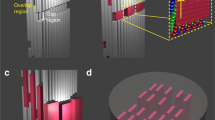
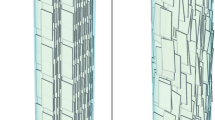
Abstract
The involvement of collagen in bone biomineralization is commonly admitted, yet its role remains unclear. Here we show that type I collagen in vitro can initiate and orientate the growth of carbonated apatite mineral in the absence of any other vertebrate extracellular matrix molecules of calcifying tissues. We also show that the collagen matrix influences the structural characteristics on the atomic scale, and controls the size and the three-dimensional distribution of apatite at larger length scales. These results call into question recent consensus in the literature on the need for Ca-rich non-collagenous proteins for collagen mineralization to occur in vivo. Our model is based on a collagen/apatite self-assembly process that combines the ability to mimic the in vivo extracellular fluid with three major features inherent to living bone tissue, that is, high fibrillar density, monodispersed fibrils and long-range hierarchical organization.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Weiner, S. & Wagner, H. D. The material bone: Structure mechanical function relations. Annu. Rev. Mater. Sci. 28, 271–298 (1998).
Landis, W. J. et al. Mineralization of collagen may occur on fibril surfaces: Evidence from conventional and high-voltage electron microscopy and three-dimensional imaging. J. Struct. Biol. 117, 24–35 (1996).
Jackson, S. F. The fine structure of developing bone in the embryonic fowl. Proc. R. Soc. Lond. B 146, 270–280 (1957).
Weiner, S. & Traub, W. Organization of hydroxyapatite crystals within collagen fibrils. FEBS Lett. 206, 262–266 (1986).
McEwen, B. F., Song, M. J. & Landis, W. J. Quantitative determination of the mineral distribution in different collagen zones of calcifying tendon using high voltage electron microscopic tomography. J. Comput. Assist. Microsc. 3, 201–210 (1991).
Glimcher, M. J. in Medical Mineralogy and Geochemistry Vol. 64 (eds Sahai, N. & Schoonen, M. A. A.) 223–282 (Reviews in Mineralogy & Geochemistry, 2006).
George, A. & Veis, A. Phosphorylated proteins and control over apatite nucleation, crystal growth, and inhibition. Chem. Rev. 108, 4670–4693 (2008).
Palmer, L. C., Newcomb, C. J., Kaltz, S. R., Spoerke, E. D. & Stupp, S. I. Biomimetic systems for hydroxyapatite mineralization inspired by bone and enamel. Chem. Rev. 108, 4754–4783 (2008).
Jahnen-Dechent, W., Heiss, A., Schäfer, C. & Ketteler, M. Fetuin-A regulation of calcified matrix metabolism. Circ. Res. 108, 1494–1509 (2011).
Hunter, G. K. & Goldberg, H. A. Nucleation of hydroxyapatite by bone sialoprotein. Proc. Natl Acad. Sci. USA 90, 8562–8565 (1993).
Frenkel-Mullerad, H. & Avnir, D. Sol–gel materials as efficient enzyme protectors: Preserving the activity of phosphatases under extreme pH conditions. J. Am. Chem. Soc. 127, 8077–8081 (2005).
Vetter, U., Eanes, E. D., Kopp, J. B., Termine, J. D. & Robey, P. G. Changes in apatite crystal size in bones of patients with osteogenesis imperfecta. Calcif. Tissue Int. 49, 248–250 (1991).
Landis, W. J. The strength of a calcified tissue depends in part on the molecular-structure and organization of its constituent mineral crystals in their organic matrix. Bone 16, 533–544 (1995).
Fratzl, P. & Weinkamer, R. Nature’s hierarchical materials. Prog. Mater. Sci. 52, 1263–1334 (2007).
Seto, J., Gupta, H. S., Zaslansky, P., Wagner, H. D. & Fratzl, P. Tough lessons from bone: Extreme mechanical anisotropy at the mesoscale. Adv. Funct. Mater. 18, 1905–1911 (2008).
Rhee, S-H., Suetsugu, Y. & Tanaka, J. Biomimetic configurational arrays of hydroxyapatite nanocrystals on bio-organics. Biomaterials 22, 2843–2847 (2001).
Olszta, M. J. et al. Bone structure and formation: A new perspective. Mater. Sci. Eng. R 58, 77–116 (2007).
Deshpande, A. S. & Beniash, E. Bioinspired synthesis of mineralized collagen fibrils. Cryst. Growth Des. 8, 3084–3090 (2008).
Liu, Y. et al. Hierarchical and non-hierarchical mineralisation of collagen. Biomaterials 32, 1291–1300 (2011).
Nudelman, F. et al. The role of collagen in bone apatite formation in the presence of hydroxyapatite nucleation inhibitors. Nature Mater. 9, 1004–1009 (2010).
Hartgerink, J. D., Beniash, E. & Stupp, S. I. Self-assembly and mineralization of peptide-amphiphile nanofibers. Science 294, 1684–1688 (2001).
Weiner, S., Traub, W. & Wagner, H. D. Lamellar bone: Structure-function relations. J. Struct. Biol. 126, 241–255 (1999).
Boskey, A. L. Biomineralization: Conflicts, challenges, and opportunities. J. Cell. Biochem. 72, 83–91 (1998).
Brown, R. A., Wiseman, M., Chuo, C. B., Cheema, U. & