Abstract
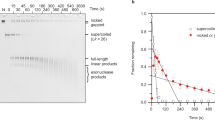
DNA in the cell is intertwined at several levels: one polynucleotide strand wraps helically around its complement and the double helix is in turn coiled in space. The higher-order intertwining most often takes the form of supercoiling of the helix axis1, but can also be observed as the wrapping of one DNA duplex around another, as in catenation2,3. We have investigated the relationship between intertwining at these three levels, the double helix, supercoiling, and catenation, using an approach that relies on comparative measurements of DNA linking numbers by gel electrophoresis. The method determines both the handedness of DNA catenanes and the change in helical repeat that accompanies catenation-induced supercoiling. For multiply-linked catenated rings of 3.5 kilobase pairs (kb), we conclude that the double helix unwinds by two-thirds of a turn for every right-handed supercoil involved in linking the two circles. Altering the geometry of the catenanes by linking rings of dissimilar size changes the effect of catenation on helical and superhelical parameters. Our experiments used intact DNA rings, but we note that linear DNA molecules, by virtue of their subdivision into closed loops or domains in vivo, can intertwine in the same ways4.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Wang, J. C. A. Rev. Biochem. 54, 665–697 (1985).
Sundin, O. & Varshavsky, A. Cell 21, 103–114 (1980); 25, 659–669 (1981).
Wasserman, S. A. & Cozzarelli, N. R. Science 232, 951–960 (1986).
Worcel, A. & Burgi, E. J. molec. Biol. 71, 127–147 (1972).
Vinograd, J., Lebowitz, J. & Watson, R. J. molec. Biol. 33, 173–192 (1968).
White, J. H., Cozzarelli, N. R. & Bauer, W. R. Science 241, 323–327 (1988).
Germond, J. E., Hirt, B., Oudet, P., Gross-Bellark, M. & Chambon, P. Proc. natn. Acad. Sci. U.S.A. 72, 1843–1847 (1975).
Nash, H. A. A. Rev. Genet. 15, 143–167 (1981).
Spengler, S. J., Stasiak, A. & Cozzarelli, N. R. Cell 42, 325–334 (1985).
Krasnow, M. A. & Cozzarelli, N. R. Cell 32, 1313–1324 (1983).
Horowitz, D. S. & Wang, J. C. J. molec. Biol. 173, 75–91 (1984).
Shore, D. & Baldwin, R. L. J. molec. Biol. 170, 983–1007 (1983).
Levitt, M. Proc. natn. Acad. Sci. U.S.A. 75, 640–644 (1978).
Klug, A. & Lutter, L. C. Nucleic Acids Res. 9, 4266–4283 (1981).
Bliska, J. B. & Cozzarelli, N. R. J. molec. Biol. 194, 205–218 (1987).
Craigie, R. & Mizuuchi, K. Cell 45, 793–800 (1986).
Kreuzer, K. N. & Jongeneel, C. V. Meth. Enzy. 100, 144–160 (1983).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Wasserman, S., White, J. & Cozzarelli, N. The helical repeat of double-stranded DNA varies as a function of catenation and supercoiling. Nature 334, 448–450 (1988). https://doi.org/10.1038/334448a0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/334448a0
This article is cited by
-
Stereoselectivity of DNA catenane fusion by resolvase
Nature (1994)
-
DNA helical repeats
Nature (1989)
-
Transactivation of the Xenopus rRNA gene promoter by its enhancer
Nature (1989)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.