Key Points
-
Advances in CRISPR technology have provided the capacity to precisely identify and define the function of genes and noncoding regulatory elements associated with disease development and susceptibility
-
CRISPR technology has made the generation of mouse models of disease much quicker and less expensive than traditional approaches, and has facilitated the development of much-needed larger animal models of disease
-
CRISPR technology has enabled the generation of gene drives, whereby genetic changes propagate rapidly through a species, providing the potential to eliminate disease vectors and thus vector-borne diseases such as malaria
-
Successful treatment of mouse models of human diseases suggests that CRISPR technology can be applied to treat human diseases in the future
-
CRISPR technology has the ability to facilitate a breakthrough in our understanding of the more common and complex human diseases, including rheumatic diseases
-
The potential of CRISPR/Cas9 technology in the development of new treatment strategies is confidently expected to have a major effect on the practice of rheumatology
Abstract
CRISPR/Cas9 genome editing technology has taken the research world by storm since its use in eukaryotes was first proposed in 2012. Publications describing advances in technology and new applications have continued at an unrelenting pace since that time. In this Review, we discuss the application of CRISPR/Cas9 for creating gene mutations — the application that initiated the current avalanche of interest — and new developments that have largely answered initial concerns about its specificity and ability to introduce new gene sequences. We discuss the new, diverse and rapidly growing adaptations of the CRISPR/Cas9 technique that enable activation, repression, multiplexing and gene screening. These developments have enabled researchers to create sophisticated tools for dissecting the function and inter-relatedness of genes, as well as noncoding regions of the genome, and to identify gene networks and noncoding regions that promote disease or confer disease susceptibility. These approaches are beginning to be used to interrogate complex and multilayered biological systems and to produce complex animal models of disease. CRISPR/Cas9 technology has enabled the application of new therapeutic approaches to treating disease in animal models, some of which are beginning to be seen in the first human clinical trials. We discuss the direct application of these techniques to rheumatic diseases, which are currently limited but are sure to increase rapidly in the near future.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Jinek, M. et al. A programmable dual-RNA-guided DNA endonuclease in adaptive bacterial immunity. Science 337, 816–821 (2012).
Mali, P. et al. RNA-guided human genome engineering via Cas9. Science 339, 823–826 (2013).
Cong, L. et al. Multiplex genome engineering using CRISPR/Cas systems. Science 339, 819–823 (2013).
Cho, S. W., Kim, S., Kim, J. M. & Kim, J. S. Targeted genome engineering in human cells with the Cas9 RNA-guided endonuclease. Nat. Biotechnol. 31, 230–232 (2013).
Hwang, W. Y. et al. Efficient genome editing in zebrafish using a CRISPR-Cas system. Nat. Biotechnol. 31, 227–229 (2013).
Jiang, W., Bikard, D., Cox, D., Zhang, F. & Marraffini, L. A. RNA-guided editing of bacterial genomes using CRISPR-Cas systems. Nat. Biotechnol. 31, 233–239 (2013).
Hsu, P. D. et al. DNA targeting specificity of RNA-guided Cas9 nucleases. Nat. Biotechnol. 31, 827–832 (2013).
Gasiunas, G., Barrangou, R., Horvath, P. & Siksnys, V. Cas9-crRNA ribonucleoprotein complex mediates specific DNA cleavage for adaptive immunity in bacteria. Proc. Natl Acad. Sci. USA 109, E2579–E2586 (2012).
Ishino, Y., Shinagawa, H., Makino, K., Amemura, M. & Nakata, A. Nucleotide sequence of the iap gene, responsible for alkaline phosphatase isozyme conversion in Escherichia coli, and identification of the gene product. J. Bacteriol. 169, 5429–5433 (1987).
Jansen, R., Embden, J. D., Gaastra, W. & Schouls, L. M. Identification of genes that are associated with DNA repeats in prokaryotes. Mol. Microbiol. 43, 1565–1575 (2002).
Sorek, R., Lawrence, C. M. & Wiedenheft, B. CRISPR-mediated adaptive immune systems in bacteria and archaea. Annu. Rev. Biochem. 82, 237–266 (2013).
Mojica, F. J., Diez-Villasenor, C., Garcia-Martinez, J. & Soria, E. Intervening sequences of regularly spaced prokaryotic repeats derive from foreign genetic elements. J. Mol. Evol. 60, 174–182 (2005).
Marraffini, L. A. CRISPR-Cas immunity in prokaryotes. Nature 526, 55–61 (2015).
