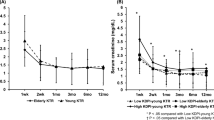
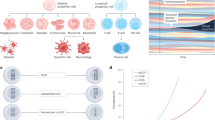
Abstract
The world population is rapidly growing and ageing at a pace that is projected to continue for at least three decades. This shift towards an older populace has invariably increased the number of individuals with diseases related to ageing, such as chronic kidney disease. The increase in chronic kidney disease is associated with a growing number of elderly patients receiving kidney transplants. Understanding how the immune system changes with increasing age will help to define the risks of rejection and infection in the elderly population and will focus attention on the need for individualized immunosuppression for patients in this age group. This Review addresses what is currently known about ageing and the immune system, highlighting age-related changes that affect the outcome of transplantation in elderly individuals. The need for new strategies to improve outcomes in this growing population of elderly renal transplant recipients is also emphasized.
Key Points
-
In the USA, the incident rate of end-stage renal disease is highest in patients aged >65 years and the ageing population is likely to result in more elderly renal transplant recipients
-
Although elderly renal transplant recipients are at increased risk of late mortality owing to comorbidities associated with ageing, early patient and graft survival are comparable to those of younger patients
-
Understanding how the immune system changes with increasing age might help clinicians to consider a more individualized immunosuppressive approach to the elderly transplant recipient to avoid inappropriate immunosuppression
-
Ageing causes thymic involution, narrowing of the T-cell repertoire, cellular senescence and reduced costimulatory signals, resulting in defects in T-cell-mediated immunity, which has an important role in allograft rejection
-
Advanced age is associated with alterations in the differentiation and function of haematopoietic stem cells, which has implications for the upper age limit of bone marrow transplant donors
-
Prospective studies of immunological function in elderly transplant recipients are needed before we can advise with certainty on the best therapeutic approach to immunosuppression in these patients
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
United States Census Bureau. World Population Ageing: 1950–2050. http://www.un.org/esa/population/publications/worldageing19502050/ (2002).
Coresh, J., Astor, B. C., Greene, T., Eknoyan, G. & Levey, A. S. Prevalence of chronic kidney disease and decreased kidney function in the adult US population: Third National Health and Nutrition Examination Survey. Am. J. Kidney Dis. 41, 1–12 (2003).
US Department of Health and Human Services. OPTN Organ Transplant and Procurement Network [online], (2012).
Danovitch, G. M. et al. Current status of kidney and pancreas transplantation in the United States, 1994–2003. Am. J. Transplant. 5, 904–915 (2005).
Wolfe, R. A. et al. Comparison of mortality in all patients on dialysis, patients on dialysis awaiting transplantation, and recipients of a first cadaveric transplant. N. Engl. J. Med. 341, 1725–1730 (1999).
Johnson, D. W. et al. A comparison of the effects of dialysis and renal transplantation on the survival of older uremic patients. Transplantation 69, 794–799 (2000).
Oniscu, G. C., Brown, H. & Forsythe, J. L. How great is the survival advantage of transplantation over dialysis in elderly patients? Nephrol. Dial. Transplant. 19, 945–951 (2004).
Giessing, M. et al. “Old-for-old” cadaveric renal transplantation: surgical findings, perioperative complications and outcome. Eur. Urol. 44, 701–708 (2003).
Rao, P. S. et al. Renal transplantation in elderly patients older than 70 years of age: results from the Scientific Registry of Transplant Recipients. Transplantation 83, 1069–1074 (2007).
Eufrásio, P. et al. Renal transplantation in recipients over 65 years old. Transplant. Proc. 43, 117–119 (2011).
Jacobsen, P. A. et al. Lower calcineurin inhibitor doses in older compared to younger kidney transplant recipients yield similar troughs. Am. J. Transplant. http://dx.doi.org/10.1111/j.1600-61432012.04232.x.
Tullius, S. G. & Milford, E. Kidney allocation and the aging immune response. N. Engl. J. Med. 364, 1369–1370 (2011).
Tullius, S. G. et al. The combination of donor and recipient age is critical in determining host immunoresponsiveness and renal transplant outcome. Ann. Surg. 252, 662–674 (2010).
Cecka, J. M. The OPTN/UNOS renal transplant registry. Clin. Transpl. 2004, 1–16 (2004).
Frei, U. et al. Prospective age-matching in elderly kidney transplant recipients—a 5-year analysis of the Eurotransplant Senior Program. Am. J. Transplant. 8, 50–57 (2008).
