Key Points
-
Neoadjuvant therapy is the standard of care for patients with locally advanced breast cancer and can improve operability of breast cancer
-
For patients with HER2-positive breast cancer, the long-term outcomes of neoadjuvant treatment with the anti-HER2 agent trastuzumab should be considered equivalent to those of adjuvant therapy with this drug
-
Despite the success of some neoadjuvant trials of dual-agent HER2 therapy for HER2-positive breast cancer, additional overall survival benefits of this approach over single-agent trastuzumab have not been documented in adjuvant trials
-
Differences in clinical-trial designs, patient characteristics, breast-cancer biology, and the sequence and schedule of drug administration might have influenced the results of neoadjuvant trials and the contrasting results seen in adjuvant trials
-
Prospective neoadjuvant trials with treatment informed by biomarkers and administration of matched experimental drug regimens should address the biological heterogeneity observed within breast-cancer subtypes
Abstract
Neoadjuvant therapy has been established as an effective therapeutic approach for patients with locally advanced breast cancer. Similar outcomes between neoadjuvant and adjuvant chemotherapy have been demonstrated in several trials. Nevertheless, neoadjuvant therapy has some advantages over adjuvant therapy, including tumour downstaging, in vivo assessment of therapeutic efficacy, reduced treatment durations, and the need to enrol fewer patients for clinical trials to reach their preplanned objectives. The number of neoadjuvant trials in patients with breast cancer has increased substantially in the past 5 years, particularly in the context of HER2-positive disease. Substantial improvements in the pathological complete response rate to anti-HER2 therapy, a proposed surrogate end point for long-term clinical benefit, have been observed with neoadjuvant dual-agent HER2 blockade. Thus, it was hypothesized that this approach would provide additional survival benefits over standard-of-care therapy with the anti-HER2 antibody trastuzumab in the adjuvant setting. Emerging data, however, are calling this notion into question. We discuss potential reasons why results of neoadjuvant trials of targeted therapies have not been mirrored in the adjuvant setting, and other than inherent differences in clinical-trial designs and statistical power, we consider how the biology of the disease, patient characteristics, and drug administration and schedule might influence the results.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
National Comprehensive Cancer Network (NCCN). Clinical practice guidelines in oncology: breast cancer. Version I.2016. [online], (2016).
Schott, A. F. & Hayes, D. F. Defining the benefits of neoadjuvant chemotherapy for breast cancer. J. Clin. Oncol. 30, 1747–1749 (2012).
Untch, M., Konecny, G. E., Paepke, S. & von Minckwitz, G. Current and future role of neoadjuvant therapy for breast cancer. Breast 23, 526–537 (2014).
von Minckwitz, G. et al. Response-guided neoadjuvant chemotherapy for breast cancer. J. Clin. Oncol. 31, 3623–3630 (2013).
Fisher, B. et al. Effect of preoperative chemotherapy on the outcome of women with operable breast cancer. J. Clin. Oncol. 16, 2672–2685 (1998).
van der Hage, J. A. et al. Preoperative chemotherapy in primary operable breast cancer: results from the European Organization for Research and Treatment of Cancer Trial 10902. J. Clin. Oncol. 19, 4224–4237 (2001).
Mauri, D., Pavlidis, N. & Ioannidis, J. P. Neoadjuvant versus adjuvant systemic treatment in breast cancer: a meta-analysis. J. Natl Cancer Inst. 97, 188–194 (2005).
Chen, A. M. et al. Breast conservation after neoadjuvant chemotherapy: the MD Anderson Cancer Center experience. J. Clin. Oncol. 22, 2303–2312 (2004).
Morrow, M. et al. Surgeon recommendations and receipt of mastectomy for treatment of breast cancer. JAMA 302, 1551–1556 (2009).
King, T. A. & Morrow, M. Surgical issues in patients with breast cancer receiving neoadjuvant chemotherapy. Nat. Rev. Clin. Oncol. 12, 335–343 (2015).