Eberl, G. Immunology: close encounters of the second type. Nature 464, 1285–1286 (2010).
Makarova, K. S. et al. An updated evolutionary classification of CRISPR-Cas systems. Nat. Rev. Microbiol. 13, 722–736 (2015).
Kim, D. et al. Digenome-seq: genome-wide profiling of CRISPR-Cas9 off-target effects in human cells. Nat. Methods 12, 237–243 (2015).
Doench, J. G. et al. Optimized sgRNA design to maximize activity and minimize off-target effects of CRISPR-Cas9. Nat. Biotechnol. 34, 184–191 (2016).
Fu, Y., Sander, J. D., Reyon, D., Cascio, V. M. & Joung, J. K. Improving CRISPR-Cas nuclease specificity using truncated guide RNAs. Nat. Biotechnol. 32, 279–284 (2014).
Ran, F. A. et al. Double nicking by RNA-guided CRISPR Cas9 for enhanced genome editing specificity. Cell 154, 1380–1389 (2013).
Kleinstiver, B. P. et al. High-fidelity CRISPR-Cas9 nucleases with no detectable genome-wide off-target effects. Nature 529, 490–495 (2016).
Slaymaker, I. M. et al. Rationally engineered Cas9 nucleases with improved specificity. Science 351, 84–88 (2016).
Gori, J. L. et al. Delivery and specificity of CRISPR-Cas9 genome editing technologies for human gene therapy. Hum. Gene Ther. 26, 443–451 (2015).
Lieber, M. R. The mechanism of double-strand DNA break repair by the nonhomologous DNA end-joining pathway. Annu. Rev. Biochem. 79, 181–211 (2010).
Hudson, D. F., Morrison, C., Ruchaud, S. & Earnshaw, W. C. Reverse genetics of essential genes in tissue-culture cells: 'dead cells talking'. Trends Cell Biol. 12, 281–287 (2002).
Rouet, P., Smih, F. & Jasin, M. Expression of a site-specific endonuclease stimulates homologous recombination in mammalian cells. Proc. Natl Acad. Sci. USA 91, 6064–6068 (1994).
Chu, V. T. et al. Increasing the efficiency of homology-directed repair for CRISPR-Cas9-induced precise gene editing in mammalian cells. Nat. Biotechnol. 33, 543–548 (2015).
Wang, B. et al. Highly efficient CRISPR/HDR-mediated knock-in for mouse embryonic stem cells and zygotes. Biotechniques 59, 201–208 (2015).
He, X. et al. Knock-in of large reporter genes in human cells via CRISPR/Cas9-induced homology-dependent and independent DNA repair. Nucleic Acids Res. 44, e85 (2016).
Richardson, C. D., Ray, G. J., DeWitt, M. A., Curie, G. L. & Corn, J. E. Enhancing homology-directed genome editing by catalytically active and inactive CRISPR-Cas9 using asymmetric donor DNA. Nat. Biotechnol. 34, 339–344 (2016).
Gilbert, L. A. et al. CRISPR-mediated modular RNA-guided regulation of transcription in eukaryotes. Cell 154, 442–451 (2013).
Dominguez, A. A., Lim, W. A. & Qi, L. S. Beyond editing: repurposing CRISPR-Cas9 for precision genome regulation and interrogation. Nat. Rev. Mol. Cell Biol. 17, 5–15 (2016).
Kiani, S. et al. Cas9 gRNA engineering for genome editing, activation and repression. Nat. Methods 12, 1051–1054 (2015).
Chavez, A. et al. Comparison of Cas9 activators in multiple species. Nat. Methods 13, 563–567 (2016).
Konermann, S. et al. Genome-scale transcriptional activation by an engineered CRISPR-Cas9 complex. Nature 517, 583–588 (2015).
Gilbert, L. A. et al. Genome-scale CRISPR-mediated control of gene repression and activation. Cell 159, 647–661 (2014).
Kearns, N. A. et al. Functional annotation of native enhancers with a Cas9-histone demethylase fusion. Nat. Methods 12, 401–403 (2015).
Hilton, I. B. et al. Epigenome editing by a CRISPR-Cas9-based acetyltransferase activates genes from promoters and enhancers. Nat. Biotechnol. 33, 510–517 (2015).
Thakore, P. I. et al. Highly specific epigenome editing by CRISPR-Cas9 repressors for silencing of distal regulatory elements. Nat. Methods 12, 1143–1149 (2015).