Pratschke, J. et al. Potent early immune response after kidney transplantation in patients of the European senior transplant program. Transplantation 87, 992–1000 (2009).
Lim, W. H. et al. Lack of impact of donor age on patient survival for renal transplant recipients ≥60years. Transpl. Int. 25, 401–408 (2012).
Palomar, R. et al. Should aging recipients of kidney grafts receive less immunosuppression? Transplant. Proc. 31, 2277–2278 (1999).
Afaneh, C. et al. Pancreas transplantation: does age increase morbidity? J. Transplant. 2011, 596801 (2011).
Daneshvar, D. et al. Heart transplantation in patients aged 70 years and older: a two-decade experience. Transplant. Proc. 43, 3851–3856 (2011).
Peraira, J. R. et al. Differential characteristics of heart transplantation in patients older than 60 years. Transplant. Proc. 35, 1959–1961 (2003).
Tjang, Y. S., van der Heijden, G. J., Tenderich, G., Körfer, R. & Grobbee, D. E. Impact of recipient's age on heart transplantation outcome. Ann. Thorac. Surg. 85, 2051–2055 (2008).
Dorshkind, K. & Swain, S. Age-associated declines in immune system development and function: causes, consequences, and reversal. Curr. Opin. Immunol. 21, 404–407 (2009).
Linton, P. J. & Dorshkind, K. Age-related changes in lymphocyte development and function. Nat. Immunol. 5, 133–139 (2004).
Yung, R. L. Changes in immune function with age. Rheum. Dis. Clin. North Am. 26, 455–473 (2000).
Koch, S. et al. Multiparameter flow cytometric analysis of CD4 and CD8 T cell subsets in young and old people. Immun. Ageing 5, 6 (2008).
Yager, E. J. et al. Age-associated decline in T cell repertoire diversity leads to holes in the repertoire and impaired immunity to influenza virus. J. Exp. Med. 205, 711–723 (2008).
Miller, R. A., Garcia, G., Kirk, C. J. & Witkowski, J. M. Early activation defects in T lymphocytes from aged mice. Immunol. Rev. 160, 79–90 (1997).
Frasca, D. & Blomberg, B. B. Effects of aging on B cell function. Curr. Opin. Immunol. 21, 425–430 (2009).
Larbi, A. et al. Impact of age on T cell signaling: a general defect or specific alterations? Ageing Res. Rev. 10, 370–378 (2011).
Bachireddy, P., Rakhra, K. & Felsher, D. W. Immunology in the clinic review series; focus on cancer: multiple roles for the immune system in oncogene addiction. Clin. Exp. Immunol. 167, 188–194 (2012).
Lynch, H. E. et al. Thymic involution and immune reconstitution. Trends Immunol. 30, 366–373 (2009).
Li, G. et al. Decline in miR-181a expression with age impairs T cell receptor sensitivity by increasing DUSP6 activity. Nat. Med. 18, 1518–1524 (2012).
Almanzar, G. et al. Long-term cytomegalovirus infection leads to significant changes in the composition of the CD8+ T-cell repertoire, which may be the basis for an imbalance in the cytokine production profile in elderly persons. J. Virol. 79, 3675–3683 (2005).
Ouyang, Q. et al. Age-associated accumulation of CMV-specific CD8+ T cells expressing the inhibitory killer cell lectin-like receptor G1 (KLRG1). Exp. Gerontol. 38, 911–920 (2003).
Ouyang, Q. et al. Large numbers of dysfunctional CD8+ T lymphocytes bearing receptors for a single dominant CMV epitope in the very old. J. Clin. Immunol. 23, 247–257 (2003).
Czesnikiewicz-Guzik, M. et al. T cell subset-specific susceptibility to aging. Clin. Immunol. 127, 107–118 (2008).
Clambey, E. T., Kappler, J. W. & Marrack, P. CD8 T cell clonal expansions & aging: a heterogeneous phenomenon with a common outcome. Exp. Gerontol. 42, 407–411 (2007).
Clambey, E. T., White, J., Kappler, J. W. & Marrack, P. Identification of two major types of age-associated CD8 clonal expansions with highly divergent properties. Proc. Natl Acad. Sci. USA 105, 12997–13002 (2008).
Campisi, J. & d'Adda di Fagagna, F. Cellular senescence: when bad things happen to good cells. Nat. Rev. Mol. Cell Biol. 8, 729–740 (2007).