De Mattos-Arruda, L. & Cortes, J. Advances in first-line treatment for patients with HER-2+ metastatic breast cancer. Oncologist 17, 631–644 (2012).
Dawood, S., Broglio, K., Buzdar, A. U., Hortobagyi, G. N. & Giordano, S. H. Prognosis of women with metastatic breast cancer by HER2 status and trastuzumab treatment: an institutional-based review. J. Clin. Oncol. 28, 92–98 (2010).
Slamon, D. J. et al. Human breast cancer: correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science 235, 177–182 (1987).
Slamon, D. J. et al. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N. Engl. J. Med. 344, 783–792 (2001).
Piccart-Gebhart, M. J. et al. Trastuzumab after adjuvant chemotherapy in HER2-positive breast cancer. N. Engl. J. Med. 353, 1659–1672 (2005).
Romond, E. H. et al. Trastuzumab plus adjuvant chemotherapy for operable HER2-positive breast cancer. N. Engl. J. Med. 353, 1673–1684 (2005).
Buzdar, A. U. et al. Significantly higher pathologic complete remission rate after neoadjuvant therapy with trastuzumab, paclitaxel, and epirubicin chemotherapy: results of a randomized trial in human epidermal growth factor receptor 2-positive operable breast cancer. J. Clin. Oncol. 23, 3676–3685 (2005).
Buzdar, A. U. et al. Neoadjuvant therapy with paclitaxel followed by 5-fluorouracil, epirubicin, and cyclophosphamide chemotherapy and concurrent trastuzumab in human epidermal growth factor receptor 2-positive operable breast cancer: an update of the initial randomized study population and data of additional patients treated with the same regimen. Clin. Cancer Res. 13, 228–233 (2007).
Slamon, D. et al. Adjuvant trastuzumab in HER2-positive breast cancer. N. Engl. J. Med. 365, 1273–1283 (2011).
Geyer, C. E. et al. Lapatinib plus capecitabine for HER2-positive advanced breast cancer. N. Engl. J. Med. 355, 2733–2743 (2006).
Swain, S. M. et al. Pertuzumab, trastuzumab, and docetaxel for HER2-positive metastatic breast cancer (CLEOPATRA study): overall survival results from a randomised, double-blind, placebo-controlled, phase 3 study. Lancet Oncol. 14, 461–471 (2013).
Verma, S. et al. Trastuzumab emtansine for HER2-positive advanced breast cancer. N. Engl. J. Med. 367, 1783–1791 (2012).
De Mattos-Arruda, L. & Cortes, J. Use of pertuzumab for the treatment of HER2-positive metastatic breast cancer. Adv. Ther. 30, 645–658 (2013).
Gianni, L. et al. Efficacy and safety of neoadjuvant pertuzumab and trastuzumab in women with locally advanced, inflammatory, or early HER2-positive breast cancer (NeoSphere): a randomised multicentre, open-label, phase 2 trial. Lancet Oncol. 13, 25–32 (2012).
Schneeweiss, A. et al. Pertuzumab plus trastuzumab in combination with standard neoadjuvant anthracycline-containing and anthracycline-free chemotherapy regimens in patients with HER2-positive early breast cancer: a randomized phase II cardiac safety study (TRYPHAENA). Ann. Oncol. 24, 2278–2284 (2013).
Scaltriti, M. et al. Lapatinib, a HER2 tyrosine kinase inhibitor, induces stabilization and accumulation of HER2 and potentiates trastuzumab-dependent cell cytotoxicity. Oncogene 28, 803–814 (2009).
Xia, W. et al. Combining lapatinib, a small molecule inhibitor of ErbB1 and ErbB2 tyrosine kinases, with therapeutic anti-ErbB2 antibodies enhances apoptosis of ErbB2-overexpressing breast cancer cells. Oncogene 24, 6213–6221 (2005).
