Abstract
Multicentric osteolysis, nodulosis and arthropathy (MONA) spectrum disorder is a rare inherited progressive skeletal disorder caused by mutations in the matrix metalloproteinase 2 (MMP2) gene. Treatment options are limited. Herein we present successful bisphosphonate therapy in three affected patients. Patients were treated with bisphosphonates (either pamidronate or zoledronate) for different time periods. The following outcome variables were assessed: skeletal pain, range of motion, bone densitometry, internal medical problems as well as neurocognitive function. Skeletal pain was dramatically reduced in all patients soon after initiation of therapy and bone mineral density increased. Range of motion did not significantly improve. One patient is still able to walk with aids at the age of 14 years. Neurocognitive development was normal in all patients. Bisphosphonate therapy was effective especially in controlling skeletal pain in MONA spectrum disorder. Early initiation of treatment seems to be particularly important in order to achieve the best possible outcome.
Similar content being viewed by others
Introduction
Multicentric osteolysis, nodulosis and arthropathy (MONA, MIM #259600) spectrum disorder syndrome is a rare genetic chronic skeletal disorder characterized by multiple peripheral osteolysis, wide metacarpals, osteoporosis, progressive joint contractures, short stature, subcutaneous nodules as well as a coarse face, skin lesions/hirsutism and ocular and cardiac manifestations. Intelligence is normal in the affected patients1,2,3,4,5,6,7,8,9,10,11. The diagnosis is based on the typical clinical features together with normal laboratory findings, which allow this disease to be distinguished from other syndromes, including fibrotic, rheumatic and lysosomal diseases. MONA spectrum disorder is caused by mutations in the MMP2 gene (16q12.2) as reported by Martignetti et al. in ref. 7. MONA spectrum disorder is considered to be a continuous clinical spectrum involving also Torg, nodulosis, arthropathy and osteolysis (NAO) and Winchester syndrome
The pathogenesis of the disorder remains unclear12. To date 43 cases from 26 families with 20 different mutations in the MMP2 gene have been reported13. The enzyme MMP2 belongs to a family of zinc-dependent matrix-degrading enzymes involved in the regulation of angiogenesis14,15, normal collagen turnover16 and tumor cell invasiveness17. MMP2 is a major degrader of gelatin and type IV collagen, the major structural component of the basement membrane, as well as elastin, laminin, fibronectin, aggrecan and fibrillin18. The actual MMP2 cytogenetic band is 16q12.2.
So far therapy of patients with MONA spectrum disorder has been mainly limited to symptomatic analgesic therapy as well as physical and occupational therapy12. Phadke et al.19 were the first to report beneficial effects of bisphosphonate therapy in MONA spectrum disorder.
In this study we retrospectively analyze the clinical course of three siblings with MONA spectrum disorder over a ten-year period, who were also treated with intravenous bisphosphonates.
Results
Characteristics of the patients
The three reported patients are the children of healthy Turkish parents, who were born after uneventful pregnancies. The parents reported not to be related to each other. A fourth male child of the family is healthy.
Patient 1 is the oldest of the reported siblings. She is currently 14 years old and developed painful swelling and contractures of the small hand and foot joints with multiple low-impact fractures, especially of the metatarsal bones, from the age of 3 years on. A coarse face and hypertrichosis but no subcutaneous nodules were present (Fig. 1). Patient 1 was diagnosed with MONA spectrum disorder only 4 years after the symptoms had started to appear, i.e. when she was 7 years old. Prior to MONA spectrum disorder a 3-methyl-crotonyl-CoA-carboxylase deficiency (3-MCC deficiency) had already been diagnosed in this patient. This is a rare autosomal recessive disorder of L-leucine metabolism20 causing neurological symptoms and acute metabolic decompensation triggered by common infections. None of these symptoms had ever been noted in patient 1. However based on published data showing that only less than 10% of the affected individuals ever develop minor symptoms and only less then 1% to 2% might be at risk for severe adverse outcome21. None of the patients with 3-MCC deficiency is reported to have a skeletal phenotype. Still, it was briefly thought to be the cause of the skeletal symptoms in Patient 1. For this reason oral L-carnitine supplementation, the recommended therapy in case of 3-MCC deficiency, was started. Dietary L-leucine restriction was not initiated as the efficacy of this approach for 3-MCC deficiency is unproven22.
