Abstract
Primary bone lymphoma(PBL) is a rare disease. To assess the clinical characteristics, outcome, and prognostic factors of this entity in Chinese population, we retrospectively analyzed 61 PBL patients initially treated in our institution between 1997 and 2014. The median age was 45 years. The most common histological subtype was diffuse large B-cell lymphoma (DLBCL) (55.7%), followed by T-cell lymphoma (18.0%). All patients underwent systemic chemotherapy as initial treatment while 24 patients (39.3%) were additionally treated with radiotherapy. The 5-year overall survival (OS) and the 5-year progression-free survival (PFS) rates of 57 cases with completed follow-up were 52.3% and 40.1%, respectively. In further analysis of the primary bone DLBCL (PB-DLBCL) subgroup, the 5-year OS and PFS rates were 53.0% and 47.0%, and a multivariable analysis revealed that baseline Eastern Cooperative Oncology Group (ECOG) score and response to initial treatment (complete remission versus no complete remission) were independent prognostic factors for both OS and PFS. The proportion of T-cell lymphoma is higher in China than in western populations. High baseline ECOG scores (≥2) and unachieved CR in initial therapy were factors for poor PB-DLBCL prognosis. The role of radiotherapy and rituximab in PLB therapy remains to be confirmed in further investigation.
Similar content being viewed by others
Introduction
Primary bone lymphoma (PBL) is a rare primary extranodal lymphoma in the skeletal system that accounts for less than 1% of all lymphomas, about 4–5% of extranodal lymphomas, and about 3% of all malignant bone tumors1,2,3. The definition of PBL was controversial in the past, but has been defined in the new version of “WHO pathology and genetics classification of soft tissue and bone tumor”4 in 2013 as: a kind of malignant tumor composed by malignant lymphocytes, forming single or multiple tumor in the bone, not associated with infringement or violation of other extranodal malignant lymph nodes outside the area. The disease can occur at any age, with a median age of onset ranging from 40–60 years old, with most of the literature suggesting that the proportion in male patients was slightly higher than in females (1.0–1.8:1), a finding also reported for children5,6. Most PBL cases belong to B cell non-Hodgkin’s lymphoma, the most common type being diffuse large B-cell lymphoma (DLBCL)7,8,9. Other types include follicular lymphoma, mantle cell lymphoma, B-lymphoblastic lymphoma, small lyphocytic lymphoma and Burkitt lymphoma. PBL’s clinical manifestations are not specific but the most common symptoms are pain and mass10,11,12,13. PBL can occur in various parts of bone tissue, with previous reports showing that the highest incidence were long bones14,15,16,17, but with the change of the PBL definition, multiple sites of bone invasion have also been included in the scope of PBL. After that, there are data suggesting the most common diseased parts were the spine or pelvis7,8,18. Current treatments include surgery, radiotherapy and chemotherapy, but there is no standard treatment due to a lack of comparability among the studies, caused by changed PBL definitions.
Since PBL is a rare disease, current studies about PBL are mostly retrospective or case reports and retrospective studies often lasted over more than ten years or even decades13. In addition, present research results are mostly from the US and Europe, but data from Asia are limited. Here we retrospectively collected and analyzed data from 61 PBL patients admitted to our center from 1997 to 2014, in order to better understand the characteristics, outcome, and prognostic factors of PBL in Chinese population. To the authors’ knowledge, this study comprises the largest sample size analyzed in Asia currently.
Patients and Methods
Patients
In this retrospective study, 61 PBL patients from the department of lymphoma of Peking University Cancer Hospital and Institute from January 1997 to January 2014 were included. All patients satisfied the 2013 WHO criteria of PBL4: lymphoma was restricted to bone and adjacent soft tissue with or without regional lymph node involvement at the time of diagnosis. Patients with lymph node involvement on the other side of the diaphragm, distant bone marrow involvement or any other site of extranodal disease were excluded in this series.
The study was approved by the Ethical committee of Peking University Cancer Hospital and Institute and written informed consent was provided by the patients. All experiments were performed in accordance with relevant guidelines and regulations.
Methods
Information Reviewed
The database was established from the medical records including: gender, age, presenting symptoms, involved sites, Eastern Cooperative Oncology Group (ECOG) score19, radiological findings, pathological diagnosis, stage, international prognostic index (IPI)20, treatment modality and treatment response. All patients were followed up by outpatient reviews or by telephone conversations; the last follow-up date was 2015-1-1. Overall survival (OS) was defined as time from pathological diagnosis until death, lost or last follow-up. Progression free survival (PFS) was defined as time from pathological diagnosis until disease progression, lost or last follow-up.
