Abstract
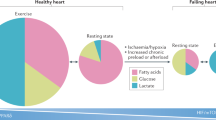
Intensive preclinical investigations have delineated a role for peroxisome proliferator-activated receptors (PPARs) in energy metabolism and inflammation. PPARs are activated by natural lipophilic ligands such as fatty acids and their derivatives. Normalization of lipid and glucose metabolism is achieved via pharmacological modulation of PPAR activity. PPARs may also alter atherosclerosis progression through direct effects on the vascular wall. PPARs regulate genes involved in the recruitment of leukocytes to endothelial cells, in vascular inflammation, in macrophage lipid homeostasis, and in thrombosis. PPARs therefore modulate metabolic and inflammatory perturbations that predispose to cardiovascular diseases and type 2 diabetes. The hypolipidemic fibrates and the antidiabetic thiazolidinediones are drugs that act via PPARα and PPARγ, respectively, and are used in clinical practice. PPARβ/δ ligands are currently in clinical evaluation. The pleiotropic actions of PPARs and the fact that chemically diverse PPAR agonists may induce distinct pharmacological responses have led to the emergence of new concepts for drug design. A more precise understanding of the molecular pathways implicated in the response to chemically distinct PPAR agonists should provide new opportunities for targeted therapeutic applications in the management of the metabolic syndrome, type 2 diabetes, and cardiovascular diseases.
Key Points
-
Peroxisome proliferator-activated receptors (PPARs) are major regulators of energy homeostasis and inflammation control
-
PPARs are modulators of cardiovascular disease risk
-
PPARs are pharmacological targets for treatment of the metabolic syndrome, diabetes, and atherosclerosis
-
Greater knowledge of the functions of PPARs in health and disease has led to new concepts of drug design
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Feige JN et al. (2006) From molecular action to physiological outputs: peroxisome proliferator-activated receptors are nuclear receptors at the crossroads of key cellular functions. Prog Lipid Res 45: 120–159
Marx N et al. (2004) Peroxisome proliferator-activated receptors and atherogenesis: regulators of gene expression in vascular cells. Circ Res 94: 1168–1178
Fu J et al. (2003) Oleylethanolamide regulates feeding and body weight through activation of the nuclear receptor PPAR-α. Nature 425: 90–93
Gervois P et al. (1999) Fibrates increase human REV-ERBα expression in liver via a novel peroxisome proliferator-activated receptor response element. Mol Endocrinol 13: 400–409
Delerive P et al. (2000) Induction of IκBα expression as a mechanism contributing to the anti-inflammatory activities of peroxisome proliferator-activated receptor-α activators. J Biol Chem 275: 36703–36707
Gervois P et al. (2004) Global suppression of IL-6-induced acute phase response gene expression after in vivo chronic treatment with the peroxisome proliferator-activated receptor α activator fenofibrate. J Biol Chem 279: 16154–16160
Gervois P et al. (2000) Regulation of lipid and lipoprotein metabolism by PPAR activators. Clin Chem Lab Med 38: 3–11
Vu-Dac N et al. (2003) Apolipoprotein A5, a crucial determinant of plasma triglyceride levels, is highly responsive to peroxisome proliferator activated receptor α. J Biol Chem 278: 17982–17985
Ross R (1999) Atherosclerosis: an inflammatory disease. N Engl J Med 340: 115–126
Marx N et al. (1999) PPARα activators inhibit cytokine-induced vascular cell adhesion molecule-1 expression in human endothelial cells. Circulation 99: 3125–3131
Shu H et al. (2000) Activation of PPARα or γ reduces secretion of matrix metalloproteinase 9 but not interleukin-8 from human monocytic THP-1 cells. Biochem Biophys Res Commun 267: 345–349
Ridker PM et al. (2000) C-reactive protein and other markers of inflammation in the prediction of cardiovascular disease in women. N Engl J Med 342: 836–843
Danesh J et al. (2005) Plasma fibrinogen level and the risk of major cardiovascular diseases and nonvascular mortality: an individual participant meta-analysis. JAMA 294: 1799–1809