Yang, M., Zhang, L., Stevens, J. & Gibson, G. CRISPR/Cas9 mediated generation of stable chondrocyte cell lines with targeted gene knockouts; analysis of an aggrecan knockout cell line. Bone 69, 118–125 (2014).
Huang, Y., Askew, E. B., Knudson, C. B. & Knudson, W. CRISPR/Cas9 knockout of HAS2 in rat chondrosarcoma chondrocytes demonstrates the requirement of hyaluronan for aggrecan retention. Matrix Biol. 56, 74–94 (2016).
Dubail, J. & Apte, S. S. Insights on ADAMTS proteases and ADAMTS-like proteins from mammalian genetics. Matrix Biol. 44–46, 24–37 (2015).
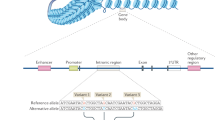
Tak, Y. G. & Farnham, P. J. Making sense of GWAS: using epigenomics and genome engineering to understand the functional relevance of SNPs in non-coding regions of the human genome. Epigenetics Chromatin 8, 57 (2015).
Yao, L., Berman, B. P. & Farnham, P. J. Demystifying the secret mission of enhancers: linking distal regulatory elements to target genes. Crit. Rev. Biochem. Mol. Biol. 50, 550–573 (2015).
Diao, Y. et al. A new class of temporarily phenotypic enhancers identified by CRISPR/Cas9-mediated genetic screening. Genome Res. 26, 397–405 (2016).
Meyer, M. B., Benkusky, N. A. & Pike, J. W. Selective distal enhancer control of the Mmp13 gene identified through clustered regularly interspaced short palindromic repeat (CRISPR) genomic deletions. J. Biol. Chem. 290, 11093–11107 (2015).
Soldner, F. et al. Parkinson-associated risk variant in distal enhancer of alpha-synuclein modulates target gene expression. Nature 533, 95–99 (2016).
Chavez, A. et al. Precise Cas9 targeting enables genomic mutation prevention. Preprint at bioRxiv http://dx.doi.org/10.1101/058974 (2016).
Kirino, Y. & Remmers, E. F. Genetic architectures of seropositive and seronegative rheumatic diseases. Nat. Rev. Rheumatol. 11, 401–414 (2015).
Birney, E. & Soranzo, N. Human genomics: the end of the start for population sequencing. Nature 526, 52–53 (2015).
Wang, T., Wei, J. J., Sabatini, D. M. & Lander, E. S. Genetic screens in human cells using the CRISPR-Cas9 system. Science 343, 80–84 (2014).
Shalem, O. et al. Genome-scale CRISPR-Cas9 knockout screening in human cells. Science 343, 84–87 (2014).
Zhou, Y. et al. High-throughput screening of a CRISPR/Cas9 library for functional genomics in human cells. Nature 509, 487–491 (2014).
Zhang, R. et al. A CRISPR screen defines a signal peptide processing pathway required by flaviviruses. Nature 535, 164–168 (2016).
Marceau, C. D. et al. Genetic dissection of Flaviviridae host factors through genome-scale CRISPR screens. Nature 535, 159–163 (2016).
Parnas, O. et al. A genome-wide CRISPR screen in primary immune cells to dissect regulatory networks. Cell 162, 675–686 (2015).
Schmid-Burgk, J. L. et al. A genome-wide CRISPR (clustered regularly interspaced short palindromic repeats) screen identifies NEK7 as an essential component of NLRP3 inflammasome activation. J. Biol. Chem. 291, 103–109 (2016).
Shi, J. et al. Cleavage of GSDMD by inflammatory caspases determines pyroptotic cell death. Nature 526, 660–665 (2015).
Reeves, W. H. Editorial: systemic lupus erythematosus: death by fire and ICE? Arthritis Rheumatol. 66, 6–9 (2014).
So, A., Ives, A., Joosten, L. A. & Busso, N. Targeting inflammasomes in rheumatic diseases. Nat. Rev. Rheumatol. 9, 391–399 (2013).
Saavedra, P. H., Demon, D., Van Gorp, H. & Lamkanfi, M. Protective and detrimental roles of inflammasomes in disease. Semin. Immunopathol. 37, 313–322 (2015).
Gaidt, M. M. et al. Human monocytes engage an alternative inflammasome pathway. Immunity 44, 833–846 (2016).
Parikh, B. A., Beckman, D. L., Patel, S. J., White, J. M. & Yokoyama, W. M. Detailed phenotypic and molecular analyses of genetically modified mice generated by CRISPR-Cas9-mediated editing. PLoS ONE 10, e0116484 (2015).