Staging
All patients underwent detailed history and physical examinations, blood tests, imaging (chest X-ray or computer tomography (CT), abdominal B-scan ultrasonography or CT, systemic superficial lymph node B-scan ultrasonography; some patients received a systemic positron emission tomography (PET)/CT examination) as well as bone marrow aspirate and biopsy. Some of the patients received a cerebrospinal fluid examination when the spine was involved. The clinical stage was determined by Ann Arbor staging criteria21. Stage IE was defined as a solitary bone lesion without lymph node involvement; stage IIE as a solitary bone lesion with regional lymph nodes involvement; and stage IV was the presence of multiple bone lesions with or without regional lymph node involvement.
Response Criteria
Response to treatment was assessed by the International Workshop to Standardize Response Criteria in 1999 (IWC), also known as the Cheson criteria22. The PET/CT review efficiency of some patients was based on the revised edition of malignant lymphoma remission criteria in 200723.
Statistical methods
All data were statistically analyzed by SPSS Statistics for Windows (Version 22.0. Armonk, NY: IBM Corp.). The Kaplan-Meier method was used for survival analysis, and the Log-rank test to analyze the survival rate between the two groups. Variables achieving significant level of P < 0.05 were entered into the COX proportional hazards regression model to complete multivariable analyses. Independent prognostic factors were determined if they had significant effect in the Cox model (P < 0.05).
Results
Clinical features
The general clinical characteristics of 61 patients are shown in Table 1. The proportion of males to females of 61 patients was 1.65:1. The median age was 45 years (range, 13–80 years). The most common initial symptom was local pain, followed by nerve compression and local mass.
The histopathological subtypes of the 61 PBL patients are shown in Table 2. DLBCL was the most common histological type, accounting for 55.7% (34 cases), followed by systemic anaplastic large cell lymphoma (ALCL) and B-lymphoblastic lymphoma (B-LBL), 13.1% (8 cases) and 11.5% (7 cases), respectively. Other rare types included: Hodgkin’s lymphoma (HL), mantle cell lymphoma (MCL), marginal zone B-cell lymphoma (MZL), T-lymphoblastic lymphoma (T-LBL), as well as two cases of T-cell origin (failed to be classified). T-cell lymphoma accounted for 18.0% of the cases.
The pathogenic sites of the 61 PBL patients are shown in Table 3. For single bone invasion, the most common site was pelvic bone (9 cases, 33.3%), followed by the long bone with a total of 8 cases (29.6%). In patients with multiple bone invasions, the most common site was the spine (25 cases, 73.5%), followed by the pelvic bones (17 cases, 50%). In all patients, the incidence of the most common sites were the spine and pelvis bones, followed by the skull, femur and humerus.
Treatments, responses and survival of patients with PBL
The treatment modality that 61 PBL patients received are shown in Table 4. All patients underwent initial therapy with systemic chemotherapy, of which 37 cases (60.7%) received chemotherapy alone and 24 patients (39.3%) were treated with combined local radiotherapy. 18 patients underwent surgery in which the purpose for 10 patients was to ease spinal cord compression or treat pathological fractures and 8 patients underwent primary lesion resections.
After the initial treatment that all patients completed, 57 patients’ clinical data can be evaluated. 32 patients achieved complete remission (CR), 18 patients obtained partial remission (PR) and the overall response rate (ORR) was 87.7% (56.1% CR + 31.6% PR). 3 cases were assessed as stable disease (SD) and 4 cases as progress disease (PD) after initial treatment.
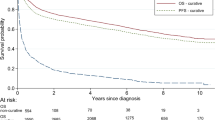
A survival analysis was done for 57 PBL patients with complete follow-up data. The median follow-up was 31 months (range, 3–216 months). By the date of the last follow-up, 35 patients survived (61.4%), 22 deaths occurred (38.6%), of which 20 patients died of tumor progression and 2 died due to the treatment. The 5-year OS was 52.3% with the 5-year PFS being 40.1% (Fig. 1).
