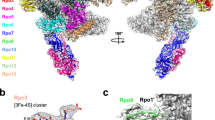
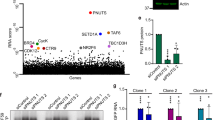
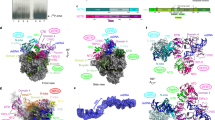
Abstract
ACTIVATOR proteins that control transcription initiation by RNA polymerase II1,2 usually have two domains: one binds to DNA, and the other activates transcription3,4. A particularly potent acidic5,6 activation domain at the C terminus of the herpes simplex virus protein VP167–9 binds directly and selectively to the human and yeast TAT A box-binding factor TFIID10. We have now investigated the biological significance of this in vitro interaction by using mutant forms of VP1611. For changes at the critical phenyl-alanine residue at position 442 of VP16 there was a good correla-tion between transactivation activity in vivo and the binding of VP16 to TFIID in vitro. In contrast, mutants with reduced negative charge were more defective for binding than for activation.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Mitchell, P. & Tjian, R. Science 245, 371–378 (1989).
Johnson, P. F. & McKnight, S. L. A. Rev. Biochem. 58, 799–839 (1989).
Brent, R. & Ptashne, M. Cell 43, 729–736 (1985).
Ptashne, M. Nature 335, 683–689 (1988).
Hope, I. A. & Struhl, K. Cell 46, 885–894 (1986).
Ma, J. & Ptashne, M. Cell 48, 847–853 (1987).
Triezenberg, S. J., Kingsbury, R. C. & McKnight, S. L. Genes Dev. 2, 718–729 (1988).
Sadowski, I., Ma, J., Triezenberg, S. & Ptashne, M. Nature 335, 563–564 (1988).
Cousens, D. J., Greaves, R., Goding, C. R. & O'Hare, P. EMBO J. 8, 2337–2342 (1989).
Stringer, K. F., Ingles, C. J. & Greenblatt, J. Nature 345, 783–786 (1990).
Cress, W. D. & Triezenberg, S. J. Science 251, 87–90 (1991).
Berger, S. L., Cress, W. D., Cress, A., Triezenberg, S. J. & Guarante, L. Cell 61, 1199–1208 (1990).
Carey, M., Lin, Y.-S., Green, M. R. & Ptashne, M. Nature 345, 361–364 (1990).
Lin, Y.-S., & Green, M. R. Cell 64, 971–981 (1991).
Morrissey, J. H. Analyt. Biochem. 117, 307–310 (1981).
Studier, F. W. & Moffat, B. A. J. molec. Biol. 189, 113–130 (1986).
Rosenberg, A. H. et al. Gene 56, 125–135 (1987).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Ingles, C., Shales, M., Cress, W. et al. Reduced binding of TFIID to transcriptionally compromised mutants of VP16. Nature 351, 588–590 (1991). https://doi.org/10.1038/351588a0
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/351588a0
This article is cited by
-
Three newly identified Immediate Early Genes of Bovine herpesvirus 1 lack the characteristic Octamer binding motif- 1
Scientific Reports (2018)
-
Plant homeodomain-leucine zipper I transcription factors exhibit different functional AHA motifs that selectively interact with TBP or/and TFIIB
Plant Cell Reports (2014)
-
Characterization of a molecular switch system that regulates gene expression in mammalian cells through a small molecule
BMC Biotechnology (2010)
-
Noninvasive Imaging of Therapeutic Gene Expression Using a Bidirectional Transcriptional Amplification Strategy
Molecular Therapy (2008)
-
HCV NS5A interacts with p53 and inhibits p53-mediated apoptosis
Oncogene (2002)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.