Henao-Mejia, J. et al. Generation of genetically modified mice using the CRISPR-Cas9 genome-editing system. Cold Spring Harb. Protoc. http://dx.doi.org/10.1101/pdb.prot090704 (2016).
Lupianez, D. G. et al. Disruptions of topological chromatin domains cause pathogenic rewiring of gene-enhancer interactions. Cell 161, 1012–1025 (2015).
Wang, H. et al. One-step generation of mice carrying mutations in multiple genes by CRISPR/Cas-mediated genome engineering. Cell 153, 910–918 (2013).
Bono, J. M., Olesnicky, E. C. & Matzkin, L. M. Connecting genotypes, phenotypes and fitness: harnessing the power of CRISPR/Cas9 genome editing. Mol. Ecol. 24, 3810–3822 (2015).
Carroll, K. J. et al. A mouse model for adult cardiac-specific gene deletion with CRISPR/Cas9. Proc. Natl Acad. Sci. USA 113, 338–343 (2016).
Reardon, S. Welcome to the CRISPR zoo. Nature 531, 160–163 (2016).
Yang, L. et al. Genome-wide inactivation of porcine endogenous retroviruses (PERVs). Science 350, 1101–1104 (2015).
Zhou, X. et al. Generation of CRISPR/Cas9-mediated gene-targeted pigs via somatic cell nuclear transfer. Cell. Mol. Life Sci. 72, 1175–1184 (2015).
Wu, M. et al. Rosa26-targeted sheep gene knock-in via CRISPR-Cas9 system. Sci. Rep. 6, 24360 (2016).
Wang, Z. Genome engineering in cattle: recent technological advancements. Chromosome Res. 23, 17–29 (2015).
Ni, W. et al. Efficient gene knockout in goats using CRISPR/Cas9 system. PLoS ONE 9, e106718 (2014).
Whitelaw, C. B., Sheets, T. P., Lillico, S. G. & Telugu, B. P. Engineering large animal models of human disease. J. Pathol. 238, 247–256 (2016).
Tu, Z., Yang, W., Yan, S., Guo, X. & Li, X. J. CRISPR/Cas9: a powerful genetic engineering tool for establishing large animal models of neurodegenerative diseases. Mol. Neurodegener. 10, 35 (2015).
Yokoyama, S. et al. A systems approach reveals that the myogenesis genome network is regulated by the transcriptional repressor RP58. Dev. Cell 17, 836–848 (2009).
Shimizu, H. et al. The AERO system: a 3D-like approach for recording gene expression patterns in the whole mouse embryo. PLoS ONE 8, e75754 (2013).
Suzuki, H. et al. Gene targeting of the transcription factor Mohawk in rats causes heterotopic ossification of Achilles tendon via failed tenogenesis. Proc. Natl Acad. Sci. USA 113, 7840–7845 (2016).
Esvelt, K. M., Smidler, A. L., Catteruccia, F. & Church, G. M. Concerning RNA-guided gene drives for the alteration of wild populations. eLife 3, e03401 (2014).
Champer, J., Buchman, A. & Akbari, O. S. Cheating evolution: engineering gene drives to manipulate the fate of wild populations. Nat. Rev. Genet. 17, 146–159 (2016).
Basu, S. et al. Silencing of end-joining repair for efficient site-specific gene insertion after TALEN/CRISPR mutagenesis in Aedes aegypti. Proc. Natl Acad. Sci. USA 112, 4038–4043 (2015).
Gantz, V. M. et al. Highly efficient Cas9-mediated gene drive for population modification of the malaria vector mosquito Anopheles stephensi. Proc. Natl Acad. Sci. USA 112, E6736–E6743 (2015).
Gantz, V. M. & Bier, E. Genome editing. The mutagenic chain reaction: a method for converting heterozygous to homozygous mutations. Science 348, 442–444 (2015).
Hammond, A. et al. A CRISPR-Cas9 gene drive system targeting female reproduction in the malaria mosquito vector Anopheles gambiae. Nat. Biotechnol. 34, 78–83 (2016).
Akbari, O. S. et al. BIOSAFETY. Safeguarding gene drive experiments in the laboratory. Science 349, 927–929 (2015).
National Academies of Sciences, Engineering, and Medicine. Gene Drives on the Horizon: Advancing Science, Navigating Uncertainty, and Aligning Research with Public Values (National Academies Press, 2016).