As Patient 1 also showed signs of myopathy and neuropathy with reduced muscle strength and deep tendon reflexes, a muscle-tendon biopsy (musculus gastrognemicus/Achilles tendon) was performed at the age of 6 years. It demonstrated mild muscle fibre atrophy (Fig. 2a) and an accumulation of mononuclear inflammatory cells, especially around vessels within the fat connective tissue at the muscle-tendon junction (Fig. 2b). The enzyme histochemical stain for nicotinamide adenine dinucleotide-tetrazolium reductase demonstrated regular enzyme activity, but slight atrophy of type II fibres (Fig. 2c). Thus, additionally to oral L-carnitine supplementation, immunosuppressive therapy with azathioprine (2 mg/kg) was commenced. Both L-carnitine supplementation and azathioprine therapy were discontinued after the diagnosis of MONA spectrum disorder had been established when the patient was 7 years old.
Hematoxylin and eosin stain of the muscle-tendon biopsy of patient 1 at the age of 4.5 years shows a mildly increased fiber size variability (a). Immuno-histochemistry using antibodies against leucocyte common antigen (LCA) revealed an accumulation of mononuclear inflammatory cells especially perivascularly at the muscle-tendon junction (b). NADH-TR stain indicates a regular enzyme activity, but the weakly stained type II fibers are smaller than the more intensely stained type I fibers (c); original magnifications x20.
Patient 2, currently 9 years old, and Patient 3, currently 7 years old, had a similar history. Both younger siblings experienced first symptoms at the age of 2 years. Clinical examination revealed decreased range of motion in the fingers, wrists, ankles and toes with contractures of the interphalangeal joints. In Patient 2 we additionally diagnosed an iris coloboma and a ventricular septal defect, which were treated conservatively. Patient 3 had a secundum atrial septal defect and a vesicoureteral reflux II°–III°, without any episodes of recurrent urinary infections. The diagnosis of MONA spectrum disorder was genetically confirmed when the patients were 7 years (Patient 2) and 5 years (Patient 3) old respectively.
All three patients received concomitant physical and occupational therapy from when first symptoms of MONA spectrum disorder appeared. Before initiation of bisphosphonate therapy all three patients needed regular analgesic therapy with non-steroidal anti-inflammatory drugs three times a day.
All three patients were found to carry the same homozygous mutation c.[1699C > T] in exon 11 of the MMP2 gene. This mutation had not been reported previously but is very likely to be pathogenic as it leads to the introduction of a premature stop codon, p.[Arg567Ter], and early truncation of the MMP2 protein. The same mutation was then identified in heterozygosity in the healthy brother as well as in the parents.
Bisphosphonate therapy
In Patient 1 bisphosphonate therapy was started already 3 years before the diagnosis of MONA spectrum disorder was established, i.e. when Patient 1 was 4 years old. She received intravenous pamidronate (1 mg/kg/d on two consecutive days every 3 months) for the treatment of osteolysis and osteopenia for eight consecutive years. Then, for convenience of the patient and because reported to be equally effective, the therapy was switched to intravenous zoledronate (0.05 mg/kg/d in a single dose), which in contrast to pamidronate has to be given not every 3 month but only every 6 month.
Patients 2 and 3 were started to treat with zoledronate after the diagnosis of MONA spectrum disorder had been genetically confirmed, which was at the age of 7 and 5 years respectively, i.e. 5 and 3 years after first clinical symptoms had appeared.
Disease progression and therapeutic success
All three patients had normal developmental milestones, were of normal intelligence and frequented regular school. During follow-up no particular internal medical problems were noted. Patient 1 transiently showed mild signs of myopathy and neuropathy, which resolved spontaneously. The other siblings did not show any neurological symptoms.
