Key Points
-
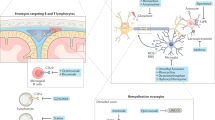
Three molecules have a key role in the remissions and relapses seen in around two thirds of patients with multiple sclerosis — α4β1 integrin, osteopontin and αB crystallin.
-
The key integrin in lymphocyte homing to the brain in multiple sclerosis is α4β1 integrin. Blockade of this integrin with a humanized monoclonal antibody, natalizumab, reduces the relapse rate by two thirds. It is the most potent approved drug for the treatment of multiple sclerosis.
-
Osteopontin is a binding partner of α4β1 integrin. It is a pro-survival molecule and inhibits apoptosis through its actions on the transcription factors forkhead box O3A (FOXO3A) and nuclear factor-κB (NF-κB).
-
Administration of osteopontin into mice with experimental autoimmune encephalomyelitis (EAE) triggers relapse of the disease. The relapses are a result of the inhibition of apoptosis and the increased production of T helper 1 (TH1)- and TH17-type cytokines from autoreactive T cells.
-
Remission in multiple sclerosis is driven by a small heat shock protein, αB crystallin. αB crystallin decreases the production of TH1- and TH17-type cytokines from autoreactive T cells.
-
Infusion of αB crystallin in animal models of multiple sclerosis resolves progressive disease and terminates relapse.
-
αB crystallin is produced in copious amounts at the sites of inflammation in the brains of patients with multiple sclerosis.
Abstract
Two thirds of patients with multiple sclerosis have the relapsing-remitting form, which often progresses to more debilitating disease. Striking clinical recovery, termed remission, often follows these periodic neurological defects, termed relapses. Recent work has revealed the role of three key molecules in relapse and remission: α4β1 integrin (also known as VLA4) is an adhesion molecule that mediates T cell migration from the blood into the brain; osteopontin binds to α4β1 integrin, stimulating the production of pro-inflammatory cytokines and inhibiting apoptosis; and αB crystallin inhibits inflammation in the brain. This Review discusses how this molecular trio interacts to initiate relapses (in the case of osteopontin and α4β1 integrin) and then to terminate them as remissions in multiple sclerosis (in the case of αB crystallin).
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Steinman L. Multiple sclerosis: a two stage disease. Nature Immunol. 2, 762–765 (2001).
Steinman, L., Martin, R., Bernard, C., Conlon, P. & Oksenberg, J. R. Multiple sclerosis: deeper understanding of its pathogenesis reveals new targets for therapy. Annu. Rev. Neurosci. 25, 491–505 (2002).
Chabas, D. et al. The influence of the pro-inflammatory cytokine, osteopontin, on autoimmune demyelinating disease. Science 294, 1731–1735 (2001).
Ashkar, S. et al. Eta-1 (osteopontin): an early component of type 1 (cell-mediated) immunity. Science 287, 860–864 (2000).
Yednock, T. et al. Prevention of experimental autoimmune encephalomyelitis by antibodies against α4β1 integrin. Nature 356, 63–66 (1992). This paper describes the history of the development of natalizumab for the treatment of relapsing-remitting multiple sclerosis.
Brocke, S., Piercy, C., Steinman, L., Weissman, I. L. & Veromaa, T. Antibodies to CD44 and integrin α4, but not L-selectin, prevent CNS inflammation and experimental encephalomyelitis by blocking secondary leukocyte recruitment. Proc. Natl Acad. Sci USA 96, 6896–6901 (1999).
Steinman, L. Blocking adhesion molecules as therapy for multiple sclerosis: natalizumab. Nature Rev. Drug Discov. 4, 510–519 (2005).
Polman, C. H. et al. A randomized, placebo-controlled trial of natalizumab for relapsing multiple sclerosis. N. Engl. J. Med. 354, 899–910 (2006).
Rudick, R. A. et al. Natalizumab plus interferon β-1a for relapsing multiple sclerosis. N. Engl. J. Med. 354, 911–923 (2006).
Hur, E. et al. Osteopontin induced relapse and progression of autoimmune brain disease via enhanced survival of activated T cells. Nature Immunol. 8, 77–86 (2007). This paper describes the pro-survival properties of osteopontin and the underlying mechanisms in inducing neurological relapses.
Stromnes, I. M. & Goverman, J. M. Osteopontin-induced survival of T cells. Nature Immunol. 8, 19–20 (2007).
Vogt, M. H., Lopatinskaya, L., Smits, M., Polman, C. H., Nagelkerken, L. Elevated osteopontin levels in active relapsing-remitting multiple sclerosis. Ann. Neurol. 53, 819–822 (2003).
Comabella, M. et al. Plasma osteopontin levels in multiple sclerosis. J. Neuroimmunol. 158, 231–239 (2005).