Baselga, J. et al. Lapatinib with trastuzumab for HER2-positive early breast cancer (NeoALTTO): a randomised, open-label, multicentre, phase 3 trial. Lancet 379, 633–640 (2012).
Guarneri, V. et al. Preoperative chemotherapy plus trastuzumab, lapatinib, or both in human epidermal growth factor receptor 2-positive operable breast cancer: results of the randomized phase II CHER-LOB study. J. Clin. Oncol. 30, 1989–1995 (2012).
Holmes, F. A. et al. Correlation of molecular effects and pathologic complete response to preoperative lapatinib and trastuzumab, separately and combined prior to neoadjuvant breast cancer chemotherapy [abstract]. J. Clin. Oncol. 29 (Suppl.), 506 (2011).
Zardavas, D., Fouad, T. M. & Piccart, M. Optimal adjuvant treatment for patients with HER2-positive breast cancer in 2015. Breast 24, S143–S148 (2015).
Piccart-Gebhart, M. et al. Adjuvant lapatinib and trastuzumab for early human epidermal growth factor receptor 2-positive breast cancer: results from the Randomized Phase III Adjuvant Lapatinib and/or Trastuzumab Treatment Optimization Trial. J. Clin. Oncol. http://dx.doi.org/10.1200/JCO.2015.62.1797 (2015).
Cameron, D. et al. Adjuvant bevacizumab-containing therapy in triple-negative breast cancer (BEATRICE): primary results of a randomised, phase 3 trial. Lancet Oncol. 14, 933–942 (2013).
Miller, K., O'Neill, A. & Dang, C. Bevacizumab (Bv) in the adjuvant treatment of HER2-negative breast cancer: final results from Eastern Cooperative Oncology Group E5103. J. Clin. Oncol. 25 (Suppl. 5), 500 (2014).
von Minckwitz, G. et al. Neoadjuvant chemotherapy and bevacizumab for HER2-negative breast cancer. N. Engl. J. Med. 366, 299–309 (2012).
Bear, H. D. et al. Bevacizumab added to neoadjuvant chemotherapy for breast cancer. N. Engl. J. Med. 366, 310–320 (2012).
von Minckwitz, G. et al. Survival after neoadjuvant chemotherapy with or without bevacizumab or everolimus for HER2-negative primary breast cancer (GBG 44-GeparQuinto). Ann. Oncol. 25, 2363–2372 (2014).
de Azambuja, E. et al. Lapatinib with trastuzumab for HER2-positive early breast cancer (NeoALTTO): survival outcomes of a randomised, open-label, multicentre, phase 3 trial and their association with pathological complete response. Lancet Oncol. 15, 1137–1146 (2014).
Giobbie-Hurder, A., Gelber, R. D. & Regan, M. M. Challenges of guarantee-time bias. J. Clin. Oncol. 31, 2963–2969 (2013).
Ring, A. E., Smith, I. E., Ashley, S., Fulford, L. G. & Lakhani, S. R. Oestrogen receptor status, pathological complete response and prognosis in patients receiving neoadjuvant chemotherapy for early breast cancer. Br. J. Cancer 91, 2012–2017 (2004).
Kurozumi, S. et al. ER, PgR, Ki67, 27Kip1, and histological grade as predictors of pathological complete response in patients with HER2-positive breast cancer receiving neoadjuvant chemotherapy using taxanes followed by fluorouracil, epirubicin, and cyclophosphamide concomitant with trastuzumab. BMC Cancer 15, 622 (2015).
Carey, L. A. et al. Clinical and translational results of CALGB 40601: A neoadjuvant phase III trial of weekly paclitaxel and trastuzumab with or without lapatinib for HER2-positive breast cancer [abstract]. J. Clin. Oncol. 31 (Suppl.), 500 (2013).
Amin, D. N. et al. Resiliency and vulnerability in the HER2-HER3 tumorigenic driver. Sci. Transl. Med. 2, 16ra7 (2010).