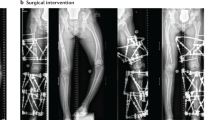
The main sequealae for the patients were the evolving orthopaedic problems related to MONA spectrum disorder. All three patients showed short stature. In Patient 1 joint destruction progressed towards proximal to involve the wrists, elbows and knees, resulting in flexion contractures. Orthopaedic surgical treatment of a radial head dislocation was necessary. Details on this orthopaedic procedure have already been reported before in the study of AlKaissi et al., were Patient 1 corresponds to Patient III23. Patient 2 and Patient 3 even had a more severe evolution in terms of joint destruction and flexion contractures as documented by radiographs of hands and feet, which demonstrate general osteopenia, multiple osteolysis, widening of the phalanges and irregular epiphyses (Figs 3 and 4) but no low impact fractures. Still, all three patients depended on regular analgesic therapy with non-steroidal anti-inflammatory drugs three times a day. Physical and occupational therapy were only slightly able to ameliorate skeletal pain in the patients.
Bisphosphonate therapy significantly reduced skeletal pain in our patients. Frequency of need for analgesic therapy diminished to a single therapeutic dose 3–4 times a month. Bone densitometry demonstrated that bisphosphonate therapy led to a steady increase in bone mineral content both in the axial and in the appendicular skeleton in all three patients. Bone mineral content was below the 3rd percentile at all measured times. It showed a steady increase, with a curve similar to that for age, sex- and ethnicity-matched healthy controls, although the discrepancy appeared to slightly grow with age24. Figure 5 exemplarily demonstrates data for Patient 1, as she has the longest observation period (8 years so far) (Fig. 5a–c). No further low-impact fractures occurred after starting bisphosphonate therapy. Patient 1 showed the best outcome. Although the nature of the disease is progressive, she is still able to walk with aids and use her hand for writing.
Bisphosphonate therapy increased overall bone mineral content (a), as well as bone mineral content in the axial (b) and appendicular skeleton (c). Bone mineral content was below the 3rd percentile at all measured time points but increase was steady and similar to age, sex and ethnicity matched healthy controls24 *. Data are given exemplarily for patient 1 including therapeutic interventions (a). Graphs demonstrate bone mineral content.
Patients 2 and 3 have a more severe disease course. Although they are pain-free, they are severely impaired in their daily life. This impairment primarily results from impaired movement of wrist and finger joints as well as from the fact that both siblings are wheelchair bound.
No adverse effects of bisphosphonate treatment, such as severe hypocalcaemia or nephrocalcinosis, were noted during this treatment period of ten years.
Discussion
In this study we report successful bisphosphonate therapy in three siblings affected from MONA spectrum disorder.
MONA spectrum disorder is characterized by short stature, severe joint contractures, peripheral corneal opacities, coarse face, osteolysis of carpal and tarsal bones as well as generalized osteopenia25. Characteristics of patients reported in the literature are summarized in Table 1 (Table 1). Only patients with a genetically confirmed diagnosis were included in the table. The underlying cause of MONA spectrum disorder is a mutation in the gene encoding MMP24,7.
The three sisters reported here presented with typical clinical findings and were diagnosed as having MONA spectrum disorder based on the molecular analysis revealing a novel stop mutation in the MMP2 gene. From the genetic findings we strongly suspect that the patients’ parents are consanguineous.
Patient 1 was identified to additionally have a 3-MCC deficiency. This was already reported in the study of AlKaissi et al., were Patient 1 corresponds to Patient III23. It is the first time that an inborn disorder of metabolism was observed in a patient with MONA spectrum disorder (Table 1). However, the association is likely to be fortuitous.
Patients 2 and 3 both show additional cardiac manifestations, i.e. a VSD and an ASD. This emphasizes a possible role of MMP2 in the pathophysiology of cardiac pathologies suggested by experimental studies26,27,28, especially as cardiac manifestations have already been reported in several patients with MONA spectrum disorder5,9.
Little information on the treatment of MONA spectrum disorder is given in the literature. Controversial data exist regarding immunosuppressive treatment. Al-Mayouf et al. reported an inhibitory effect of methotrexate combined with D-penicillamines on progression of joint contractures29. Tuysuz et al. noted an improvement in contractures in only one patient under prednisolone therapy, but not in his similarly affected cousin30.