Effros, R. B. Telomerase induction in T cells: a cure for aging and disease? Exp. Gerontol. 42, 416–420 (2007).
Macaulay, R., Akbar, A. N. & Henson, S. M. The role of the T cell in age-related inflammation. Age (Dordr.) http://dx.doi.org/10.1007/s11357-012-9381-2.
Gorgas, G., Butch, E. R., Guan, K. L. & Miller, R. A. Diminished activation of the MAP kinase pathway in CD3-stimulated T lymphocytes from old mice. Mech. Ageing Dev. 94, 71–83 (1997).
Du, W., Shen, H., Galan, A. & Goldstein, D. R. An age-specific CD8+ T cell pathway that impairs the effectiveness of strategies to prolong allograft survival. J. Immunol. 187, 3631–3640 (2011).
Haynes, L., Linton, P. J., Eaton, S. M., Tonkonogy, S. L. & Swain, S. L. Interleukin 2, but not other common γ chain-binding cytokines, can reverse the defect in generation of CD4 effector T cells from naive T cells of aged mice. J. Exp. Med. 190, 1013–1024 (1999).
Cancro, M. P. et al. B cells and aging: molecules and mechanisms. Trends Immunol. 30, 313–318 (2009).
Frasca, D., Diaz, A., Romero, M., Landin, A. M. & Blomberg, B. B. Age effects on B cells and humoral immunity in humans. Ageing Res. Rev. 10, 330–335 (2011).
Kolibab, K., Smithson, S. L., Rabquer, B., Khuder, S. & Westerink, M. A. Immune response to pneumococcal polysaccharides 4 and 14 in elderly and young adults: analysis of the variable heavy chain repertoire. Infect. Immun. 73, 7465–7476 (2005).
Chong, Y. et al. Age-related accumulation of Ig VH gene somatic mutations in peripheral B cells from aged humans. Clin. Exp. Immunol. 133, 59–66 (2003).
Kolar, G. R., Mehta, D., Wilson, P. C. & Capra, J. D. Diversity of the Ig repertoire is maintained with age in spite of reduced germinal centre cells in human tonsil lymphoid tissue. Scand. J. Immunol. 64, 314–324 (2006).
Wang, X. & Stollar, B. D. Immunoglobulin VH gene expression in human aging. Clin. Immunol. 93, 132–142 (1999).
Josefowicz, S. Z., Lu, L. F. & Rudensky, A. Y. Regulatory T cells: mechanisms of differentiation and function. Annu. Rev. Immunol. 30, 531–564 (2012).
Zhao, L. et al. Changes of CD4+CD25+Foxp3+ regulatory T cells in aged Balb/c mice. J. Leukoc. Biol. 81, 1386–1394 (2007).
Denecke, C. et al. Prolonged graft survival in older recipient mice is determined by impaired effector T-cell but intact regulatory T-cell responses. PLoS ONE 5, e9232 (2010).
Di Ianni, M. et al. Tregs prevent GVHD and promote immune reconstitution in HLA-haploidentical transplantation. Blood 117, 3921–3928 (2011).
Rahimpour, A. et al. γδ T cells augment rejection of skin grafts by enhancing cross-priming of CD8 T cells to skin-derived antigen. J. Invest. Dermatol. 132, 1656–1664 (2012).
Cheung, K. P., Taylor, K. R. & Jameson, J. M. Immunomodulation at epithelial sites by obesity and metabolic disease. Immunol. Res. 52, 182–199 (2012).
Giachino, C. et al. Clonal expansions of Vδ1+ and Vδ2+ cells increase with age and limit the repertoire of human γδ T cells. Eur. J. Immunol. 24, 1914–1918 (1994).
Argentati, K. et al. Numerical and functional alterations of circulating γδ T lymphocytes in aged people and centenarians. J. Leukoc. Biol. 72, 65–71 (2002).
Re, F. et al. Skewed representation of functionally distinct populations of Vγ9Vδ2 T lymphocytes in aging. Exp. Gerontol. 40, 59–66 (2005).
Della Bella, S. et al. Peripheral blood dendritic cells and monocytes are differently regulated in the elderly. Clin. Immunol. 122, 220–228 (2007).
Agrawal, A., Tay, J., Ton, S., Agrawal, S. & Gupta, S. Increased reactivity of dendritic cells from aged subjects to self-antigen, the human DNA. J. Immunol. 182, 1138–1145 (2009).