Chien, A. J. et al. Phase I dose-escalation study of 5-day intermittent oral lapatinib therapy in patients with human epidermal growth factor receptor 2-overexpressing breast cancer. J. Clin. Oncol. 32, 1472–1479 (2014).
White, J. & DeMichele, A. Neoadjuvant therapy for breast cancer: controversies in clinical trial design and standard of care. Am. Soc. Clin. Oncol. Educ. Book 35, e17–23 (2015).
Goldhirsch, A. et al. 2 years versus 1 year of adjuvant trastuzumab for HER2-positive breast cancer (HERA): an open-label, randomised controlled trial. Lancet 382, 1021–1028 (2013).
von Minckwitz, G. et al. Definition and impact of pathologic complete response on prognosis after neoadjuvant chemotherapy in various intrinsic breast cancer subtypes. J. Clin. Oncol. 30, 1796–1804 (2012).
U.S. Department of Health and Human Services. Guidance for industry. Pathological complete response in neoadjuvant treatment of high-risk early-stage breast cancer: use as an endpoint to support accelerated approval. FDA [online], (2014).
von Minckwitz, G. et al. Doxorubicin with cyclophosphamide followed by docetaxel every 21 days compared with doxorubicin and docetaxel every 14 days as preoperative treatment in operable breast cancer: the GEPARDUO study of the German Breast Group. J. Clin. Oncol. 23, 2676–2685 (2005).
Untch, M. et al. Neoadjuvant treatment with trastuzumab in HER2-positive breast cancer: results from the GeparQuattro study. J. Clin. Oncol. 28, 2024–2031 (2010).
Gianni, L. et al. Neoadjuvant and adjuvant trastuzumab in patients with HER2-positive locally advanced breast cancer (NOAH): follow-up of a randomised controlled superiority trial with a parallel HER2-negative cohort. Lancet Oncol. 15, 640–647 (2014).
Untch, M. et al. Intensive dose-dense compared with conventionally scheduled preoperative chemotherapy for high-risk primary breast cancer. J. Clin. Oncol. 27, 2938–2945 (2009).
Esposito, A., Criscitiello, C. & Curigliano, G. Highlights from the 14th St Gallen International Breast Cancer Conference 2015 in Vienna: dealing with classification, prognostication, and prediction refinement to personalize the treatment of patients with early breast cancer. ecancermedicalscience 9, 518 (2015).
Liedtke, C. et al. Response to neoadjuvant therapy and long-term survival in patients with triple-negative breast cancer. J. Clin. Oncol. 26, 1275–1281 (2008).
Robidoux, A. et al. Lapatinib as a component of neoadjuvant therapy for HER2-positive operable breast cancer (NSABP protocol B-41): an open-label, randomised phase 3 trial. Lancet Oncol. 14, 1183–1192 (2013).
Berruti, A. et al. Pathologic complete response as a potential surrogate for the clinical outcome in patients with breast cancer after neoadjuvant therapy: a meta-regression of 29 randomized prospective studies. J. Clin. Oncol. 32, 3883–3891 (2014).
Berry, D. A. & Hudis, C. A. Neoadjuvant therapy in breast cancer as a basis for drug approval. JAMA Oncol. 1, 875–876 (2015).
Cortazar, P. et al. Pathological complete response and long-term clinical benefit in breast cancer: the CTNeoBC pooled analysis. Lancet 384, 164–172 (2014).
Prentice, R. L. Surrogate endpoints in clinical trials: definition and operational criteria. Stat. Med. 8, 431–440 (1989).
Freedman, L. S., Graubard, B. I. & Schatzkin, A. Statistical validation of intermediate endpoints for chronic diseases. Stat. Med. 11, 167–178 (1992).
Fleming, T. R., Prentice, R. L., Pepe, M. S. & Glidden, D. Surrogate and auxiliary endpoints in clinical trials, with potential applications in cancer and AIDS research. Stat. Med. 13, 955–968 (1994).
