Abstract
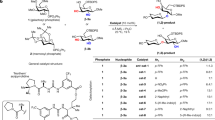
3-Deoxysugars are important constituents of complex carbohydrates. For example, 2-keto-3-deoxy-D-manno-octulosonic acid (KDO) is an essential component of lipopolysaccharides in Gram-negative bacteria, 2-keto-3-deoxy-D-glycero-D-galacto-nonulosonic acid (KDN) is widely found in carbohydrates of the bacterial cell wall and in lower vertebrates, and sialic acid is a common cap of mammalian glycoproteins. Although ready access to such sugars would benefit the creation of vaccine candidates, antibiotics and small-molecule drugs, their chemical synthesis is difficult. Here we present a simple chemoenzymatic method for preparing differentially protected 3-deoxysugar derivatives from readily available starting materials. It exploits the promiscuous aldolase activity of the enzyme macrophomate synthase (MPS) to add pyruvate enolate diastereoselectively to a wide range of structurally complex aldehydes. A short synthesis of KDN illustrates the utility of this approach. Enzyme promiscuity, which putatively fosters large functional leaps in natural evolution, has great promise as a source of synthetically useful catalytic transformations.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Serafimov, J. M., Gillingham, D., Kuster, S. & Hilvert, D. The putative Diels-Alderase macrophomate synthase is an efficient aldolase. J. Am. Chem. Soc. 130, 7798–7799 (2008).
Li, L. S. & Wu, Y. L. Recent progress in syntheses of higher 3-deoxy-2-ulosonic acids and their derivatives. Curr. Org. Chem. 7, 447–475 (2003).
Cornforth, J. W., Firth, M. E. & Gottschalk, A. The synthesis of N-acetylneuraminic acid. Biochem. J. 68, 57–61 (1958).
Shirai, R. & Ogura, H. Improved syntheses of two 3-deoxyald-2-ulosonic acids (KDN, KDO) by condensation of oxalacetic acid with aldoses followed by Ni2+ catalyzed decarboxylation. Tetrahedron Lett. 30, 2263–2264 (1989).
Dean, S. M., Greenberg, W. A. & Wong, C. H. Recent advances in aldolase-catalyzed asymmetric synthesis. Adv. Synth. Catal. 349, 1308–1320 (2007).
Sugai, T., Shen, G. J., Ichikawa, Y. & Wong, C. H. Synthesis of 3-deoxy-D-manno-2-octulosonic acid (KDO) and its analogs based on KDO aldolase-catalyzed reactions. J. Am. Chem. Soc. 115, 413–421 (1993).
Williams, G. J., Woodhall, T., Farnsworth, L. M., Nelson, A. & Berry, A. Creation of a pair of stereochemically complementary biocatalysts. J. Am. Chem. Soc. 128, 16238–16247 (2006).
Fong, S., Machajewski, T. D., Mak, C. C. & Wong, C. H. Directed evolution of D-2-keto-3-deoxy-6-phosphogluconate aldolase to new variants for the efficient synthesis of D- and l-sugars. Chem. Biol. 7, 873–883 (2000).
Masamune, S., Choy, W., Petersen, J. S. and Sita, L. R. Double asymmetric synthesis and a new strategy for stereochemical control in organic synthesis. Angew. Chem. Int. Ed. 24, 1–76 (1985).
Weitz, D. J. & Bednarski, M. D. Synthesis of acyclic sugar aldehydes by ozonolysis of oximes. J. Org. Chem. 54, 4957–4959 (1989).
Inoue, S. & Kitajima, K. KDN (deaminated neuraminic acid): Dreamful past and exciting future of the newest member of the sialic acid family. Glycoconjugate J. 23, 277–290 (2006).
Ose, T. et al. Insight into a natural Diels-Alder reaction from the structure of macrophomate synthase. Nature 422, 185–189 (2003).
Denmark, S. E. & Henke, B. R. Investigations on transition-state geometry in the aldol condensation. J. Am. Chem. Soc. 113, 2177–2194 (1991).
Zimmerman, H. E. & Traxler, M. D. The stereochemistry of the Ivanov and Reformatsky reactions. I. J. Am. Chem. Soc. 79, 1920–1923 (1957).
Jäckel, C., Kast, P. & Hilvert, D. Protein design by directed evolution. Ann. Rev. Biophys. 37, 153–173 (2008).
Mugford, P. F., Wagner, U. G., Jiang, Y., Faber, K. & Kazlauskas, R. J. Enantiocomplementary enzymes: Classification, molecular basis for their enantiopreference, and prospects for mirror-image biotransformations. Angew. Chem. Int. Ed. 47, 8782–8793 (2008).
Acknowledgements
This work was supported by the ETH Zürich, a Marie Curie International Incoming Fellowship within the 7th European Community Framework Programme to D.G.G. (IIF-AEOM) and a PhD fellowship from the Studienstiftung des Deutschen Volkes to P.S.
Author information
Authors and Affiliations
Contributions
D.G.G., P.S., P.H.S. and D.H. designed research. D.G.G, P.S. and A.A. performed the experiments. D.G.G., P.S., P.H.S and D.H. analysed data. D.G.G., P.S., P.H.S. and D.H. wrote the paper.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary information
Supplementary information (PDF 3202 kb)
Rights and permissions
About this article
Cite this article
Gillingham, D., Stallforth, P., Adibekian, A. et al. Chemoenzymatic synthesis of differentially protected 3-deoxysugars. Nature Chem 2, 102–105 (2010). https://doi.org/10.1038/nchem.504
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nchem.504
This article is cited by
-
A novel enzyme-catalyzed synthesis of N-substituted pyrrole derivatives
Molecular Diversity (2013)
-
A Novel Enzymatic Synthesis of Quinoline Derivatives
Catalysis Letters (2012)
-
Sweet flexibility
Nature Chemistry (2010)