Shodell, M. & Siegal, F. P. Circulating, interferon-producing plasmacytoid dendritic cells decline during human ageing. Scand. J. Immunol. 56, 518–521 (2002).
Agrawal, A. et al. Altered innate immune functioning of dendritic cells in elderly humans: a role of phosphoinositide 3-kinase-signaling pathway. J. Immunol. 178, 6912–6922 (2007).
Mahbub, S., Brubaker, A. L. & Kovacs, E. J. Aging of the innate immune system: an update. Curr. Immunol. Rev. 7, 104–115 (2011).
Gomez, C. R., Boehmer, E. D. & Kovacs, E. J. The aging innate immune system. Curr. Opin. Immunol. 17, 457–462 (2005).
Nomellini, V., Gomez, C. R. & Kovacs, E. J. Aging and impairment of innate immunity. Contrib. Microbiol. 15, 188–205 (2008).
El Mezayen, R., El Gazzar, M., Myer, R. & High, K. P. Aging-dependent upregulation of IL-23p19 gene expression in dendritic cells is associated with differential transcription factor binding and histone modifications. Aging Cell 8, 553–565 (2009).
Stout-Delgado, H. W., Yang, X., Walker, W. E., Tesar, B. M. & Goldstein, D. R. Aging impairs IFN regulatory factor 7 up-regulation in plasmacytoid dendritic cells during TLR9 activation. J. Immunol. 181, 6747–6756 (2008).
Takahashi, I. et al. Monocyte chemiluminescence and macrophage precursors in the aged. Acta Med. Okayama 39, 447–451 (1985).
Ogawa, T., Kitagawa, M. & Hirokawa, K. Age-related changes of human bone marrow: a histometric estimation of proliferative cells, apoptotic cells, T cells, B cells and macrophages. Mech. Ageing Dev. 117, 57–68 (2000).
Herrero, C., Marqués, L., Lloberas, J. & Celada, A. IFN-γ-dependent transcription of MHC class II IA is impaired in macrophages from aged mice. J. Clin. Invest. 107, 485–493 (2001).
Chaves, M. M. et al. Role of inositol 1,4,5-triphosphate and p38 mitogen-activated protein kinase in reactive oxygen species generation by granulocytes in a cyclic AMP-dependent manner: an age-related phenomenon. Gerontology 53, 228–233 (2007).
Chelvarajan, R. L., Collins, S. M., Van Willigen, J. M. & Bondada, S. The unresponsiveness of aged mice to polysaccharide antigens is a result of a defect in macrophage function. J. Leukoc. Biol. 77, 503–512 (2005).
Clark, J. A. & Peterson, T. C. Cytokine production and aging: overproduction of IL-8 in elderly males in response to lipopolysaccharide. Mech. Ageing Dev. 77, 127–139 (1994).
Delpedro, A. D., Barjavel, M. J., Mamdouh, Z., Faure, S. & Bakouche, O. Signal transduction in LPS-activated aged and young monocytes. J. Interferon Cytokine Res. 18, 429–437 (1998).
Mariani, E. et al. RANTES and MIP-1α production by T lymphocytes, monocytes and NK cells from nonagenarian subjects. Exp. Gerontol. 37, 219–226 (2002).
Gon, Y. et al. Lower serum concentrations of cytokines in elderly patients with pneumonia and the impaired production of cytokines by peripheral blood monocytes in the elderly. Clin. Exp. Immunol. 106, 120–126 (1996).
Ligthart, G. J. et al. Admission criteria for immunogerontological studies in man: the SENIEUR protocol. Mech. Ageing Dev. 28, 47–55 (1984).
van Duin, D. et al. Age-associated defect in human TLR-1/2 function. J. Immunol. 178, 970–975 (2007).
Kong, K. F. et al. Dysregulation of TLR3 impairs the innate immune response to West Nile virus in the elderly. J. Virol. 82, 7613–7623 (2008).
Fortin, C. F., McDonald, P. P., Lesur, O. & Fülöp, T. Jr. Aging and neutrophils: there is still much to do. Rejuvenation Res. 11, 873–882 (2008).
Lord, J. M., Butcher, S., Killampali, V., Lascelles, D. & Salmon, M. Neutrophil ageing and immunesenescence. Mech. Ageing Dev. 122, 1521–1535 (2001).