Gervois P et al. (2001) Negative regulation of human fibrinogen gene expression by peroxisome proliferator-activated receptor α agonists via inhibition of CCAAT box/enhancer-binding protein β. J Biol Chem 276: 33471–33477
Kleemann R et al. (2003) Fibrates down-regulate IL-1-stimulated C-reactive protein gene expression in hepatocytes by reducing p50-NF-κB-C/EBP-β complex formation. Blood 101: 545–551
Wu KK et al. (2005) Increased hypercholesterolemia and atherosclerosis in mice lacking both ApoE and leptin receptor. Atherosclerosis 181: 251–259
Tordjman K et al. (2001) PPARα deficiency reduces insulin resistance and atherosclerosis in apoE-null mice. J Clin Invest 107: 1025–1034
Staels B and Fruchart JC (2005) Therapeutic roles of peroxisome proliferator-activated receptor agonists. Diabetes 54: 2460–2470
Frick MH et al. (1987) Helsinki Heart Study: primary prevention trial with gemfibrozil in middle-aged men with dyslipidemia. Safety of treatment, changes in risk factors, and incidence of coronary heart disease. N Engl J Med 317: 1237–1245
Elkeles RS et al. (1998) Cardiovascular outcomes in type 2 diabetes. A double-blind placebo-controlled study of bezafibrate: the St. Mary's, Ealing, Northwick Park Diabetes Cardiovascular Disease Prevention (SENDCAP) Study. Diabetes Care 21: 641–648
FIELD-Investigators (2005) Effects of long-term fenofibrate therapy on cardiovascular events in 9795 people with type 2 diabetes mellitus (the FIELD study): randomised controlled trial. Lancet 366: 1849–1861
ACCORD Trial Homepage [http://www.accordtrial.org/public/index.cfm] (accessed 8 September 2006)
He W et al. (2003) Adipose-specific peroxisome proliferator-activated receptor γ knockout mice causes insulin resistance in fat and liver but not in muscle. Proc Natl Acad Sci USA 100: 15712–15717
Barak Y et al. (1999) PPAR γ is required for placenta, cardiac, and adipose tissue development. Mol Cell 4: 585–595
Kubota N et al. (1999) PPAR γ mediates high fat diet-induced adipocyte hypertrophy and insulin resistance. Mol Cell 4: 597–609
Rosen ED et al. (1999) PPAR γ is required for the differentiation of adipose tissue in vitro and in vivo . Mol Cell 4: 611–617
Savage DB et al. (2003) Human metabolic syndrome resulting from dominant-negative mutations in the nuclear receptor peroxisome proliferator-activated receptor-γ. Diabetes 52: 910–917
Chao L et al. (2000) Adipose tissue is required for the antidiabetic, but not for the hypolipidemic, effect of thiazolidinediones. J Clin Invest 106: 1221–1228
Walczak R and Tontonoz P (2002) PPARadigms and PPARadoxes: expanding roles for PPARγ in the control of lipid metabolism. J Lipid Res 43: 177–186
De Vos P et al. (1996) Thiazolidinediones repress ob gene expression in rodents via activation of peroxisome proliferator-activated receptor γ. J Clin Invest 98: 1004–1009
Iwaki M et al. (2003) Induction of adiponectin, a fat-derived antidiabetic and antiatherogenic factor, by nuclear receptors. Diabetes 52: 1655–1663
Nawrocki AR et al. (2006) Mice lacking adiponectin show decreased hepatic insulin sensitivity and reduced responsiveness to peroxisome proliferator-activated receptor γ agonists. J Biol Chem 281: 2654–2660
Weisberg SP et al. (2003) Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest 112: 1796–1808
Xu H et al. (2003) Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J Clin Invest 112: 1821–1830
Pasceri V et al. (2000) Modulation of vascular inflammation in vitro and in vivo by peroxisome proliferator-activated receptor-γ activators. Circulation 101: 235–238
Li AC and Palinski W (2006) Peroxisome proliferator-activated receptors: how their effects on macrophages can lead to the development of a new drug therapy against atherosclerosis. Annu Rev Pharmacol Toxicol 46: 1–39
Pascual G et al. (2005) A SUMOylation-dependent pathway mediates transrepression of inflammatory response genes by PPAR-γ. Nature 437: 759–763
Chen Z et al. (2001) Troglitazone inhibits atherosclerosis in apolipoprotein E-knockout mice:pleiotropic effects on CD36 expression and HDL. Arterioscler Thromb Vasc Biol 21: 372–377
Li AC et al. (2000) Peroxisome proliferator-activated receptor γ ligands inhibit development of atherosclerosis in LDL receptor-deficient mice. J Clin Invest 106: 523–531