Reardon, S. First CRISPR clinical trial gets green light from US panel. Nature http://www.nature.com/news/first-crispr-clinical-trial-gets-green-light-from-us-panel-1.20137 (2016).
Cyranoski, D. Chinese scientists to pioneer first human CRISPR trial. Nature 535, 476–477 (2016).
Ren, J. et al. Multiplex Cripsr/Cas9 genome editing to generate potent universal CART and PD1-deficient cells against leukemia. Blood 126, 4280–4280 (2015).
Tabebordbar, M. et al. In vivo gene editing in dystrophic mouse muscle and muscle stem cells. Science 351, 407–411 (2016).
Nelson, C. E. et al. In vivo genome editing improves muscle function in a mouse model of Duchenne muscular dystrophy. Science 351, 403–407 (2016).
Long, C. et al. Postnatal genome editing partially restores dystrophin expression in a mouse model of muscular dystrophy. Science 351, 400–403 (2016).
Juliano, R. L. The delivery of therapeutic oligonucleotides. Nucleic Acids Res. 44, 6518–6548 (2016).
Li, L., He, Z. Y., Wei, X. W., Gao, G. P. & Wei, Y. Q. Challenges in CRISPR/CAS9 delivery: potential roles of nonviral vectors. Hum. Gene Ther. 26, 452–462 (2015).
Ran, F. A. et al. In vivo genome editing using Staphylococcus aureus Cas9. Nature 520, 186–191 (2015).
Yin, H. et al. Therapeutic genome editing by combined viral and non-viral delivery of CRISPR system components in vivo. Nat. Biotechnol. 34, 328–333 (2016).
Yan, H. et al. Suppression of NF-kappaB activity via nanoparticle-based siRNA delivery alters early cartilage responses to injury. Proc. Natl Acad. Sci. USA 113, E6199–E6208 (2016).
Liang, X. et al. Rapid and highly efficient mammalian cell engineering via Cas9 protein transfection. J. Biotechnol. 208, 44–53 (2015).
Wang, S., Wiley, G. B., Kelly, J. A. & Gaffney, P. M. Disease mechanisms in rheumatology — tools and pathways: defining functional genetic variants in autoimmune diseases. Arthritis Rheumatol. 67, 1–10 (2015).
Roederer, M. et al. The genetic architecture of the human immune system: a bioresource for autoimmunity and disease pathogenesis. Cell 161, 387–403 (2015).
Cotsapas, C. & Hafler, D. A. Immune-mediated disease genetics: the shared basis of pathogenesis. Trends Immunol. 34, 22–26 (2013).
Yang, Y. et al. A dual AAV system enables the Cas9-mediated correction of a metabolic liver disease in newborn mice. Nat. Biotechnol. 34, 334–338 (2016).
Bassuk, A. G., Zheng, A., Li, Y., Tsang, S. H. & Mahajan, V. B. Precision medicine: genetic repair of retinitis pigmentosa in patient-derived stem cells. Sci. Rep. 6, 19969 (2016).
Shinkuma, S., Guo, Z. & Christiano, A. M. Site-specific genome editing for correction of induced pluripotent stem cells derived from dominant dystrophic epidermolysis bullosa. Proc. Natl Acad. Sci. USA 113, 5676–5681 (2016).
Osborn, M. J., Belanto, J. J., Tolar, J. & Voytas, D. F. Gene editing and its application for hematological diseases. Int. J. Hematol. 104, 18–28 (2016).
Zhen, S. et al. Harnessing the clustered regularly interspaced short palindromic repeat (CRISPR)/CRISPR-associated Cas9 system to disrupt the hepatitis B virus. Gene Ther. 22, 404–412 (2015).
DeWitt, M. A. et al. Selection-free genome editing of the sickle mutation in human adult hematopoietic stem/progenitor cells. Sci. Transl Med. 8, 360ra134 (2016).
Xie, F. et al. Seamless gene correction of beta-thalassemia mutations in patient-specific iPSCs using CRISPR/Cas9 and piggyBac. Genome Res. 24, 1526–1533 (2014).
Firth, A. L. et al. Functional gene correction for cystic fibrosis in lung epithelial cells generated from patient iPSCs. Cell Rep. 12, 1385–1390 (2015).
Mandal, P. K. et al. Efficient ablation of genes in human hematopoietic stem and effector cells using CRISPR/Cas9. Cell Stem Cell 15, 643–652 (2014).