Fulop, T. et al. Signal transduction and functional changes in neutrophils with aging. Aging Cell 3, 217–226 (2004).
Fortin, C. F., Lesur, O. & Fulop, T. Jr. Effects of TREM-1 activation in human neutrophils: activation of signaling pathways, recruitment into lipid rafts and association with TLR4. Int. Immunol. 19, 41–50 (2007).
Gomez, C. R. et al. Advanced age exacerbates the pulmonary inflammatory response after lipopolysaccharide exposure. Crit. Care Med. 35, 246–251 (2007).
Mocchegiani, E. & Malavolta, M. NK and NKT cell functions in immunosenescence. Aging Cell 3, 177–184 (2004).
Borrego, F. et al. NK phenotypic markers and IL2 response in NK cells from elderly people. Exp. Gerontol. 34, 253–265 (1999).
Krishnaraj, R. Senescence and cytokines modulate the NK cell expression. Mech. Ageing Dev. 96, 89–101 (1997).
Krishnaraj, R. Immunosenescence of human NK cells: effects on tumor target recognition, lethal hit and interferon sensitivity. Immunol. Lett. 34, 79–84 (1992).
Kutza, J. & Murasko, D. M. Effects of aging on natural killer cell activity and activation by interleukin-2 and IFN-α. Cell. Immunol. 155, 195–204 (1994).
Kutza, J. & Murasko, D. M. Age-associated decline in IL-2 and IL-12 induction of LAK cell activity of human PBMC samples. Mech. Ageing Dev. 90, 209–222 (1996).
Mariani, E. et al. Age-associated changes in CD8+ and CD16+ cell reactivity: clonal analysis. Clin. Exp. Immunol. 81, 479–484 (1990).
Solana, R. & Mariani, E. NK and NK/T cells in human senescence. Vaccine 18, 1613–1620 (2000).
Peralbo, E., Alonso, C. & Solana, R. Invariant NKT and NKT-like lymphocytes: two different T cell subsets that are differentially affected by ageing. Exp. Gerontol. 42, 703–708 (2007).
DelaRosa, O. et al. Vα24+ NKT cells are decreased in elderly humans. Exp. Gerontol. 37, 213–217 (2002).
Peralbo, E. et al. Decreased frequency and proliferative response of invariant Vα24Vβ11 natural killer T (iNKT) cells in healthy elderly. Biogerontology 7, 483–492 (2006).
Faunce, D. E., Palmer, J. L., Paskowicz, K. K., Witte, P. L. & Kovacs, E. J. CD1d-restricted NKT cells contribute to the age-associated decline of T cell immunity. J. Immunol. 175, 3102–3109 (2005).
Chen, J., Astle, C. M. & Harrison, D. E. Genetic regulation of primitive hematopoietic stem cell senescence. Exp. Hematol. 28, 442–450 (2000).
Geiger, H., True, J. M., de Haan, G. & Van Zant, G. Age- and stage-specific regulation patterns in the hematopoietic stem cell hierarchy. Blood 98, 2966–2972 (2001).
Brusnahan, S. K. et al. Human blood and marrow side population stem cell and Stro-1 positive bone marrow stromal cell numbers decline with age, with an increase in quality of surviving stem cells: correlation with cytokines. Mech. Ageing Dev. 131, 718–722 (2010).
de Haan, G. & Van Zant, G. Dynamic changes in mouse hematopoietic stem cell numbers during aging. Blood 93, 3294–3301 (1999).
Cho, R. H., Sieburg, H. B. & Muller-Sieburg, C. E. A new mechanism for the aging of hematopoietic stem cells: aging changes the clonal composition of the stem cell compartment but not individual stem cells. Blood 111, 5553–5561 (2008).
Muller-Sieburg, C. & Sieburg, H. B. Stem cell aging: survival of the laziest? Cell Cycle 7, 3798–3804 (2008).
Roeder, I. et al. Characterization and quantification of clonal heterogeneity among hematopoietic stem cells: a model-based approach. Blood 112, 4874–4883 (2008).
Rossi, D. J. et al. Cell intrinsic alterations underlie hematopoietic stem cell aging. Proc. Natl Acad. Sci. USA 102, 9194–9199 (2005).
Chambers, S. M. et al. Aging hematopoietic stem cells decline in function and exhibit epigenetic dysregulation. PLoS Biol. 5, e201 (2007).
Liang, Y., Van Zant, G. & Szilvassy, S. J. Effects of aging on the homing and engraftment of murine hematopoietic stem and progenitor cells. Blood 106, 1479–1487 (2005).
