Abstract
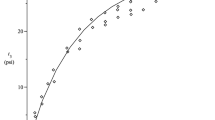
IT is well known that collagens from various sources have characteristic shrinkage temperatures (T s), at which samples will contract abruptly in water or in 0.9 per cent saline solution. The T s values of fish-skin collagens range from about 35° C. to 57° C., according to the mean temperature of the environment1, while mammalian collagen has a T s value of about 62° C., apparently irrespective of source. By an examination of the complete amino-acid analyses, these shrinkage temperatures have been correlated with the hydroxyproline content of the collagen; the lower the amount of hydroxyproline the lower the value of T s (ref. 1). Hydroxyproline is considered to consolidate the collagen structure by forming hydrogen bonds between molecular chains. The data of ref. 1 have been plotted by us in Fig. 1 using the mean values of the shrinkage temperature quoted. These data are represented by circles and include the additional values, hydroxyproline = 12.0 per cent and 13.0 per cent for cattle hide and rat tail tendon respectively. The continuous straight line in Fig. 1 has been fitted by the method of least squares to these data on the assumption that the relation between hydroxyproline and T s is linear.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Takahashi, T., quoted in “The Chemistry and Reactivity of Collagen”, by Gustavson, K. H., 224 (Academic Press, Inc., New York, 1956).
Hall, D. A., and Reed, R., Nature, 180, 243 (1957).
Gustavson, K. H., Nature, 175, 70 (1955).
Bowes, J. H., Elliott, R. G., and Moss, J. A., in “Nature and Structure of Collagen”, edit. by Randall, 199 (Butterworths, London, 1953).
Weir, C. E., J. Amer. Leather Chem. Assoc., 44, 108 (1949).
Rigby, B. J., Hirai, N., Spikes, J. D., and Eyring, H., J. Gen. Physiol., 43, 265 (1959).
Briscoe, A. M., and Loring, W. E., 136th Meeting Amer. Chem. Soc., Sept. 13–18, 1959.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
RIGBY, B., SPIKES, J. Hydroxyproline and the Shrinkage Temperature of Collagen. Nature 187, 150–151 (1960). https://doi.org/10.1038/187150a0
Issue Date:
DOI: https://doi.org/10.1038/187150a0
This article is cited by
-
Near-infrared laser thermal conjunctivoplasty
Scientific Reports (2018)
-
Zur �tiologie der Kontraktur beim Morbus Dupuytren
Virchows Archiv A Pathological Anatomy and Histology (1976)
-
Isotonic and Isometric Thermal Contraction of Human Dermis. I. Technic and Controlled Study*†
Journal of Investigative Dermatology (1964)
-
Hydroxyproline and the Shrinkage Temperature of Collagen
Nature (1962)
-
Rat Tail Tendon Denaturation and Rheumatic Diseases
Nature (1962)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.