Effect of the histopathological subtypes on the prognosis of patients with PBL
In this group of patients, in addition to DLBCL, other pathology samples were few and thus the survival among all histopathological subtypes could not be compared. The histopathological subtypes B-cell lymphoma, T-cell lymphoma and Hodgkin’s lymphoma showed no significant difference for the survival among this three groups. There was also no significant difference in the survival between patients with DLBCL and non-DLBCL. In addition, no significant difference was found for OS and PFS between ALCL and non-ALCL patients, or between DLBCL patients and ALCL patients (Table 5).
Analysis of Survival and prognostic factors in patients with PB-DLBCL
Given the effects of different histopathological subtypes on survival and the sample size limitation of other pathological types, we only analyzed prognostic factors of 34 DLBCL patients. The median follow-up was 38 months (range, 3–216 months). The 5-year OS was 53.0%, 5-year PFS was 47.0% (Fig. 2).
The Kaplan-Meier method and log-rank test was used to analyze the following factors for univariable analysis: gender, age, pathogenic sites, pathological fractures, B symptoms, ECOG score, LDH levels, soft tissue invasion, lymph node involvement, stage, IPI score, molecular subtypes (germinal center B-cell-like (GCB) vs. non-GCB), treatment modality, response to initial therapy and rituximab use. High ECOG score (≥2), stage IV (stage IV vs. stage I&II), high IPI score (>2) and unachieved CR in initial therapy were associated with worse OS (Fig. 3 and Table 6), whereas older age (≥60), B symptoms, high ECOG score (≥2), elevated LDH and unachieved CR in initial therapy were associated with worse PFS (Figs 3, 4 and Table 6).
(A) OS according to ECOG score. (B) PFS according to ECOG score. (C) OS according to respose to initial therapy. (D) PFS according to respose to initial therapy. (E) OS according to International Prognostic Index. (F) PFS according to International Prognostic Index. (G) OS according to stage. (H) PFS according to stage.
23 cases out of a total of 34 PB-DLBCL patients were further divided into GCB type (11 cases) and non-GCB type (12 cases), with 5-year OS rates of the 2 groups being 70.1% and 20.0% respectively, but the apparent difference did not reach statistical significance (P = 0.193) and also for PFS there was no statistical significance (P = 0.299). 20 patients received combined rituximab treatments, but compared with the group without rituximab, OS and PFS were not significantly different (5-year OS 48.4% vs 60.6%, P = 0.494; 5-year PFS 44.9% vs 44.0%, P = 0.432). The OS rate of patients with complicated pathologic fractures appeared to be inferior to patients without pathological fractures, but the trend did not reach statistical significance (5-year OS 31.7% vs 58.9%, P = 0.066) and also PFS rates did not significantly differ between the two groups (P = 0.240) (Table 6).
Multivariable analysis using a COX proportional hazards regression model showed that the baseline ECOG score and response to initial treatment were independent factors for the OS of PB-DLBCL patients. Response to initial treatment was also an independent risk factor for PFS of the patients (Table 7).
Discussion
In previous studies, the definition, clinical characteristics, treatment modalities, and prognosis of PBL remain controversial and most of the present research results are from the US and Europe. In this report, we described a series of Chinese PBL patients using the new 2013 WHO criteria4. This study comprises the largest sample size analyzed in Asia.
According to previous studies, the majority of PBL belongs to B-cell NHL, the most common type being DLBCL, occurring in about ≥80% of all cases, followed by follicular lymphoma7,8,9 while T-cell NHL accounts for about 1–5% of all PBL patients in the US and Europe7,8,14,24. In our study, although DLBCL was the most common histological type (55.7%), the incidence seems to be lower than previous reports in the US and Europe. T-cell lymphoma accounted for 18.0% of all PBL patients in our study. The incidence of T-cell lymphoma was higher than previous reports in the US and Europe but similar to other Asia series18,25,26. We believe that this differences are due to regional differences of T-cell lymphoma incidences being higher in Asia than in the US and Europe27.
Most previous studies suggested a predominance of long bone involvement in PBL14,15,16,17. However, Ramadan et al.8 reported that the spine was the most commonly involved site, accounting for one-third of 131 cases. Others studies from China and Japan showed that the pelvis was the most common site of PLB involvement18,26,28. In the present study, the most commonly involved sites were the spine and pelvis (both accounting for 42.6%, respectively). The preponderance of pelvis involvement may be a unique characteristic of Asian patients with PBL.
