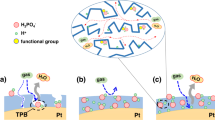
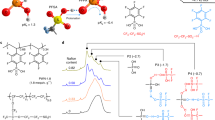
Abstract
Modern electrochemical energy conversion devices require more advanced proton conductors for their broad applications. Phosphonated polymers have been proposed as anhydrous proton conductors for fuel cells. However, the anhydride formation of phosphonic acid functional groups lowers proton conductivity and this prevents the use of phosphonated polymers in fuel cell applications. Here, we report a poly(2,3,5,6-tetrafluorostyrene-4-phosphonic acid) that does not undergo anhydride formation and thus maintains protonic conductivity above 200 °C. We use the phosphonated polymer in fuel cell electrodes with an ion-pair coordinated membrane in a membrane electrode assembly. This synergistically integrated fuel cell reached peak power densities of 1,130 mW cm−2 at 160 °C and 1,740 mW cm−2 at 240 °C under H2/O2 conditions, substantially outperforming polybenzimidazole- and metal phosphate-based fuel cells. Our result indicates a pathway towards using phosphonated polymers in high-performance fuel cells under hot and dry operating conditions.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
All the data represented in Figs. 1–5 and Extended Data Figs. 2–9 are provided with the paper as source data. All other data that support results in this Article are available from the corresponding author upon reasonable request. Source data are provided with this paper.
References
Choi, S. et al. Exceptional power density and stability at intermediate temperatures in protonic ceramic fuel cells. Nat. Energy 3, 202–210 (2018).
Chandan, A. et al. High temperature (HT) polymer electrolyte membrane fuel cells (PEMFC)—a review. J. Power Sources 231, 264–278 (2013).
Lee, K. S., Spendelow, J. S., Choe, Y. K., Fujimoto, C. & Kim, Y. S. An operationally flexible fuel cell based on quaternary ammonium-biphosphate ion pairs. Nat. Energy 1, 16120 (2016).
Wilson, M. S. & Gottesfeld, S. Thin-film catalyst layers for polymer electrolyte fuel cell electrode. J. Appl. Electrochem. 22, 1–7 (1992).
Wang, L. et al. An optimised synthesis of high performance radiation-grafted anion-exchange membranes. Green Chem. 19, 831–843 (2017).
Huang, G. et al. Composite poly(norbornene) anion conducting membranes for achieveing durability, water management and high power (3.4 W/cm2) in hydrogen/oxygen alkaline fuel cells. J. Electrochem. Soc. 166, F637 (2019).
Li, D. et al. Highly quaternized polystyrene ionomers for high performance anion exchange membrane water electrolysers. Nat. Energy 5, 378–385 (2020).
Liu, B. J. et al. Fluorinated poly(aryl ether) containing a 4-bromophenyl pendant group and its phosphonated derivative. Macromol. Rapid. Comm. 27, 1411–1417 (2006).
Parvole, J. & Jannasch, P. Polysulfones grafted with poly(vinylphosphonic acid) for highly proton conducting fuel cell membranes in the hydrated and nominally dry state. Macromolecules 41, 3893–3903 (2008).
Markova, D., Kumar, A., Klapper, M. & Mullen, K. Phosphonic acid-containing homo-, AB and BAB block copolymers via ATRP designed for fuel cell applications. Polymer 50, 3411–3421 (2009).
Abu-Thabit, N. Y., Ali, S. A. & Zaidi, S. M. J. New highly phosphonated polysulfone membranes for PEM fuel cells. J. Membr. Sci. 360, 26–33 (2010).
Atanasov, V. & Kerres, J. Highly phosphonated polypentafluorostyrene. Macromolecules 44, 6416–6423 (2011).
Tamura, Y., Sheng, L., Nakazawa, S., Higashihara, T. & Ueda, M. Polymer electrolyte membranes based on polystyrenes with phosphonic acid via long alkyl side chains. J. Polym. Sci. Pol. Chem. 50, 4334–4340 (2012).