Buyse, M., Molenberghs, G., Burzykowski, T., Renard, D. & Geys, H. The validation of surrogate endpoints in meta-analyses of randomized experiments. Biostatistics 1, 49–67 (2000).
Venkatraman, E. S. & Begg, C. B. Properties of a nonparametric test for early comparison of treatments in clinical trials in the presence of surrogate endpoints. Biometrics 55, 1171–1176 (1999).
Li, Y. & Taylor, J. M. Predicting treatment effects using biomarker data in a meta-analysis of clinical trials. Stat. Med. 29, 1875–1889 (2010).
Bonnefoi, H. et al. Neoadjuvant treatment with docetaxel plus lapatinib, trastuzumab, or both followed by an anthracycline-based chemotherapy in HER2-positive breast cancer: results of the randomised phase II EORTC 10054 study. Ann. Oncol. 26, 325–332 (2015).
Parker, J. S. et al. Supervised risk predictor of breast cancer based on intrinsic subtypes. J. Clin. Oncol. 27, 1160–1167 (2009).
Untch, M. et al. Lapatinib versus trastuzumab in combination with neoadjuvant anthracycline-taxane-based chemotherapy (GeparQuinto, GBG 44): a randomised phase 3 trial. Lancet Oncol. 13, 135–144 (2012).
Majewski, I. J. et al. PIK3CA mutations are associated with decreased benefit to neoadjuvant human epidermal growth factor receptor 2-targeted therapies in breast cancer. J. Clin. Oncol. 33, 1334–1339 (2015).
Salgado, R. et al. Tumor-infiltrating lymphocytes and associations with pathological complete response and event-free survival in HER2-positive early-stage breast cancer treated with lapatinib and trastuzumab: a secondary analysis of the NeoALTTO Trial. JAMA Oncol. 1, 448–454 (2015).
Zardavas, D., Phillips, W. A. & Loi, S. PIK3CA mutations in breast cancer: reconciling findings from preclinical and clinical data. Breast Cancer Res. 16, 201 (2014).
Ali, H. R. et al. Association between CD8+ T-cell infiltration and breast cancer survival in 12,439 patients. Ann. Oncol. 25, 1536–1543 (2014).
Salgado, R. et al. The evaluation of tumor-infiltrating lymphocytes (TILs) in breast cancer: recommendations by an International TILs Working Group 2014. Ann. Oncol. 26, 259–271 (2015).
Savas, P. et al. Clinical relevance of host immunity in breast cancer: from TILs to the clinic. Nat. Rev. Clin. Oncol. http://dx.doi.org/10.1038/nrclinonc.2015.215 (2015).
Luria, S. E. & Delbruck, M. Mutations of bacteria from virus sensitivity to virus resistance. Genetics 28, 491–511 (1943).
Michor, F. et al. Dynamics of chronic myeloid leukaemia. Nature 435, 1267–1270 (2005).
Leder, K. et al. Fitness conferred by BCR–ABL kinase domain mutations determines the risk of pre-existing resistance in chronic myeloid leukemia. PLoS ONE 6, e27682 (2011).
Foo, J., Chmielecki, J., Pao, W. & Michor, F. Effects of pharmacokinetic processes and varied dosing schedules on the dynamics of acquired resistance to erlotinib in EGFR-mutant lung cancer. J. Thorac. Oncol. 7, 1583–1593 (2012).
Diaz, L. A. Jr et al. The molecular evolution of acquired resistance to targeted EGFR blockade in colorectal cancers. Nature 486, 537–540 (2012).
Iwasa, Y., Nowak, M. A. & Michor, F. Evolution of resistance during clonal expansion. Genetics 172, 2557–2566 (2006).
Durrett, R. & Moseley, S. Evolution of resistance and progression to disease during clonal expansion of cancer. Theor. Popul. Biol. 77, 42–48 (2010).
Komarova, N. L. & Wodarz, D. Drug resistance in cancer: principles of emergence and prevention. Proc. Natl Acad. Sci. USA 102, 9714–9719 (2005).