Ousman, S. S. et al. Protective and therapeutic role for αB-crystallin in autoimmune demyelination. Nature 448, 474–479 (2007). This paper describes the guardian properties of αB crystallin in autoimmune demyelination.
Han, M. H. et al. Proteomic analysis of active multiple sclerosis lesions reveals therapeutic targets. Nature 451, 1076–1081 (2008).
Cannella, B. & Raine, C. S. The adhesion molecule and cytokine profile of multiple sclerosis lesions. Ann. Neurol. 37, 424–435 (1995).
Fisher, L. W., Torchia, D. A., Fohr, B., Young, M. F. & Fedarko, N. S. Flexible structures of SIBLING proteins, bone sialoprotein, and osteopontin. Biochem. Biophys. Res. Commun. 280, 460–465 (2001). This paper describes the properties of the Sibling family of proteins.
Jansson, M., Panoutsakopoulou, V., Baker, J., Klein, L. & Cantor, H. Attenuated experimental autoimmune encephalomyelitis in Eta-1/osteopontin-deficient mice. J. Immunol. 168, 2096–2099 (2002).
Shinohara, M. L., Kim, J.-H., Garcia, V. A. & Cantor, H. Engagement of the type-I interferon receptor on dendritic cells inhibits promotion of Th17 cells: role of intracellular osteopontin. Immunity 29, 68–78 (2008).
Murugaiyan, G., Mittal, A. & Weiner, H. L. Increased osteopontin expression in dendritic cells amplifies IL-17 production by CD4+ T cells in experimental autoimmune encephalomyelitis and in multiple sclerosis. J. Immunol. 181, 7480–7488 (2008).
Oldberg, A., Franzén, A. & Heinegård, D. Cloning and sequence analysis of rat bone sialoprotein (osteopontin) cDNA reveals an Arg–Gly–Asp cell-binding sequence. Proc. Natl Acad. Sci. USA 83, 8819–8823 (1986).
Lederberg, J. H1N1-influenza as Lazarus: genomic resurrection from the tomb of an unknown. Proc. Natl Acad. Sci. USA 98, 2115–2116 (2001).
Senger, D. R., Wirth, D. F. & Hynes, R. O. Transformed mammalian cells secrete specific proteins and phosphoproteins. Cell 16, 885–893 (1979).
Singh, R. P., Patarca, R., Schwartz, J., Singh, P., & Cantor, H. Definition of a specific interaction between the early T lymphocyte activation 1 (Eta-1) protein and murine macrophages in vitro and its effect upon macrophages in vivo. J. Exp. Med. 171, 1931–1942 (1990).
Smith, J. H. & Denhardt, D. T. Molecular cloning of a tumor promoter-inducible mRNA found in JB6 mouse epidermal cells: induction is stable at high, but not at low, cell densities. J. Cell Biochem. 34, 13–22 (1987).
Sinclair, C., Mirakhur, M., Kirk, J., Farrell, M. & McQuaid, S. Up-regulation of osteopontin and aB-crystallin in the normal-appearing white matter of multiple sclerosis: an immunohistochemical study utilizing tissue microarrays. Neuropathol. Appl. Neurobiol. 31, 292–303 (2005).
Diaz-Sanchez, M., Williams, K., DeLuca, G. C. & Esiri, M. M. Protein co-expression with axonal injury in multiple sclerosis plaques. Acta Neuropathol. 111, 289–299 (2006).
Caillier, S. et al.. Osteopontin polymorphisms and disease course in MS. Genes Immun. 4, 312–315 (2003).
Miller, D. H. et al. A controlled trial of natalizumab for relapsing multiple sclerosis. N. Engl. J. Med. 348, 15–23 (2003).
Bettelli, E., Oukka, M. & Kuchroo, V. K. TH-17 cells in the circle of immunity and autoimmunity. Nature Immunol. 8, 345–350 (2007).
Steinman, L. A rush to judgment on Th17. J. Exp. Med. 205, 1517–1522 (2008).
Pepinsky, R. B. et al. Comparative assessment of the ligand and metal ion binding properties of integrins α9β1 and α4β1. Biochemistry 41, 7125–7141 (2002).
Davis, G., Bayless, K., Davis, M. J. & Meininger, G. A. Regulation of tissue injury responses by the exposure of matricryptic sites within extracellular matrix molecules. Am. J. Pathol. 156, 1489–1498 (2000).
Leung, L. L., Nishimura, T. & Myles, T. Regulation of tissue inflammation by thrombin-activatable carboxypeptidase B or (TAFI). Adv. Exp. Med. Biol. 632, 61–69 (2008).
de Jong, W. W., Caspers, G. J. & Leunissen, J. A. Genealogy of the α-crystallin — small heat-shock protein superfamily. Int. J. Biol. Macromol. 22, 151–162 (1998).
Kappe, G., Leunissen, J. A. & de Jong, W. W. Evolution and diversity of prokaryotic small heat shock proteins. Prog. Mol. Subcell. Biol. 28, 1–17 (2002).