Abouzari-Lotf, E., Ghassemi, H., Mehdipour-Ataei, S. & Shockravi, A. Phosphonated polyimides: enhancement of proton conductivity at high temperatures and low humidity. J. Membr. Sci. 516, 74–82 (2016).
Sun, J. et al. Morphology and proton transport in humidified phosphonated peptoid block copolymers. Macromolecules 49, 3083–3090 (2016).
Jang, S., Kim, S. Y., Jung, H. Y. & Park, M. J. Phosphonated polymers with fine-tuned ion clustering behavior: toward efficient proton conductors. Macromolecules 51, 1120–1128 (2018).
Date, B. et al. Synthesis and morphology study of SEBS Triblock copolymers functionalized with sulfonate and phosphonate groups for proton exchange membrane fuel cells. Macromolecules 51, 1020–1030 (2018).
Thompson, T. N. & Arnett, N. Y. Effect of phosphonated triazine monomer additive in disulfonated poly (arylene ether sulfone) composite membranes for proton exchange membrane fuel cells. Polymer 171, 34–44 (2019).
Schuster, M., Kreuer, K.-D., Steininger, H. & Maier, J. Proton conductivity and diffusion study of molten phosphonic acid H3PO3. Solid State Ion. 179, 523–528 (2008).
Schuster, M., Rager, T., Noda, A., Kreuer, K.-D. & Maier, J. About the choice of the protogenic group in PEM separator materials for intermediate temperature, low humidity operation: a critical comparison of sulfonic acid, phosphonic acid and imidazole functionalized model compounds. Fuel Cells 5, 355–365 (2005).
Bingöl, B., Meyer, W. H., Wagner, M. & Wegner, G. Synthesis, microstruture, and acidity of poly(vinylphosphonic acid). Macromol. Rapid Comm. 27, 1719–1724 (2006).
Rager, T., Schuster, M., Steininger, H. & Kreuer, K.-D. Poly(1,3-phenylene-5-phosphonic acid), a fully aromatic polyelectrolyte with high ion exchange capacity. Adv. Mater. 19, 3317–3321 (2007).
Vilciauskas, L., de Araujo, C. C. & Kreuer, K.-D. Proton conductivity and difffusion in molten phosphinic acid (H3PO2): the last member of the phosphorus oxoacid proton conductor family. Solid State Ion. 212, 6–9 (2012).
Melchior, J.-P., Majer, G. & Kreuer, K.-D. Why do proton conducting polybenzimidazole phosphoric acid membranes perform well in high-temperature PEM fuel cells? Phys. Chem. Chem. Phys. 19, 601 (2017).
Wu, Q. & Weiss, R. A. Synthesis and characterization of poly (styrene-co-vinyl phosphonate) ionomers. J. Polym. Sci. Pol. Phys. 42, 3628–3641 (2004).
Debe, M. K., Schmoeckel, A. K., Vernstrorn, G. D. & Atanasoski, R. High voltage stability of nanostructured thin film catalysts for PEM fuel cells. J. Power Sources 161, 1002–1011 (2006).
Kim, Y. S. et al. Origin of toughness in dispersion-cast Nafion membranes. Macromolecules 48, 2161–2172 (2015).
Frisch, M. J. et al. Gaussian 09 Revision C.01 (Gaussian, Inc., 2010).
Hochstrasser, R. M. & King, D. S. Theoretical calculations of the hydrolysis energies of some “high energy” molecules. I. The phosphoric and carboxylic anhydrides. J. Am. Chem. Soc. 97, 4762–4763 (1975).
Zhang, S., Baker, J. & Pulay, P. A reliable and efficient first principles-based method for predicting pKa values. 2. Organic acids. J. Phys. Chem. A 114, 432–442 (2010).
Atanasov, V., Oleynikov, A., Xia, J. B., Lyonnard, S. & Kerres, J. Phosphonic acid functionalized poly(pentafluorostyrene) as polyelectrolyte membrane for fuel cell application. J. Power Sources 343, 364–372 (2017).