Montagut, C. et al. Identification of a mutation in the extracellular domain of the Epidermal Growth Factor Receptor conferring cetuximab resistance in colorectal cancer. Nat. Med. 18, 221–223 (2012).
Maheswaran, S. et al. Detection of mutations in EGFR in circulating lung-cancer cells. N. Engl. J. Med. 359, 366–377 (2008).
Turke, A. B. et al. Preexistence and clonal selection of MET amplification in EGFR mutant NSCLC. Cancer Cell 17, 77–88 (2010).
Garcia-Murillas, I. et al. Mutation tracking in circulating tumor DNA predicts relapse in early breast cancer. Sci. Transl. Med. 7, 302ra133 (2015).
Olsson, E. et al. Serial monitoring of circulating tumor DNA in patients with primary breast cancer for detection of occult metastatic disease. EMBO Mol. Med. 7, 1034–1047 (2015).
De Mattos-Arruda, L. et al. Capturing intra-tumor genetic heterogeneity by de novo mutation profiling of circulating cell-free tumor DNA: a proof-of -principle. Ann. Oncol. 25, 1729–1735 (2014).
De Mattos-Arruda, L. et al. Circulating tumour cells and cell-free DNA as tools for managing breast cancer. Nat. Rev. Clin. Oncol. 10, 377–389 (2013).
Crowley, E., Di Nicolantonio, F., Loupakis, F. & Bardelli, A. Liquid biopsy: monitoring cancer-genetics in the blood. Nat. Rev. Clin. Oncol. 10, 472–484 (2013).
De Mattos-Arruda et al. Cerebrospinal fluid-derived circulating tumour DNA better represents the genomic alterations of brain tumours than plasma. Nat. Commun. 6, 8839 (2015).
Chen, Z. et al. Analysis of cancer mutation signatures in blood by a novel ultra-sensitive assay: monitoring of therapy or recurrence in non-metastatic breast cancer. PLoS ONE 4, e7220 (2009).
Beaver, J. A. et al. Detection of cancer DNA in plasma of patients with early-stage breast cancer. Clin. Cancer Res. 20, 2643–2650 (2014).
Gerlinger, M. et al. Intratumor heterogeneity and branched evolution revealed by multiregion sequencing. N. Engl. J. Med. 366, 883–892 (2012).
Geyer, F. C. et al. Molecular analysis reveals a genetic basis for the phenotypic diversity of metaplastic breast carcinomas. J. Pathol. 220, 562–573 (2010).
Navin, N. et al. Tumour evolution inferred by single-cell sequencing. Nature 472, 90–94 (2011).
Janiszewska, M. et al. In situ single-cell analysis identifies heterogeneity for PIK3CA mutation and HER2 amplification in HER2-positive breast cancer. Nat. Genet. (2015).
Shah, S. P. et al. Mutational evolution in a lobular breast tumour profiled at single nucleotide resolution. Nature 461, 809–813 (2009).
Ding, L. et al. Genome remodelling in a basal-like breast cancer metastasis and xenograft. Nature 464, 999–1005 (2010).
Gonzalez-Angulo, A. M. et al. PI3K pathway mutations and PTEN levels in primary and metastatic breast cancer. Mol. Cancer Ther. 10, 1093–1101 (2011).
Nik-Zainal, S. et al. The life history of 21 breast cancers. Cell 149, 994–1007 (2012).
Yates, L. R. et al. Subclonal diversification of primary breast cancer revealed by multiregion sequencing. Nat. Med. 21, 751–759 (2015).
Niikura, N. et al. Changes in tumor expression of HER2 and hormone receptors status after neoadjuvant chemotherapy in 21,755 patients from the Japanese Breast Cancer Registry. Ann. Oncol. http://dx.doi.org/10.1093/annonc/mdv611 (2015).
