Abstract
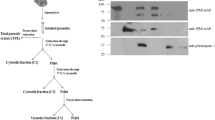
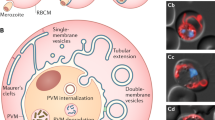
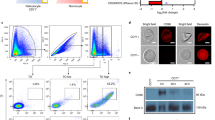
The parasite Plasmodium berghei imports the enzyme δ-aminolevulinate dehydratase (ALAD), and perhaps the subsequent enzymes of the pathway from the host red blood cell to sustain heme synthesis. Here we have studied the mechanism of this import. A 65-kDa protein on the P. berghei membrane specifically bound to mouse red blood cell ALAD, and a 93-amino-acid fragment (ALAD-ΔNC) of the host erythrocyte ALAD was able to compete with the full-length enzyme for binding to the P. berghei membrane. ALAD-ΔNC was taken up by the infected red blood cell when added to a culture of P. falciparum and this led to a substantial decrease in ALAD protein and enzyme activity and, subsequently, heme synthesis in the parasite, resulting in its death.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Surolia, N. & Padmanaban, G. De novo biosynthesis of heme offers a new chemotherapeutic target in the human malarial parasite. Biochem. Biophys. Res. Comm. 187, 744–750 (1992).
Slater, A.F. Malarial pigment. Experimental Parasitol. 74, 362–365 (1992).
Sullivan, D.J., Gluzman, I.Y. & Goldberg, D.E. Plasmodium hemozoin formation mediated by histidine-rich proteins. Science 271, 219–222 (1996).
Surolia, N. & Padmanaban, G. Chloroquine inhibits heme dependent protein synthesis in Plasmodium falciparum. Proc. Natl. Acad. Sci. USA 88, 4788–4790 (1991).
Bonday, Z.Q., Taketani, S., Gupta, P.D. & Padmanaban, G. Heme biosynthesis by the malarial parasite: Import of δ-aminolevulinate dehydrase from the host red cell. J. Biol. Chem. 272, 21839–21846 (1997).
Wilson, C.M., Smith, A.B. & Baylon, R.V. Characterization of the δ-aminolevulinate synthase gene homologue in P. falciparum. Mol. Biochem. Parasitol. 79, 135–140 (1996).
Reece, R.J., Rickles, R.J. & Ptashne, M. Overproduction and single step purification of GAL4 fusion proteins from Escherichia coli. Gene 15, 105–107 (1993).
Lingelbach, K. & Joiner, K.A. The parasitophorous vacuole membrane surrounding Plasmodium and Toxoplasma: an usual compartment in infected cells. J. Cell. Sci. 111, 1467–1475 (1998).
Pouvelle, B., Gormley, J.A. & Taraschi, T.F. Characterization of trafficking pathways and membrane genesis in malaria infected erythrocytes. Mol. Biochem. Parasitol. 66, 83–96 (1994).
Haldar, K. Ducts, Channels and transporters in Plasmodium-infected erythrocytes. Parasitol. Today 10, 393–395 (1994).
Hibbs, A.R., Stenzel, D.J. & Saul, A. Macromolecular transport in malaria - does the duct exist? Eur. J. Cell Biol. 72, 182–188 (1997).
Hibbs, A.R. & Saul, A.J. Plasmodium falciparum highly mobile small vesicles in the malaria infected red blood cell cytoplasm. Exp. Parasitol. 79, 260–269 (1994).
Trager, W. Parasitophorous duct? Still more questions than answers. Parasitol. Today 11, 69 (1995).
Jensen, J.B. & Trager, W. Plasmodium falciparum in culture: Use of outdated erythrocytes and description of the candle-jar method. J. Parasitol. 63, 883–886 (1977).
Sokhanekova, T.L., Sergacheva, I. & Soprunov, F.F. Sensitivity of the erythrocytes of mice infected with Plasmodium berghei to saponin and a hypotonic solution. Med. Parasitol (Mosc) 1, 46–49 (1984).
Anderson, P.M. & Desnick, R.J. Purification and properties of δ-aminolevulinate dehydrase from human erythrocytes. J. Biol. Chem. 254, 6924–6930 (1979).
Schmitt, J., Hess, H. & Stunnenberg. H.G. Affinity purification of histidine-tagged proteins. Mol. Biol. Rep. 18, 223–226 (1993).
Bonniec, S. Le. et al. Plasmepsin II, an acidic hemoglobinase from the Plasmodium falciparum food vacuole, is active at neutral pH on the host erythrocyte membrane skeleton. J. Biol. Chem. 274, 14218–14223 (1999).
Goldberg, D.E., Slater, A.F.G., Cerami, A. & Henderson, B. Hemoglobin degradation in the malaria parasite Plasmodium falciparum: An ordered process in a unique organelle. Proc. Natl. Acad. Sci. USA 87, 2931–2935 (1990).
Mauzerall, D. and Granick, S. The occurrence and determination of δ-aminolevulinic acid and porphobilinogen in urine. J. Biol. Chem. 219, 435–446(1956).
Santiyanont, R. Parasite Identification Counting and Staining. Application of Genetic Engineering to Research on Tropical Disease Pathogens, With Special Reference to Plasmodia—A Laboratory Manual of Selected Techniques (eds. Panyim, S., Wilairat, P. and Yuthavong, Y.) 413 (Mahidol University, Bangkok, Thailand, 1985).
Acknowledgements
This work was supported by grants from the Department of Biotechnology and the Council of Scientific & Industrial Research (New Delhi, India).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bonday, Z., Dhanasekaran, S., Rangarajan, P. et al. Import of host δ-aminolevulinate dehydratase into the malarial parasite: Identification of a new drug target. Nat Med 6, 898–903 (2000). https://doi.org/10.1038/78659
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/78659
This article is cited by
-
Malaria parasite heme biosynthesis promotes and griseofulvin protects against cerebral malaria in mice
Nature Communications (2022)
-
Malaria parasites both repress host CXCL10 and use it as a cue for growth acceleration
Nature Communications (2021)
-
Disruption of Plasmodium falciparum histidine-rich protein 2 may affect haem metabolism in the blood stage
Parasites & Vectors (2020)
-
Griseofulvin impairs intraerythrocytic growth of Plasmodium falciparum through ferrochelatase inhibition but lacks activity in an experimental human infection study
Scientific Reports (2017)
-
The influence of host genetics on erythrocytes and malaria infection: is there therapeutic potential?
Malaria Journal (2015)