Atanasov, V., Gudat, D., Ruffmann, B. & Kerres, J. Highly phosphonated polypentafluorostyrene: characterization and blends with polybenzimidazole. Eur. Polym. J. 49, 3977–3985 (2013).
Wilkie, C. A., Thomsen, J. R. & Mittleman, M. L. Interaction of poly (methyl-methacrylate) and Nafions. J. Appl. Polym. Sci. 42, 901–909 (1991).
Lee, A. S., Choe, Y. K., Matanovic, I. & Kim, Y. S. The energetics of phosphoric acid interactions reveals a new acid loss mechanism. J. Mater. Chem. A 7, 9867–9876 (2019).
Park, J. O. et al. Role of binders in high temperature PEMFC electrode. J. Electrochem. Soc. 158, B675–B681 (2011).
Su, H. N., Pasupathi, S., Bladergroen, B., Linkov, V. & Pollet, B. G. Optimization of gas diffusion electrode for polybenzimidazole-based high temperature proton exchange membrane fuel cell: evaluation of polymer binders in catalyst layer. Int. J. Hydrogen Energy 38, 11370–11378 (2013).
Venugopalan, G. et al. Stable and highly conductive polycation-polybenimidazole membrane blends for intermediate temperature polymer electrolyte membrane fuel cells. ACS Appl. Energy Mater. 3, 573–585 (2020).
Kerres, J. & Atanasov, V. Cross-linked PBI-based high temperature membranes: stability, conductivity and fuel cell performance. Int. J. Hydrogen Energy 40, 14723–14735 (2015).
De Castro, E. PBI-phosphoric acid based membrane electrode assemblies: status update. In MCFC and PAFC R&D Workshop Summary Report. https://www1.eere.energy.gov/hydrogenandfuelcells/pdfs/mcfc_pafc_workshop_summary.pdf (US Department of Energy, 2010).
Lee, K. S. et al. Intermediate temperature fuel cells via an ion-pair coordinated polymer electrolyte. Energy Environ. Sci. 11, 979–987 (2018).
Hibbs, M. R. Alkaline stability of poly(phenylene)-based anion exchange membranes with various cations. J. Polym. Sci. B 51, 1736–1742 (2013).
Park, E. J. et al. Alkaline stability of quaternized Diels-Alder polyphenylenes. Macromolecules 52, 5419–5428 (2019).
Frisch, M. J., Head-Gordon, M. & Pople, J. A. Direct MP2 gradient method. Chem. Phys. Lett. 166, 275–280 (1990).
Frisch, M. J., Head-Gordon, M. & Pople, J. A. Semi-direct algorithms for the MP2 energy and gradient. Chem. Phys. Lett. 166, 281–289 (1990).
Head-Gordon, M., Pople, J. A. & Frisch, M. J. MP2 energy evaluation by direct methods. Chem. Phys. Lett. 153, 503–506 (1988).
Saebø, S. & Almlöf, J. Avoiding the integral storage bottleneck in LCAO calculations of electron correlation. Chem. Phys. Lett. 154, 83–89 (1989).
Head-Gordon, M. & Head-Gordon, T. Analytic MP2 frequencies without fifth order storage: theory and application to bifurcated hydrogen bonds in the water hexamer. Chem. Phys. Lett. 220, 122–128 (1994).
Zhang, S. M., Baker, J. & Pulay, P. A reliable and efficient first principles-based method for predicting pKa values. 1. Methodology. J. Phys. Chem. A 114, 425–431 (2010).
Marenich, A. V., Cramer, C. J. & Truhlar, D. G. Universal solvation model based on solute electron density and on a continuum model of the solvent defined by the bulk dielectric constant and atomic surface tensions. J. Phys. Chem. B 113, 6378–6396 (2009).
Zhao, Y. & Truhlar, D. G. The M06 suite of density functionals for main group thermochemistry, thermochemical kinetics, noncovalent interactions, excited states, and transition elements: two new functionals and systematic testing of four M06-class functionals and 12 other functionals. Theor. Chem. Acc. 120, 215–241 (2008).