Chawla A et al. (2001) A PPAR γ-LXR-ABCA1 pathway in macrophages is involved in cholesterol efflux and atherogenesis. Mol Cell 7: 161–171
Xiang AH et al. (2006) Effect of pioglitazone on pancreatic-β cell function and diabetes risk in Hispanic women with prior gestational diabetes. Diabetes 55: 517–522
Mayerson AB et al. (2002) The effects of rosiglitazone on insulin sensitivity, lipolysis, and hepatic and skeletal muscle triglyceride content in patients with type 2 diabetes. Diabetes 51: 797–802
Bajaj M et al. (2003) Pioglitazone reduces hepatic fat content and augments splanchnic glucose uptake in patients with type 2 diabetes. Diabetes 52: 1364–1370
Turner RC et al. (1998) Risk factors for coronary artery disease in non-insulin dependent diabetes mellitus: United Kingdom Prospective Diabetes Study (UKPDS: 23). BMJ 316: 823–828
Malmberg K et al. (2000) Impact of diabetes on long-term prognosis in patients with unstable angina and non-Q-wave myocardial infarction: results of the OASIS (Organization to Assess Strategies for Ischemic Syndromes) Registry. Circulation 102: 1014–1019
Sunayama S et al. (1999) Effects of troglitazone on atherogenic lipoprotein phenotype in coronary patients with insulin resistance. Atherosclerosis 146: 187–193
Goldberg RB et al. (2005) A comparison of lipid and glycemic effects of pioglitazone and rosiglitazone in patients with type 2 diabetes and dyslipidemia. Diabetes Care 28: 1547–1554
Langenfeld MR et al. (2005) Pioglitazone decreases carotid intima-media thickness independently of glycemic control in patients with type 2 diabetes mellitus: results from a controlled randomized study. Circulation 111: 2525–2531
Sidhu JS et al. (2004) Effect of rosiglitazone on common carotid intima-media thickness progression in coronary artery disease patients without diabetes mellitus. Arterioscler Thromb Vasc Biol 24: 930–934
Choi D et al. (2004) Preventative effects of rosiglitazone on restenosis after coronary stent implantation in patients with type 2 diabetes. Diabetes Care 27: 2654–2660
Marx N et al. (2005) Pioglitazone reduces neointima volume after coronary stent implantation: a randomized, placebo-controlled, double-blind trial in nondiabetic patients. Circulation 112: 2792–2798
Meisner F et al. (2006) Effect of rosiglitazone treatment on plaque inflammation and collagen content in nondiabetic patients. Data from a randomized placebo-controlled trial. Arterioscler Thromb Vasc Biol 26: 845–850
Dormandy JA et al. (2005) Secondary prevention of macrovascular events in patients with type 2 diabetes in the PROactive Study (PROspective pioglitAzone Clinical Trial In macroVascular Events): a randomised controlled trial. Lancet 366: 1279–1289
PROactive (online 9 February 2006) The official PROactive results [http://www.proactive-results.com/html/results.htm] (accessed 9 September 2006)
Home PD et al. (2005) Rosiglitazone Evaluated for Cardiac Outcomes and Regulation of Glycaemia in Diabetes (RECORD): study design and protocol. Diabetologia 48: 1726–1735
Xu HE et al. (1999) Molecular recognition of fatty acids by peroxisome proliferator-activated receptors. Mol Cell 3: 397–403
Bastie C et al. (1999) Expression of peroxisome proliferator-activated receptor PPARδ promotes induction of PPARγ and adipocyte differentiation in 3T3C2 fibroblasts. J Biol Chem 274: 21920–21925
Barak Y et al. (2002) Effects of peroxisome proliferator-activated receptor δ on placentation, adiposity, and colorectal cancer. Proc Natl Acad Sci USA 99: 303–308
Tanaka T et al. (2003) Activation of peroxisome proliferator-activated receptor δ induces fatty acids oxidation in skeletal muscle and attenuates metabolic syndrome. Proc Natl Acad Sci USA 100: 15924–15929
Wang YX et al. (2003) Peroxisome-proliferator-activated receptor δ activates fat metabolism to prevent obesity. Cell 113: 159–170
Lee CH et al. (2003) Transcriptional repression of atherogenic inflammation: modulation by PPARδ. Science 302: 453–457
Van der Veen JN et al. (2005) Reduced cholesterol absorption upon PPARδ activation coincides with decreased intestinal expression of NPC1L1. J Lipid Res 46: 526–534