The overall outcome of PBL is controversial. In our study, the overall 5-year OS and PFS of 57 PBL patients was 52.3% and 40.5% and the 5-year OS and PFS of 34 PB-DLBCL patients among them were 54.7% and 49.1%. According to previous reports, 5-year OS of PBL patients were 88%15, 76%7, 57.8%29 and 55%18. However, although DLBCL accounts for a large proportion (68–83%), these studies did not exclude the effect of histological heterogeneity on survival of PBL; we therefore consider that these data lack comparability. To exclude the effect of different histological type on prognosis, fewer studies have discussed the prognosis of PB-DLBCL alone and suggested that PB-DLBCL has a better prognosis than other types of DLBCL. Wu et al.9 reported the 5-year OS of 53 PB-DLBCL cases was 81.1%. Small sample data from India30 showed an 8-year OS and DFS of 21 PB-DLBCL patients of 95.2% and 100%. Heyning et al.31 reported that in a group of 36 PB-DLBCL cases from the Netherlands, the 5-year OS was 75%. However, our study did not suggest such a good prognosis of PB-DLBCL, as reported by some other authors. Jawad et al.24 reported the 5-year and 10-year OS of 994 PB-DLBCL cases were 61% and 48%, respectively. Ramadan et al.8 reported on 131 PBL patients from which the 5-year and 10-year OS of 103 (79%) PB-DLBCL patients were 62% and 41%, respectively. Considering variations in the definition and the treatment of PBL and selection bias in retrospective studies, it is perhaps not surprising that there were quite different outcomes between independent studies.
The prognostic factors of PBL have not been well established. In nodal lymphoma, pathological type is one of the most important prognostic factors. Superior prognosis of PB-DLBCL compared to non-DLBCL patients has been noted by Hsieh et al.25 who documented 14 cases of PBL in Taiwan and concluded that the prognosis of B-cell PBL was better than T-cell PBL (P = 0.016). Other studies also reported histological type to be a prognostic factor16,32. However, in our series of PBL patients including B-cell lymphoma, T-cell lymphoma, and Hodgkin’s lymphoma, no statistical difference in prognosis was found between the three groups. And neither DLBCL group nor ALCL group showed a superior prognosis when compared to other pathological subtypes. Due to the small sample size, it was not possible to compare further differences in prognosis between the various pathological types. Thus, we believe that the impact of pathological type on prognosis of PBL remains an open question.
To exclude the effect of the pathological type on prognosis, in our study we used univariable and multivariable analyses only on PB-DLBCL patients. The IPI system was developed to assess prognosis in patients with aggressive NHL. High IPI score had been considered as a poor prognostic factor of PBL by Ramadan et al.8 , Wu et al.9 and Huang et al.18, but not by Catlett et al.33 and Alencar et al.14. In the present study, IPI and its variants(age, ECOG score, LDH levels, number of extranodal sites and Ann Arbor stage) were analyzed. Univariable analyses showed that IPI score, tumor stage and ECOG score had a significant impact on prognosis, but only the ECOG score was identified to be an independent prognostic factor in multivariable Cox analysis. The prognostic impact of IPI on patients with PLB still requires further discussion. Some studies have suggested that age was an important factor affecting the prognosis of PBL8,15,16,34,35. In our study, although age had an impact on the PFS of PB-DLBCL patients, there was no significant effect of age on OS rates and also multivariable analysis showed no age effect. In addition to ECOG score, whether CR in initial treatment was an independent prognostic factor determining both OS and PFS, which was consistent with some previous reports7,36. A previous study aimed at nodal DLBCL, suggested that the prognosis of the GCB subtype is better than that of the non-GCB subtype in using standard chemotherapy37. In our study, 23 cases of 34 PB-DLBCL patients could be further divided into GCB type (11 cases) and non-GCB type (12 cases), but the 5-year OS and PFS between the 2 groups did not reach statistical significance, which is in accordance with reports from Bhagavathi et al.38 and Heyning et al.31, but the sample sizes were small and the influence of molecular subtypes on prognosis remains to be elucidated.