Acknowledgements
This work was supported by the US Department of Energy, Energy Efficiency and Renewable Energy, Hydrogen and Fuel Cell Technologies Office (HFTO) and the Advanced Research Project Agency-Energy (ARPA-E). This work was also partly supported from the Bundesministerium für Bildung und Forschung (BMBF) on account of the ‘HT-Linked’ project with Förderkennzeichen: 03SF0531C. Los Alamos National Laboratory is operated by Triad National Security, LLC under US Department of Energy contract number 89233218CNA000001. Sandia National Laboratories is a multi-programme laboratory operated by Sandia Corporation, a wholly owned subsidiary of Lockheed Martin Company, for the US Department of Energy’s National Nuclear Security Administration under contract DE-AC04-94AL85000. I.M. acknowledges the computational resources from the Tri-Lab computing resources of Los Alamos National Laboratory and University of New Mexico Center for Advanced Research Computing. We thank E. De Castro and C. Kreller for providing the commercial PA-PBI MEA and SnP2O7, respectively. We also thank A. Muenchinger and K.-D. Kreuer for providing PWN70 conductivity data and discussions. This paper describes objective technical results and analysis. Any subjective views or opinions that might be expressed in the paper do not necessarily represent the views of the US Department of Energy of the United States Government.
Author information
Authors and Affiliations
Contributions
Y.S.K. designed and directed the study. V.A. and J.K. prepared the phosphonated polymers. A.S.L. performed electrochemical measurements. E.J.P., E.D.B., C.F. and M.H. prepared the ion-pair polymers. A.S.L., V.A., E.J.P., S.M., E.D.B., C.F., M.H. and Y.S.K. characterized polymers. I.M. performed the first principles calculations. A.S.L. and Y.S.K. wrote the paper, with contributions from all co-authors.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Anhydride formation of phosphonic acid.
Gibbs free energy diagrams for the anhydride formation at 240 °C: phosphoric (black), methylphosphonic (orange), and pentafluorophenylphosphonic acid (light blue).
Extended Data Fig. 2 Property change of PWN70 during high-temperature treatment.
a, The conductivity change of PWN70 after exposure at 200 °C. The proton conductivity was measured in 5 wt.% DMSO solution at 80 °C. b, The solubility change of PWN70 after exposing at 240 °C. c, Electrochemical impedance analysis of PWN70 and PVPA MEAs at 160 °C. The impedance spectra were obtained at 0.8 V and a frequency range of 0.1 Hz to 1 MHz.
Extended Data Fig. 3 Proton conductivity of PWN70 as a function of temperature.
The conductivity was measured in an open system in ambient air (RH: ~35% at room temperature).
Extended Data Fig. 4 Thermal oxidative stability of PWN70 and polypentafluorostyrene.
a, TGA profiles (solid lines) and Gram Schmidt profiles (dashed lines) of PWN70 (black) and polypentafluorostyrene (red). b,c, FTIR spectra at selected temperatures for PWN70 (b) and for polypentafluorostyrene (c).
Extended Data Fig. 5 FTIR spectra of proton conductors at selected temperatures.
a, PWN70 (red), PA-QASOH (green), PA-QAPOH (black) and PA-PBI (blue) and b, TPP/Nafion composite as a function of temperature.
Extended Data Fig. 6 Electrochemical impedance analysis of MEAs at 160 °C as a function of partial water vapour pressure.
The impedance spectra were obtained at a frequency range of 0.1 Hz to 1 MHz.
Extended Data Fig. 7 Comparison of H2/O2 fuel cell performance between Nafion-based LT-PEMFC and PWN70-based HT-PEMFC.
