Abstract
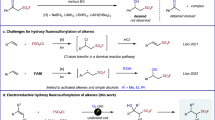
IN contrast to the observations of Human and Mills1 on the use of pyridine in the interaction of thionyl chloride and the carboxylic group, we found that instead of one molecular proportion of tertiary base in the Darzens' procedure2 for hydroxy compounds, much less than 0·1 mol. of base or its hydrochloride was sufficient. Thionyl chloride (1·0 mol.) was added to a cooled mixture of the hydroxy compound (iso-amyl, n-butyl, Î-phenylethyl alcohols, or ethyl lactate) (1·0 mol.), and a few drops of base (pyridine, quinoline, or dimethylaniline) or a small amount of the corresponding hydrochloride. An excellent yield of the chloride (RC1) was distilled directly from the reaction mixture, after it had been heated for four hours at 65–80° (depending on the base). Yields approaching 95 per cent were obtained.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Human and Mills, Nature, 158, 877 (1946).
Darzens, C.R. Acad. Sci., Paris, 152, 1314, 1601 (1911).
Gerrard, J. Chem. Soc., 688 (1936); 99 (1939); 218 (1940); 85 (1944).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
GERRARD, W., FRENCH, K. Influence of Tertiary Bases on the Interaction of Thionyl Chloride and Hydroxy Compounds. Nature 159, 263–264 (1947). https://doi.org/10.1038/159263b0
Issue Date:
DOI: https://doi.org/10.1038/159263b0
This article is cited by
-
Interaction of Thionyl Chloride and Hydroxy Compounds
Nature (1947)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.