In our patients we detected significant clinical improvement under bisphosphonate therapy. Bisphosphonates decrease osteoclastic activity, thereby favouring bone formation31. In paediatric patients they are used to treat conditions like osteoporosis and osteogenesis imperfecta. These agents have been reported to have analgesic effects, improve range of movement, function and bone mineral content32. With bisphosphonate therapy we were able to reduce the need for non-steroidal analgesics from three times a day to 3–4 times a month in our patient, which dramatically improved their quality of life. Bisphosphonate therapy was not able to significantly improve range of motion. Still, one of the reported patients is able to walk with aids at the age of 14 years. This has been reported only in three other adult patients by Azzollini et al.1 and Jeong et al.6, which again underlies the broad inter- and intrafamilial variability of MONA spectrum disorder. We are also aware that our study suffers from the limitation of a retrospective design.
Bisphosphonate therapy also led to an increase in bone mineral content in the axial and the appendicular skeleton in all three patients. Bone mineral content in the patients was always below the 3rd percentile, but increase rate was similar to that of age-, sex- and ethnicity-matched healthy controls (Fig. 5), although the discrepancy with these controls appeared to slightly grow with age regardless of the site of measurement. The reason is unclear. We hypothesize that it might be related to a decreasing level of physical activity of the patients as the disease progresses. It might also be related to the changing hormonal regulation during puberty, which eventually has a negative impact on bone mineral density in patients with MONA spectrum disorders. However this is highly speculative and further studies will be needed to clarify this point.
After initiation of bisphosphonate treatment no further low-impact fractures occurred in the herein reported patients. Effects were similar for both agents used (pamidronate and zoledronate). We do not attribute the more favourable clinical outcome of patient 1 to the fact that she was initially treated with pamidornate while treatment in the siblings was started with zoledronate. We hypothesize that the more benign course might be related to early initiation of bisphosphonate therapy in this patient (one year after appearance of first clinical symptoms). In contrast in Patient 2 and 3 bisphosphonate therapy was only started 5 and 3 years respectively after clinical symptoms had appeared. Furthermore Patient 1 developed first symptoms of MONA spectrum disorder at the age of 4 years whereas Patient 2 and 3 showed first symptoms already when they were 2 years old. This reflects intrafamilial variability in the disease course with siblings 2 and 3 being more severely affected. Also we do not suspect that the favourable course of Patient 1 was due to the initial combination of immunosuppressive therapy with azathioprin and bisphosphonate therapy. This hypothesis is supported by the fact that also the two other reported sisters, who haven’t had immunosuppressive treatment, clinically improved after bisphosphonate therapy was started. Furthermore MMP2 has not been reported to trigger auto-inflammatory cascades, thus patients with MONA spectrum disorder might hardly benefit from immunosuppression33.
Besides our study there are two more reports on the use of bisphosphonates for the treatment of MONA spectrum disorder. Phadke et al.19 administered intravenous pamidronate in a dose of 1 mg/kg/d on three consecutive days at four-monthly intervals for 3 years in two siblings affected with MONA spectrum disorder. Similarly to our study they noticed a subjective decrease in pain, tenderness and joint contractures at the wrist as well as increase of bone mineral density of the axial skeleton but no increase at the radius and ulna. Al-Mayouf et al.34 treated seven children with NAO syndrome with intravenous pamidronate. The dosis was 2 mg/kg on each of three consecutive days every three month for one year. The patients were maintained on 800 IU/day vitamin D and at least 800 mg/day of elemental calcium supplement. The authors also noticed a decrease in limb and joint pain and improvement in functional ability although not significant. However bone mineral density had increase significantly in all patients at the end of treatment34.
Promising results of bisphosphonate therapy have been already reported multiple times in other inherited osteolysis syndromes in children, i.e. inherited multicentric osteolysis35. Three siblings were treated with intravenous pamidronate at an initial dose of 0.5 mg/kg, which was increased to 1 mg/kg administered monthly for the next six months. Pain improved dramatically, no new fractures or focal osteolytic areas occurred. Similar to our patients, range of motion did not improve. Positive effects of bisphosphonates were also observed in patients with complex regional pain syndrome (CRPS), especially if they had osteopenic or osteoporotic changes in the affected limb. Amelioration of symptoms was observed both in patients who were treated orally (eg. alendronate) and intravenously (eg. clodronate or pamidronate)36,37,38,39,40. However the positive effects of bisphosphonates in CRPS are probably not related to their antiresorptive properties but to the fact that they might be able to modulate various inflammatory mediators that are up-regulated in CRPS-I40. This fact might also contribute to the positive effect of bisphosphonates observed in the treatment of MONA spectrum disorder.