Ng, C. K. et al. Intra-tumor genetic heterogeneity and alternative driver genetic alterations in breast cancers with heterogeneous HER2 gene amplification. Genome Biol. 16, 107 (2015).
Burrell, R. A. & Swanton, C. Tumour heterogeneity and the evolution of polyclonal drug resistance. Mol. Oncol. 8, 1095–1111 (2014).
Yap, T. A., Gerlinger, M., Futreal, P. A., Pusztai, L. & Swanton, C. Intratumor heterogeneity: seeing the wood for the trees. Sci. Transl. Med. 4, 127ps10 (2012).
Fisher, R., Pusztai, L. & Swanton, C. Cancer heterogeneity: implications for targeted therapeutics. Br. J. Cancer 108, 479–485 (2013).
Alizadeh, A. A. et al. Toward understanding and exploiting tumor heterogeneity. Nat. Med. 21, 846–853 (2015).
Merlo, L. M., Pepper, J. W., Reid, B. J. & Maley, C. C. Cancer as an evolutionary and ecological process. Nat. Rev. Cancer 6, 924–935 (2006).
Almendro, V. et al. Inference of tumor evolution during chemotherapy by computational modeling and in situ analysis of genetic and phenotypic cellular diversity. Cell Rep. 6, 514–527 (2014).
Mayer, E. L. et al. Long-term follow-up after preoperative trastuzumab and chemotherapy for HER2-overexpressing breast cancer. Clin. Breast Cancer 15, 24–30 (2015).
Rouzier, R. et al. Incidence and prognostic significance of complete axillary downstaging after primary chemotherapy in breast cancer patients with T1 to T3 tumors and cytologically proven axillary metastatic lymph nodes. J. Clin. Oncol. 20, 1304–1310 (2002).
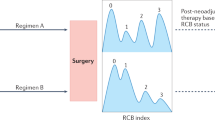
Symmans, W. F. et al. Measurement of residual breast cancer burden to predict survival after neoadjuvant chemotherapy. J. Clin. Oncol. 25, 4414–4422 (2007).
De Mattos-Arruda, L. & Rodon, J. Pilot studies for personalized cancer medicine: focusing on the patient for treatment selection. Oncologist 18, 1180–1188 (2013).
Park, J. W. & Liu, M. C. Neratinib plus standard neoadjuvant therapy for high-risk breast cancer: efficacy results from the I-SPY 2 trial [abstract]. 72 (Suppl.), CT227 (2014).
Sahin, O. et al. Biomarker-guided sequential targeted therapies to overcome therapy resistance in rapidly evolving highly aggressive mammary tumors. Cell Res. 24, 542–559 (2014).
Foulkes, W. D., Smith, I. E. & Reis-Filho, J. S. Triple-negative breast cancer. N. Engl. J. Med. 363, 1938–1948 (2010).
Author information
Authors and Affiliations
Contributions
All authors contributed to researching the data for the article, discussions of content, and the writing, review and editing of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
De Mattos-Arruda, L., Shen, R., Reis-Filho, J. et al. Translating neoadjuvant therapy into survival benefits: one size does not fit all. Nat Rev Clin Oncol 13, 566–579 (2016). https://doi.org/10.1038/nrclinonc.2016.35
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrclinonc.2016.35
This article is cited by
-
Evaluation of predictive and prognostic value of androgen receptor expression in breast cancer subtypes treated with neoadjuvant chemotherapy
Discover Oncology (2023)
-
The temporal mutational and immune tumour microenvironment remodelling of HER2-negative primary breast cancers
npj Breast Cancer (2021)
-
RNA disruption indicates CHOP therapy efficacy in canine lymphoma
BMC Veterinary Research (2019)
-
Mammographic density is a potential predictive marker of pathological response after neoadjuvant chemotherapy in breast cancer
BMC Cancer (2019)
-
Intracellular tracking of drug release from pH-sensitive polymeric nanoparticles via FRET for synergistic chemo-photodynamic therapy
Journal of Nanobiotechnology (2019)