Nafion LT-PEMFC: cell temperature: 80 °C, membrane: Nafion 211 (25 μm thickness), Anode: Pt/C (0.6 mgPt cm−2), Cathode: Pt/C (0.6 mgPt cm−2), absolute backpressure varied from 78 to 285 kPa; PWN70-based HT-PEMFC: cell temperature: 240 °C, membrane: QAPOH−PA ion pair (40 μm thickness), Anode: PtRu/C (0.5 mgPt cm−2), Cathode: Pt/C (0.6 mgPt cm−2), absolute backpressure of 147 kPa.
Extended Data Fig. 8 H2/air fuel cell performance of MEA4.
The performance measured at 160, 200 and 240 °C under backpressure of 147 kPa. Membrane: QAPOH-PA ion pair (40 μm thickness), Ionomeric binder: PWN70, Anode: PtRu/C (0.5 mgPt cm−2), Cathode: Pt/C (0.6 mgPt cm−2).
Extended Data Fig. 9 Structural characterization of polymers.
a, 1H NMR spectrum of QASOH in DMSO-d6. b, 19F NMR spectrum of PWN70 in DMSO-d6, RT. The phosphonation degree calculated from 19F NMR: 66–69 mol% and the phosphonation degree calculated from titration: 50 mol% (equivalent IEC = 2.21 mequiv. g-1). 1H-NMR (400 MHz, DMSO-d6, ppm) δ = 8.36 (s, H), 4.33 (s, H), 3.77 (m, H), 2.94 (s, H), 2.78 (s, H), 1.95 (s, H), 1.02 (s, H) 19F-NMR (250 MHz, DMSO-d6, ppm) δ = −133.10 (bp 2 F), −143.14 (bp, 2 F). 31P-NMR (101.2 MHz, DMSO-d6, ppm) δ = -1.09 (bp,1 P). c, GPC profile of PWN70. Eluent: water, standard: PSSNa, detector: Shodex RI 101. Mn 97 kg mol−1, Mw 136 kg mol−1, PDI 1.40.
Extended Data Fig. 10 Conductivity measurement cell.
a, Liquid cell. b, Window cells for polymer film. Left: window opening: 2 cm; Right: window opening 0.5 cm.
Source data
Source Data Fig. 1
Numerical data for graphs
Source Data Fig. 2
Numerical data for graphs
Source Data Fig. 3
Numerical data for graphs
Source Data Fig. 4
Numerical data for graphs
Source Data Fig. 5
Numerical data for graphs
Source Data Extended Data Fig. 1
Molecular structure and DFT calculation data
Source Data Extended Data Fig. 2
Numerical data for graphs
Source Data Extended Data Fig. 3
Numerical data for graphs
Source Data Extended Data Fig. 4
Numerical data for graphs
Source Data Extended Data Fig. 5
Numerical data for graphs
Source Data Extended Data Fig. 6
Numerical data for graphs
Source Data Extended Data Fig. 7
Numerical data for graphs
Source Data Extended Data Fig. 8
Numerical data for graphs
Source Data Extended Data Fig. 9
Numerical data for graphs
Source Data Extended Data Fig. 10
Conductivity cell picture
Rights and permissions
About this article
Cite this article
Atanasov, V., Lee, A.S., Park, E.J. et al. Synergistically integrated phosphonated poly(pentafluorostyrene) for fuel cells. Nat. Mater. 20, 370–377 (2021). https://doi.org/10.1038/s41563-020-00841-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41563-020-00841-z
This article is cited by
-
Overcoming the Electrode Challenges of High-Temperature Proton Exchange Membrane Fuel Cells
Electrochemical Energy Reviews (2023)
-
Development and Challenges of Electrode Ionomers Used in the Catalyst Layer of Proton-Exchange Membrane Fuel Cells: A Review
Transactions of Tianjin University (2023)
-
Protonated phosphonic acid electrodes for high power heavy-duty vehicle fuel cells
Nature Energy (2022)
-
Fuel cells with an operational range of –20 °C to 200 °C enabled by phosphoric acid-doped intrinsically ultramicroporous membranes
Nature Energy (2022)
-
Low Pt loading for high-performance fuel cell electrodes enabled by hydrogen-bonding microporous polymer binders
Nature Communications (2022)