Author information
Authors and Affiliations
Contributions
G.J.G. contributed to researching data for the article, writing, and reviewing and editing the manuscript before submission. M.Y. contributed to discussion of content and writing the article.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Glossary
- CRISPR
-
Segments of prokaryotic DNA containing short repetitions of DNA sequences, which are interrupted by so-called spacer DNA derived from past invaders. CRISPR serves as the bacterial adaptive immune system that protects against invading genetic materials.
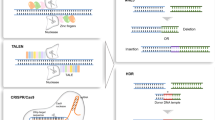
- CRISPR/Cas9
-
RNA-guided gene editing platform based on the Cas9–gRNA ribonucleoprotein complex. The two-component complex can mediate gRNA-programmed recognition of specific DNA sequences and create a site-specific double-strand cleavage of the targeted DNA.
- Cas9
-
An RNA-guided DNA endonuclease enzyme that is the universal component of the RNA-guided CRISPR/Cas9 gene editing machinery. Cas9 by itself is inactive; upon binding to the gRNA scaffold, Cas9 goes through conformational changes that initiate its target recognition, binding and cleavage activity.
- Spacer
-
The spacer sequence refers to the 5′ end, ∼20 nucleotide variable sequence of the targeting gRNA construct. The spacer contains a targeting sequence that matches a region of DNA substrate and guides Cas9 nuclease activity.
- Protospacer
-
The protospacer sequence refers to the targeted site on the DNA substrate. The nucleotide sequences of the spacer and the corresponding protospacer are identical.
- Protospacer adjacent motif (PAM)
-
A three base pair DNA sequence immediately following the protospacer or the DNA sequence targeted by the Cas9/gRNA ribonuclease. The canonical PAM sequence for CRISPR/Cas9 gene editing machinery is 5′-NGG-3′.
- Guide RNA (gRNA)
-
gRNA, also known as short guide RNA (sgRNA) is a short synthetic RNA sequence consisting of a scaffold structure and a programmable ∼20 nucleotide spacer at the 5′ end. The ∼80 nucelotide RNA scaffold structure is essential for mediating both Cas9 protein binding and activation. The unique spacer sequence dictates the DNA target site to be recognized and cleaved by Cas9 protein.
- Non-homologous end joining (NHEJ)
-
A cellular pathway that repairs double-strand breaks in DNA. NHEJ is active throughout the cell cycle and requires no repair template. NHEJ is frequently imprecise and the repair process can generate an open reading frame shift with insertions, deletions or mutations at the site of double-strand breaks. The inaccurate nature of the NHEJ repair process forms the basis of the CRISPR/Cas knockout strategy.
- Homology-directed repair (HDR)
-
The HDR pathway (also known as homologous recombination), involving a homologous template (either a sister chromatid or an exogenous DNA template), repairs double-strand DNA breaks accurately according to the template. The template or donor DNA consists of left and right arms identical to sequences flanking the double-strand break. Between the arms, any DNA sequence or marker can be inserted and HDR will force the additional genetic material to be knocked in to the particular locus. HDR is usually believed to be active only during S and G2 phases of the cell cycle.
- CRISPR/Cas9 genomic screen
-
CRISPR/Cas9-mediated genome-wide knockout screen system. In this platform, a gRNA library targeting most genes in the genome with multiple sites per gene is cloned into lentiviral vectors and delivered as a pool into target cells that express Cas9. A low multiplicity of infection is used to ensure that each cell will receive no more than one gRNA or viral particle. By proper phenotype selection, gRNAs that are enriched or depleted in cells are determined and, correspondingly, genes that are required for that particular phenotype can be systematically identified.
- Gene drives
-
Gene drives are genetic manipulations that enable a gene to force its inheritance to all, rather than half, of its offspring.
Rights and permissions
About this article
Cite this article
Gibson, G., Yang, M. What rheumatologists need to know about CRISPR/Cas9. Nat Rev Rheumatol 13, 205–216 (2017). https://doi.org/10.1038/nrrheum.2017.6
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrrheum.2017.6
This article is cited by
-
Harnessing the potential of mutation breeding, CRISPR genome editing, and beyond for sustainable agriculture
Functional & Integrative Genomics (2024)
-
Exosomes for CRISPR-Cas9 Delivery: The Cutting Edge in Genome Editing
Molecular Biotechnology (2023)
-
Genome editing to define the function of risk loci and variants in rheumatic disease
Nature Reviews Rheumatology (2021)
-
Therapeutic applications of CRISPR/Cas9 system in gene therapy
Biotechnology Letters (2018)