Conclusion
From the presented data we conclude that bisphosphonates are a suitable treatment option to reduce skeletal pain thereby increasing quality of life in patients with MONA spectrum disorder. Early initiation of therapy, i.e. when first symptoms of the disease appear seems to be particularly important to achieve the most favourable outcome. From our experience we suggest that bisphosphonates are a treatment option for patients suffering from a MONA spectrum disorder. Although range of motion does not improve, bisphosphonate therapy seems to be able to reduce skeletal pain in these patients.
Methods
Assessment of the patients
After informed consent had been obtained from the parents we assessed the patients regarding the following characteristics: consanguinity, clinical symptoms at diseases on-set, age at on-set of symptoms and age at diagnosis, cognitive development, progression of clinical symptoms related to the diagnosis, molecular investigations, associated disorders as well as therapies besides bisphosphonate therapy. Informed consent from the parents was also obtained to publish the patient’s photographs. All investigations were performed according to the relevant ethical guidelines. The experimental protocols were approved by the Department of Pediatrics of the Medical University of Innsbruck, Austria. The trial was registered in the registry and results database ClinicalTrials.gov on 1st July 2016 (registration number NCT02823925).
Bisphosphonate therapy
The reported patients received intravenous bisphosphonate therapy either with pamidronate (1 mg/kg/d on two consecutive days every 3 months) or zoledronate (a single dose of 0.05 mg/kg/day every 6 month).
Evaluation of disease progression and therapeutic success
To assess both progression of MONA spectrum disorder and therapeutic success the patients were regularly evaluated clinically in 3 to 6 month intervals. Clinical evaluation comprised an internal, neurological and orthopaedic status as well as a general assessment of neurocognitive function. Additionally, need for oral analgesic therapy was documented. In irregular intervals, depending also on the clinical symptoms, x-rays of hands and feet were taken and a densitometry of the total body, lumbar spine and hip was performed.
Additional Information
How to cite this article: Pichler, K. et al. Bisphosphonates in multicentric osteolysis, nodulosis and arthropathy (MONA) spectrum disorder – an alternative therapeutic approach. Sci. Rep. 6, 34017; doi: 10.1038/srep34017 (2016).
References
Azzollini, J. et al. Functional characterisation of a novel mutation affecting the catalytic domain of MMP2 in siblings with multicentric osteolysis, nodulosis and arthropathy. J Hum Genet 59, 631–637 (2014).
Castberg, F. C. et al. Multicentric osteolysis with nodulosis and arthropathy (MONA) with cardiac malformation, mimicking polyarticular juvenile idiopathic arthritis: case report and literature review. Eur J Pediatr 172, 1657–1663 (2013).
Ekbote, A. V. et al. Patient with mutation in the matrix metalloproteinase 2 (MMP2) gene - a case report and review of the literature. J Clin Res Pediatr Endocrinol 6, 40–46 (2014).
Evans, B. R. et al. Mutation of membrane type-1 metalloproteinase, MT1-MMP, causes the multicentric osteolysis and arthritis disease Winchester syndrome. Am J Hum Genet 91, 572–576 (2012).
Gok, F. et al. Clinical and radiographic findings in two brothers affected with a novel mutation in matrix metalloproteinase 2 gene. Eur J Pediatr 169, 363–367 (2010).
Jeong, S. Y., Kim, B. Y., Kim, H. J., Yang, J. A. & Kim, O. H. A novel homozygous MMP2 mutation in a patient with Torg-Winchester syndrome. J Hum Genet 55, 764–766 (2010).
Martignetti, J. A. et al. Mutation of the matrix metalloproteinase 2 gene (MMP2) causes a multicentric osteolysis and arthritis syndrome. Nat Genet 28, 261–265 (2001).