Treatment modalities for PBL include chemotherapy, radiotherapy and surgery which is mainly applied for diagnostic biopsy, to repair pathologic fractures or for spinal cord compression therapies. In our PB-DLBCL group of patients, the prognosis of patients accepting excisions was not better than the prognosis of patients who did not accept excisions and chemotherapy was the main form of treatment for the PBL patients. Various studies have noted that combined modality therapy (CMT) was better than radio/chemotherapy alone for PBL7,11,15,17. However, there is still controversy in whether CMT is superior to chemotherapy alone. Cai et al.7 reported 116 early PBL cases, with 5-year OS rates of 79% for the CMT group and 69% for the radio/chemotherapy alone groups (P = 0.05) and a multivariable analysis showed that CMT was an independent factor that affects OS. Report by Beal et al.15 also revealed that CMT was an independent prognostic risk factor for PBL, with 5-year OS rate of 95% for the CMT group and 78% for the solely radio/chemotherapy groups (P = 0.001). In our study, all of 34 PB-DLBCL patients received chemotherapy and 18 patients (42.1%) received CMT. However, the addition of radiotherapy to chemotherapy did not improve the prognosis of PB-DLBCL in our study. Similar data were also reported in a study by Alencar et al.14 and Ramadan et al.8 Taken together we believe, regardless of the stage at diagnosis, PBL should still be regarded as a systemic disease like other lymphomas, with systemic chemotherapy being the main treatment. Combined radiotherapy in the present study failed to improve the prognosis of PBL patients, but because of the limited sample size and the results of previous studies, the role of radiotherapy still needs further verification. Whether PBL patients need CMT, the treatment modality should be selected in the clinic individually according to the actual condition of the patient. However, we suggest that surgical resection of the lesion is not appropriate as a preferred treatment for PBL patients.
Rituximab in combination with chemotherapy has been used as standard protocol for CD20 + B-cell non-Hodgkin’s lymphoma. The vast majority of the pathological types of PBL are B-cell related, but whether the addition of rituximab can improve the prognosis of PBL patients remains controversial. Ramadan et al.8 compared the prognosis between PB-DLBCL patients receiving CHOP/CHOP-like chemotherapy and those receiving an R-CHOP program, and found that rituximab significantly improved PFS. In the report of Alencar et al.,14 the trend of prolonged PFS has been apparently improved in PB-DLBCL patients receiving rituximab-CHOP compared to those on a CHOP regime alone, but statistical significance (P = 0.062) was not reached. Also Catlett et al.33 and Kim et al.39 reported non-significant trend towards improved OS with rituximab combination therapy, which is in agreement with our result. Thus, the role of rituximab in PBL treatments requires further investigation.
Conclusion
By retrospective analysis 61 PBL patients in our single institution, we identified the clinical characteristics and prognosis of PBL in Chinese population. The results showed that the most common pathological type was DLBCL, but the proportion of the T-cell type cases was higher than in the US and Europe. The most common sites invaded were the bones of the spine and the pelvis. High baseline ECOG scores and unachieved CR in initial therapy result in poor prognosis of PB-DLBCL patients. Chemotherapy plays a central role in the treatment of PBL. Though the present result failed to support the use of combined modality for the treatment of PBL, the role of radiotherapy and optimal treatment strategy for PBL warrants further investigation by larger prospective multicenter studies.
Additional Information
How to cite this article: Zhang, X.Y. et al. Clinical characterization and outcome of primary bone lymphoma: a retrospective study of 61 Chinese patients. Sci. Rep. 6, 28834; doi: 10.1038/srep28834 (2016).
Change history
15 February 2017
The version of this Article previously published incorrectly listed XuanYe Zhang, and not Jun Zhu, as a corresponding author. This has now been corrected in the PDF and HTML versions of the Article.
References
Limb, D., Dreghorn, C., Murphy, J. K. & Mannion, R. Primary lymphoma of bone. International orthopaedics 18, 180–183 (1994).
Freeman, C., Berg, J. W. & Cutler, S. J. Occurrence and prognosis of extranodal lymphomas. Cancer 29, 252–260 (1972).
Rosenberg, S. A., Diamond, H. D., Jaslowitz, B. & Craver, L. F. Lymphosarcoma: a review of 1269 cases. Medicine 40, 31–84 (1961).
Fletcher, C. D., Brigde, J. A., Hogendoorn, P. C. & Mertens, F. World Health Organization Classification of Tumours of Soft Tissue and Bone. Lyon: IARC Press. (2013).
Furman, W. L., Fitch, S., Hustu, H. O., Callihan, T. & Murphy, S. B. Primary lymphoma of bone in children. Journal of Clinical Oncology: Official Journal of the American Society of Clinical Oncology 7, 1275–1280 (1989).
