Data). HCUP Methods Series Report #2017-04, 17 October 2017 (US Agency for Healthcare Research and Quality, accessed 4 January 2019); www.hcup-us.ahrq.gov/reports/methods/2017-04.pdf.9.
Agency for Healthcare Research and Quality. HCUP National Inpatient Sample (NIS). Healthcare Utilization Project (HCUP) (Agency for Healthcare Research and Quality); https://www.hcupus.ahrq.gov/nisoverview.jsp (2018).
Agency for Healthcare Research and Quality. Overview of the National (Nationwide) Inpatient Sample (NIS) (Agency for Healthcare Research and Quality, accessed 20 September 2019); https://www.hcupus.ahrq.gov/nisoverview.jsp.
Wickremasinghe, A. C., Kuzniewicz, M. W. & Newman, T. B. Black race is not protective against hazardous bilirubin levels. J. Pediatr. 162, 1068–9 (2013).
Tioseco, J. A., Aly, H., Essers, J., Patel, K. & El-Mohandes, A. A. E. Male sex and intraventricular hemorrhage. Pediatr. Crit. Care Med. 7, 40–4 (2006).
Mage, D. T. & Donner, M. Female resistance to hypoxia: Does it explain the sex difference in mortality rates? J. Women’s Health 15, 786–94 (2006).
Gilmour, S. M. et al. Prolonged neonatal jaundice: when to worry and what to do. Paediatr. Child Health 9, 700–4 (2004).
Author information
Authors and Affiliations
Contributions
I.Q. conceptualized and designed the study, conducted the analysis, drafted the initial manuscript, and reviewed and revised the manuscript. M.A.A.F. drafted the initial manuscript, and reviewed and revised the manuscript. M.E. drafted the initial manuscript, and reviewed and revised the manuscript. M.A.M. critically revised the manuscript and statistical analyses and approved the final draft of the manuscript. H.A. conceptualized and designed the study, interpreted the analysis, drafted the initial manuscript, and reviewed and revised the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Qattea, I., Farghaly, M.A.A., Elgendy, M. et al. Neonatal hyperbilirubinemia and bilirubin neurotoxicity in hospitalized neonates: analysis of the US Database. Pediatr Res 91, 1662–1668 (2022). https://doi.org/10.1038/s41390-021-01692-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41390-021-01692-3
This article is cited by
-
Obstetric and neonatal outcomes following taxane use during pregnancy: a systematic review
BMC Cancer (2024)
-
Bilirubin impairs neuritogenesis and synaptogenesis in NSPCs by downregulating NMDAR-CREB-BDNF signaling
In Vitro Cellular & Developmental Biology - Animal (2024)
-
Chemoprevention of bilirubin encephalopathy with a nanoceutical agent
Pediatric Research (2023)
-
Chorioamnionitis, Cesarean Deliveries, and Racial Disparities in the USA
Journal of Racial and Ethnic Health Disparities (2023)
-
Neonatal hyperbilirubinemia and bilirubin neurotoxicity: what can be learned from the database analysis?
Pediatric Research (2022)