Rouzier, C. et al. A novel homozygous MMP2 mutation in a family with Winchester syndrome. Clin Genet 69, 271–276 (2006).
Temtamy, S. A. et al. A report of three patients with MMP2 associated hereditary osteolysis. Genet Couns 23, 175–184 (2012).
Zankl, A., Bonafé, L., Calcaterra, V., Di Rocco, M. & Superti-Furga, A. Winchester syndrome caused by a homozygous mutation affecting the active site of matrix metalloproteinase 2. Clin Genet 67, 261–266 (2005).
Zankl, A. et al. Torg syndrome is caused by inactivating mutations in MMP2 and is allelic to NAO and Winchester syndrome. J Bone Miner Res 22, 329–333 (2007).
Orphanet: Multicentric osteolysis nodulosis arthropathy spectrum MONA spectrum; Available at: http://www.orpha.net/consor/cgi-bin/Disease_Search.php?lng=EN&data_id=22539&Disease_Disease_Search_diseaseGroup=mona&Disease_Disease_Search_diseaseType=Pat&Disease(s)/group%20of%20diseases=Multicentric-osteolysis-nodulosis-arthropathy-spectrum--MONA-spectrum-&title=Multicentric-osteolysis-nodulosis-arthropathy-spectrum--MONA-spectrum-&search=Disease_Search_Simple. (Accessed 18th October 2015).
Bhavani, G. S. et al. Clinical and mutation profile of multicentric osteolysis nodulosis and arthropathy. Am J Med Genet A 170A, 410–417 (2016).
Massova, I., Kotra, L. P., Fridman, R. & Mobashery, S. Matrix metalloproteinases: structures, evolution, and diversification. FASEB J 12, 1075–1095 (1998).
Murphy, G. et al. Evaluation of some newer matrix metalloproteinases. Ann N Y Acad Sci 878, 25–39 (1999).
Creemers, L. B. et al. Gelatinase A (MMP-2) and cysteine proteinases are essential for the degradation of collagen in soft connective tissue. Matrix Biol 17, 35–46 (1998).
Chen, W. T. Proteases associated with invadopodia, and their role in degradation of extracellular matrix. Enzyme Protein 49, 59–71 (1996).
Stetler-Stevenson, W. G. & Yu, A. E. Proteases in invasion: matrix metalloproteinases. Semin Cancer Biol 11, 143–152 (2001).
Phadke, S. R., Ramirez, M., Difeo, A., Martignetti, J. A. & Girisha, K. M. Torg-Winchester syndrome: lack of efficacy of pamidronate therapy. Clin Dysmorphol 16, 95–100 (2007).
Stadler, S. C. et al. Newborn screening for 3-methylcrotonyl-CoA carboxylase deficiency: population heterogeneity of MCCA and MCCB mutations and impact on risk assessment. Hum Mutat 27, 748–759 (2006).
Grünert, S. C. et al. 3-methylcrotonyl-CoA carboxylase deficiency: clinical, biochemical, enzymatic and molecular studies in 88 individuals. Orphanet J Rare Dis 7, 31 (2012).
Arnold, G. L. et al. A Delphi-based consensus clinical practice protocol for the diagnosis and management of 3-methylcrotonyl CoA carboxylase deficiency. Mol Genet Metab 93, 363–370 (2008).
Al Kaissi, A. et al. The diagnosis and management of patients with idiopathic osteolysis. Pediatr Rheumatol Online J 9, 31 (2011).
Zemel, B. S. et al. Revised reference curves for bone mineral content and areal bone mineral density according to age and sex for black and non-black children: results of the bone mineral density in childhood study. J Clin Endocrinol Metab 96, 3160–3169 (2011).
OMIM #259600 MULTICENTRIC OSTEOLYSIS, NODULOSIS, AND ARTHROPATHY; MONA; Available at: http://omim.org/entry/259600. (Accessed 21st June 2016).
Lovett, D. H. et al. N-terminal truncated intracellular matrix metalloproteinase-2 induces cardiomyocyte hypertrophy, inflammation and systolic heart failure. PLoS One 8, e68154 (2013).