Glotzbecker, M. P. et al. Primary non-Hodgkin’s lymphoma of bone in children. The Journal of bone and joint surgery. American volume 88, 583–594, doi: 10.2106/JBJS.D.01967 (2006).
Cai, L. et al. Early-stage primary bone lymphoma: a retrospective, multicenter Rare Cancer Network (RCN) Study. International journal of radiation oncology, biology, physics 83, 284–291, doi: 10.1016/j.ijrobp.2011.06.1976 (2012).
Ramadan, K. M., Shenkier, T., Sehn, L. H., Gascoyne, R. D. & Connors, J. M. A clinicopathological retrospective study of 131 patients with primary bone lymphoma: a population-based study of successively treated cohorts from the British Columbia Cancer Agency. Annals of oncology: official journal of the European Society for Medical Oncology / ESMO 18, 129–135, doi: 10.1093/annonc/mdl329 (2007).
Wu, H. et al. Prognostic significance of soft tissue extension, international prognostic index, and multifocality in primary bone lymphoma: a single institutional experience. British journal of haematology 166, 60–68, doi: 10.1111/bjh.12841 (2014).
Baar, J. et al. Primary non-Hodgkin’s lymphoma of bone. A clinicopathologic study. Cancer 73, 1194–1199 (1994).
Fidias, P. et al. Long-term results of combined modality therapy in primary bone lymphomas. International journal of radiation oncology, biology, physics 45, 1213–1218 (1999).
Gianelli, U. et al. Lymphomas of the bone: a pathological and clinical study of 54 cases. International journal of surgical pathology 10, 257–266 (2002).
Stein, M. E. et al. Primary lymphoma of bone–a retrospective study. Experience at the Northern Israel Oncology Center (1979–2000). Oncology 64, 322–327, doi: 70288 (2003).
Alencar, A., Pitcher, D., Byrne, G. & Lossos, I. S. Primary bone lymphoma–the University of Miami experience. Leukemia & lymphoma 51, 39–49, doi: 10.3109/10428190903308007 (2010).
Beal, K., Allen, L. & Yahalom, J. Primary bone lymphoma: treatment results and prognostic factors with long-term follow-up of 82 patients. Cancer 106, 2652–2656, doi: 10.1002/cncr.21930 (2006).
Heyning, F. H. et al. Primary non-Hodgkin’s lymphoma of bone: a clinicopathological investigation of 60 cases. Leukemia 13, 2094–2098 (1999).
Zinzani, P. L. et al. Primary bone lymphoma: experience with 52 patients. Haematologica 88, 280–285 (2003).
Huang, J. J. et al. Clinical characterization and prognostic factors of primary lymphoma of bone in case of Chinese patients. Medical oncology 28 Suppl 1, S476–482, doi: 10.1007/s12032-010-9666-1 (2011).
Oken, M. M. et al. Toxicity and response criteria of the Eastern Cooperative Oncology Group. American journal of clinical oncology 5, 649–655 (1982).
Shipp, M. A. et al. A predictive model for aggressive non-Hodgkin’s lymphoma. The International Non-Hodgkin’s Lymphoma Prognostic Factors Project. The New England journal of medicine 329, 987–994, doi: 10.1056/NEJM199309303291402 (1993).
Carbone, P. P., Kaplan, H. S., Musshoff, K., Smithers, D. W. & Tubiana, M. Report of the Committee on Hodgkin’s Disease Staging Classification. Cancer research 31, 1860–1861 (1971).
Cheson, B. D. et al. Report of an international workshop to standardize response criteria for non-Hodgkin’s lymphomas. NCI Sponsored International Working Group. Journal of clinical oncology: official journal of the American Society of Clinical Oncology 17, 1244 (1999).
Cheson, B. D. et al. Revised response criteria for malignant lymphoma. Journal of clinical oncology: official journal of the American Society of Clinical Oncology 25, 579–586, doi: 10.1200/JCO.2006.09.2403 (2007).
Jawad, M. U. et al. Primary lymphoma of bone in adult patients. Cancer 116, 871–879, doi: 10.1002/cncr.24828 (2010).
Hsieh, P. P. et al. Primary non-Hodgkin’s lymphoma of bone: a rare disorder with high frequency of T-cell phenotype in southern Taiwan. Leukemia & lymphoma 47, 65–70, doi: 10.1080/10428190500272705 (2006).