Kim, K. K., Kim, R. & Kim, S. H. Crystal structure of a small heat-shock protein. Nature 394, 595–599 (1998).
van Noort, J. M. et al. The small heat-shock protein αB-crystallin as candidate autoantigen in multiple sclerosis. Nature 375, 798–801 (1995).
Ransohoff, R. M. Inflammatory disease: assault on the guardian. Nature 448, 421–422 (2007).
Van Veen, T. et al. αB crystallin genotype has impact on the multiple sclerosis phenotype. Neurology 61, 1245–1249 (2003).
Stoevring, B., Fredericksen, J. L. & Christiansen, M. Cryab promoter polymorphisms: influence on multiple sclerosis susceptibility and clinical presentation. Clin. Chim. Acta. 375, 57–62 (2007).
Bellachene, A. et al. Small integrin-binding ligand N-linked glycoprotein's (SIBLINGs): multifunctional proteins in cancer. Nature Rev. Cancer 8, 212–226 (2008).
Chelouche-Lev, D., Kluger, H. M., Berger, A. J., Rimm, D. L. & Price, J. E. αB crystallin as a marker of lymph node involvement in breast carcinoma. Cancer 100, 2543–2548 (2004).
Sato, T. et al. Osteopontin/Eta-1 upregulated in Crohn's disease regulates the Th1 immune response. Gut 54, 1254–1262 (2005).
Wong, C. K., Lit, L. C., Tam, L. S., Li, E. K. & Lam, C. W. Elevation of plasma osteopontin concentration is correlated with disease activity in patients with systemic lupus erythematosus. Rheumatology (Oxford) 44, 602–606 (2005).
De Groot, C. J. et al. Expression of TGF-β1, -β2, and -β3 isoforms and TGF-β type I and type II receptors in multiple sclerosis lesions and adult astrocyte cultures. J. Neuropathol. Exp. Neurol. 58, 174–187 (1999).
Luo, J. et al. Glia-dependent TFG-β signaling, independent of TH17 pathway is critical for initiation of autoimmune encephalomyelitis. J. Clin. Invest. 117, 3306–3315 (2007).
Steinman, L. Nuanced roles of cytokines in three major human brain disorders. J. Clin. Invest. 118, 3557–2563 (2009).
Acknowledgements
L. Steinman acknowledges the fruitful collaboration with D.T. Denhardt over the past decade.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
L. Steinman has patent applications and issued patents on therapeutic uses of osteopontin and αB crystallin. He consults for Bayhill Therapeutics and Cardinal, which own interests in these patents. L. Steinman founded both companies and serves on their board of directors.
Related links
Glossary
- Gadolinium-enhanced lesion
-
A lesion that is visualized with magnetic resonance imaging and that is enhanced with the element gadolinium, which has a magnetic spin. These lesions correlate with areas of inflammation in the brains of patients with multiple sclerosis.
- Perivascular cuff
-
An area surrounding an inflamed blood vessel, containing inflammatory lymphocytes, and delimited by endothelium on one side and basement membrane on the other side.
- Myelin oligodendrocyte glycoprotein
-
One of the myelin proteins and a member of the immunoglobulin superfamily.
- TH1 cell
-
An inflammatory cell, the signature cytokine of which is interferon-γ.
- TH17 cell
-
An inflammatory cell, the signature cytokine of which is interleukin-17.
- Reactive astrocyte
-
A type of glial cell within the brain that has many properties of macrophages.
- Microglial cell
-
A type of macrophage-like glial cell that is derived from bone marrow and resides in the central nervous system.
- Coagulation cascade
-
A biochemical sequence of reactions that regulates coagulation by a series of proteases acting on substrates such as fibrinogen and fibrin.
- Laser capture microdissection
-
A technique in which tissues are dissected with a laser and then subjected to biochemical analysis. The pathological stage of the specimen can be correlated with biochemistry.
Rights and permissions
About this article
Cite this article
Steinman, L. A molecular trio in relapse and remission in multiple sclerosis. Nat Rev Immunol 9, 440–447 (2009). https://doi.org/10.1038/nri2548
Issue Date:
DOI: https://doi.org/10.1038/nri2548
This article is cited by
-
FoxO3 and oxidative stress: a multifaceted role in cellular adaptation
Journal of Molecular Medicine (2023)
-
Osteopontin in autoimmune disorders: current knowledge and future perspective
Inflammopharmacology (2022)
-
Transcriptional heterogeneity between primary adult grey and white matter astrocytes underlie differences in modulation of in vitro myelination
Journal of Neuroinflammation (2020)
-
Mast cells and angiogenesis in multiple sclerosis
Inflammation Research (2020)
-
The role of osteopontin in the progression of solid organ tumour
Cell Death & Disease (2018)