Leibowitz MD et al. (2000) Activation of PPARδ alters lipid metabolism in db/db mice. FEBS Lett 473: 333–336
Akiyama TE et al. (2004) Peroxisome proliferator-activated receptor β/δ regulates very low density lipoprotein production and catabolism in mice on a Western diet. J Biol Chem 279: 20874–20881
Dressel U et al. (2003) The peroxisome proliferator-activated receptor β/δ agonist, GW501516, regulates the expression of genes involved in lipid catabolism and energy uncoupling in skeletal muscle cells. Mol Endocrinol 17: 2477–2493
Rival Y et al. (2002) PPARα and PPARδ activators inhibit cytokine-induced nuclear translocation of NF-κB and expression of VCAM-1 in EAhy926 endothelial cells. Eur J Pharmacol 435: 143–151
Li AC et al. (2004) Differential inhibition of macrophage foam-cell formation and atherosclerosis in mice by PPARα, β/δ, and γ. J Clin Invest 114: 1538–1540
Graham TL et al. (2005) The PPARδ agonist GW0742X reduces atherosclerosis in LDLR(−/−) mice. Atherosclerosis 181: 29–37
Camp HS et al. (2000) Differential activation of peroxisome proliferator-activated receptor-γ by troglitazone and rosiglitazone. Diabetes 49: 539–547
Derosa G et al. (2004) Metabolic effects of pioglitazone and rosiglitazone in patients with diabetes and metabolic syndrome treated with glimepiride: a twelve-month, multicenter, double-blind, randomized, controlled, parallel-group trial. Clin Ther 26: 744–754
Mukherjee R et al. (2000) A selective peroxisome proliferator-activated receptor-γ (PPARγ) modulator blocks adipocyte differentiation but stimulates glucose uptake in 3T3-L1 adipocytes. Mol Endocrinol 14: 1425–1433
Berger JP et al. (2003) Distinct properties and advantages of a novel peroxisome proliferator-activated protein-γ selective modulator. Mol Endocrinol 17: 662–676
Schiffrin EL et al. (2005) Peroxisome proliferator-activated receptors and cardiovascular remodeling. Am J Physiol Heart Circ Physiol 288: 1037–1043
Finck BN et al. (2002) The cardiac phenotype induced by PPARα overexpression mimics that caused by diabetes mellitus. J Clin Invest 109: 121–130
Chakrabarti R et al. (2003) Ragaglitazar: a novel PPAR α PPAR γ agonist with potent lipid-lowering and insulin-sensitizing efficacy in animal models. Br J Pharmacol 140: 527–537
Hegarty BD et al. (2004) Peroxisome proliferator-activated receptor (PPAR) activation induces tissue-specific effects on fatty acid uptake and metabolism in vivo—a study using the novel PPARα/γ agonist tesaglitazar. Endocrinology 145: 3158–3164
Skrumsager BK et al. (2003) Ragaglitazar: the pharmacokinetics, pharmacodynamics, and tolerability of a novel dual PPAR α and γ agonist in healthy subjects and patients with type 2 diabetes. J Clin Pharmacol 43: 1244–1256
Saad MF et al. (2004) Ragaglitazar improves glycemic control and lipid profile in type 2 diabetic subjects: a 12-week, double-blind, placebo-controlled dose-ranging study with an open pioglitazone arm. Diabetes Care 27: 1324–1329
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Gervois, P., Fruchart, JC. & Staels, B. Drug Insight: mechanisms of action and therapeutic applications for agonists of peroxisome proliferator-activated receptors. Nat Rev Endocrinol 3, 145–156 (2007). https://doi.org/10.1038/ncpendmet0397
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/ncpendmet0397
This article is cited by
-
Design & synthesis of hybrid pharmacophore of β-lactam, 1,8-naphthyridine, and secondary amines; Discover their in vitro antimicrobial, anticancer properties & DFT and ADMET prediction studies
Medicinal Chemistry Research (2023)
-
Structural Basis for PPARα Activation by 1H-pyrazolo-[3,4-b]pyridine Derivatives
Scientific Reports (2020)
-
Thiazolidinediones: the Forgotten Diabetes Medications
Current Diabetes Reports (2019)
-
VCE-003.2, a novel cannabigerol derivative, enhances neuronal progenitor cell survival and alleviates symptomatology in murine models of Huntington’s disease
Scientific Reports (2016)
-
Selective targeting of PPARγ by the natural product chelerythrine with a unique binding mode and improved antidiabetic potency
Scientific Reports (2015)