Kollman, C. et al. Donor characteristics as risk factors in recipients after transplantation of bone marrow from unrelated donors: the effect of donor age. Blood 98, 2043–2051 (2001).
Wei, J., Xu, H., Davies, J. L. & Hemmings, G. P. Increase of plasma IL-6 concentration with age in healthy subjects. Life Sci. 51, 1953–1956 (1992).
Daynes, R. A. et al. Altered regulation of IL-6 production with normal aging. Possible linkage to the age-associated decline in dehydroepiandrosterone and its sulfated derivative. J. Immunol. 150, 5219–5230 (1993).
Gori, A. M. et al. A proinflammatory state is associated with hyperhomocysteinemia in the elderly. Am. J. Clin. Nutr. 82, 335–341 (2005).
Salvioli, S. et al. Inflamm-aging, cytokines and aging: state of the art, new hypotheses on the role of mitochondria and new perspectives from systems biology. Curr. Pharm. Des. 12, 3161–3171 (2006).
Grimm, R. H. Jr, Neaton, J. D. & Ludwig, W. Prognostic importance of the white blood cell count for coronary, cancer, and all-cause mortality. JAMA 254, 1932–1937 (1985).
Schmaltz, H. N. et al. Chronic cytomegalovirus infection and inflammation are associated with prevalent frailty in community-dwelling older women. J. Am. Geriatr. Soc. 53, 747–754 (2005).
Leng, S. X., Xue, Q. L., Tian, J., Walston, J. D. & Fried, L. P. Inflammation and frailty in older women. J. Am. Geriatr. Soc. 55, 864–871 (2007).
Leng, S. X. et al. White blood cell counts, insulin-like growth factor-1 levels, and frailty in community-dwelling older women. J. Gerontol. A Biol. Sci. Med. Sci. 64, 499–502 (2009).
Leng, S. X. et al. Associations of neutrophil and monocyte counts with frailty in community-dwelling disabled older women: results from the Women's Health and Aging Studies I. Exp. Gerontol. 44, 511–516 (2009).
Ruggiero, C. et al. White blood cell count and mortality in the Baltimore Longitudinal Study of Aging. J. Am. Coll. Cardiol. 49, 1841–1850 (2007).
Lumeng, C. N. et al. Aging is associated with an increase in T cells and inflammatory macrophages in visceral adipose tissue. J. Immunol. 187, 6208–6216 (2011).
Gupta, S., Su, H., Bi, R., Agrawal, S. & Gollapudi, S. Life and death of lymphocytes: a role in immunesenescence. Immun. Ageing 2, 12 (2005).
van de Berg, P. J. et al. Human cytomegalovirus induces systemic immune activation characterized by a type 1 cytokine signature. J. Infect. Dis. 202, 690–699 (2010).
Acknowledgements
The authors' work is supported by National Institutes of Health grant DK080048 (J. Jameson), DK075718 (D. McKay), DK091136 (D. McKay), CIRM RM1-01709 (D. McKay), and Price Charities Foundation/Scripps Clinic and Green Hospital (D. McKay)
Author information
Authors and Affiliations
Contributions
Both authors researched the data for the article, provided a substantial contribution to discussions of the content, wrote the article and reviewed and/or edited the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
D. McKay and J. Jameson have received research funding from Novartis.
Rights and permissions
About this article
Cite this article
McKay, D., Jameson, J. Kidney transplantation and the ageing immune system. Nat Rev Nephrol 8, 700–708 (2012). https://doi.org/10.1038/nrneph.2012.242
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrneph.2012.242
This article is cited by
-
The aging of the immune system and its implications for transplantation
GeroScience (2023)
-
A Comprehensive Review of Infections in Older Kidney Transplant Recipients
Current Transplantation Reports (2021)
-
Rationale and design of the OPTIMIZE trial: OPen label multicenter randomized trial comparing standard IMmunosuppression with tacrolimus and mycophenolate mofetil with a low exposure tacrolimus regimen In combination with everolimus in de novo renal transplantation in Elderly patients
BMC Nephrology (2021)
-
Age-associated decrease in de novo donor-specific antibodies in renal transplant recipients reflects changing humoral immunity
Immunity & Ageing (2019)
-
Circulating small non-coding RNAs associated with age, sex, smoking, body mass and physical activity
Scientific Reports (2018)