Mahimkar, R. et al. Cardiac transgenic matrix metalloproteinase-2 expression induces myxomatous valve degeneration: a potential model of mitral valve prolapse disease. Cardiovasc Pathol 18, 253–261 (2009).
Soini, Y., Satta, J., Määttä, M. & Autio-Harmainen, H. Expression of MMP2, MMP9, MT1-MMP, TIMP1, and TIMP2 mRNA in valvular lesions of the heart. J Pathol 194, 225–231 (2001).
Al-Mayouf, S. M., Majeed, M., Hugosson, C. & Bahabri, S. New form of idiopathic osteolysis: nodulosis, arthropathy and osteolysis (NAO) syndrome. Am J Med Genet 93, 5–10 (2000).
Tuysuz, B. et al. A novel matrix metalloproteinase 2 (MMP2) terminal hemopexin domain mutation in a family with multicentric osteolysis with nodulosis and arthritis with cardiac defects. Eur J Hum Genet 17, 565–572 (2009).
Russell, R. G. Bisphosphonates: mode of action and pharmacology. Pediatrics 119 Suppl 2, S150–S162 (2007).
Phillipi, C. A., Remmington, T. & Steiner, R. D. Bisphosphonate therapy for osteogenesis imperfecta. Cochrane Database Syst Rev CD005088 (2008).
Faber, M. R. et al. Inherited multicentric osteolysis with carpal-tarsal localisation mimicking juvenile idiopathic arthritis. Eur J Pediatr 163, 612–618 (2004).
Al-Mayouf, S. M., Madi, S. M. & Bin-Abbas, B. S. Cyclic intravenous pamidronate treatment in children with nodulosis, arthropathy and osteolysis syndrome. Ann Rheum Dis 65, 1672–3 (2006).
Lee, S. J., Whitewood, C. & Murray, K. J. Inherited multicentric osteolysis: case report of three siblings treated with bisphosphonate. Pediatr Rheumatol Online J 8, 12 (2010).
Robinson, J. N., Sandom, J. & Chapman, P. T. Efficacy of pamidronate in complex regional pain syndrome type I. Pain Med 5, 276–280 (2004).
Manicourt, D. H., Brasseur, J. P., Boutsen, Y., Depreseux, G. & Devogelaer, J. P. Role of alendronate in therapy for posttraumatic complex regional pain syndrome type I of the lower extremity. Arthritis Rheum 50, 3690–3697 (2004).
Adami, S., Fossaluzza, V., Gatti, D., Fracassi, E. & Braga, V. Bisphosphonate therapy of reflex sympathetic dystrophy syndrome. Ann Rheum Dis 56, 201–204 (1997).
Varenna, M. et al. Treatment of complex regional pain syndrome type I with neridronate: a randomized, double-blind, placebo-controlled study. Rheumatology (Oxford) 52, 534–542 (2013).
Varenna, M., Adami, S. & Sinigaglia, L. Bisphosphonates in Complex Regional Pain syndrome type I: how do they work? Clin Exp Rheumatol 32, 451–454 (2014).
Author information
Authors and Affiliations
Contributions
K.P., D. Karall, E.S.-G., A.R.-W., K.M. and S.S.-B. were involved in diagnosis and treatment of the reported patients as well as in data collection. D. Kotzot, L.M.-C., L.B. and A.S.-F. carried out the molecular genetic studies. J.W. performed muscle staining. K.P. performed statistical data analysis and drafted the manuscript helped by D. Karall and S.S.-B. All authors have read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Pichler, K., Karall, D., Kotzot, D. et al. Bisphosphonates in multicentric osteolysis, nodulosis and arthropathy (MONA) spectrum disorder – an alternative therapeutic approach. Sci Rep 6, 34017 (2016). https://doi.org/10.1038/srep34017
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep34017
This article is cited by
-
Clinical, radiographic and molecular characterization of two unrelated families with multicentric osteolysis, nodulosis, and arthropathy
BMC Musculoskeletal Disorders (2023)
-
Do Bisphosphonates Alleviate Pain in Children? A Systematic Review
Current Osteoporosis Reports (2020)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.