Maruyama, D. et al. Primary bone lymphoma: a new and detailed characterization of 28 patients in a single-institution study. Japanese journal of clinical oncology 37, 216–223, doi: 10.1093/jjco/hym007 (2007).
Anderson, J. R., Armitage, J. O. & Weisenburger, D. D. Epidemiology of the non-Hodgkin’s lymphomas: distributions of the major subtypes differ by geographic locations. Non-Hodgkin’s Lymphoma Classification Project. Annals of oncology: official journal of the European Society for Medical Oncology / ESMO 9, 717–720 (1998).
Ueda, T. et al. Malignant lymphomas of bone in Japan. Cancer 64, 2387–2392 (1989).
Lewis, V. O. et al. Oncologic outcomes of primary lymphoma of bone in adults. Clinical orthopaedics and related research, 90–97, doi: 10.1097/01.blo.0000093901.12372.ad (2003).
Pellegrini, C. et al. Primary bone lymphoma: evaluation of chemoimmunotherapy as front-line treatment in 21 patients. Clinical lymphoma, myeloma & leukemia 11, 321–325, doi: 10.1016/j.clml.2011.03.021 (2011).
Heyning, F. H. et al. Primary lymphoma of bone: extranodal lymphoma with favourable survival independent of germinal centre, post-germinal centre or indeterminate phenotype. Journal of clinical pathology 62, 820–824, doi: 10.1136/jcp.2008.063156 (2009).
Clayton, F., Butler, J. J., Ayala, A. G., Ro, J. Y. & Zornoza, J. Non-Hodgkin’s lymphoma in bone. Pathologic and radiologic features with clinical correlates. Cancer 60, 2494–2501 (1987).
Catlett, J. P., Williams, S. A., O’Connor, S. C., Krishnan, J. & Malkovska, V. Primary lymphoma of bone: an institutional experience. Leukemia & lymphoma 49, 2125–2132, doi: 10.1080/10428190802404030 (2008).
Horsman, J. M., Thomas, J., Hough, R. & Hancock, B. W. Primary bone lymphoma: a retrospective analysis. International journal of oncology 28, 1571–1575 (2006).
Marshall, D. T., Amdur, R. J., Scarborough, M. T., Mendenhall, N. P. & Virkus, W. W. Stage IE primary non-Hodgkin’s lymphoma of bone. Clinical orthopaedics and related research, 216–222 (2002).
Liu, Y. C. et al. Prognostic factors and treatment efficacy in patients with primary diffuse large B-cell lymphoma of the bone: single institute experience over 11 years. Internal medicine 53, 95–101 (2014).
Fu, K. et al. Addition of rituximab to standard chemotherapy improves the survival of both the germinal center B-cell-like and non-germinal center B-cell-like subtypes of diffuse large B-cell lymphoma. Journal of clinical oncology: official journal of the American Society of Clinical Oncology 26, 4587–4594, doi: 10.1200/JCO.2007.15.9277 (2008).
Bhagavathi, S. et al. Primary bone diffuse large B-cell lymphoma: clinicopathologic study of 21 cases and review of literature. The American journal of surgical pathology 33, 1463–1469, doi: 10.1097/PAS.0b013e3181b314ce (2009).
Kim, S. Y. et al. Clinical characteristics and outcomes of primary bone lymphoma in Korea. The Korean journal of hematology 47, 213–218, doi: 10.5045/kjh.2012.47.3.213 (2012).
Acknowledgements
We appreciate the great help from Dr. Shaodong Hong who have provided statistical aids. We also thank the patients and their families in supporting the study.
Author information
Authors and Affiliations
Contributions
X.Y.Z. and J.Z. designed research; X.Y.Z. and J.Z. conducted research; Y.S., L.Y.P. and W.Z. analyzed data; and X.Y.Z., Y.S., L.Y.P, W.Z. and J.Z. wrote & revised the paper. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Zhang, X., Zhu, J., Song, Y. et al. Clinical characterization and outcome of primary bone lymphoma: a retrospective study of 61 Chinese patients. Sci Rep 6, 28834 (2016). https://doi.org/10.1038/srep28834
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep28834
This article is cited by
-
Primary lymphoma of the lumbar vertebrae: a case report and review of the literature
Journal of Medical Case Reports (2023)
-
Primary Bone Lymphoma: A 13 Year Retrospective Institutional Analysis in the Chemo-Immunotherapy Era
Indian Journal of Hematology and Blood Transfusion (2021)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.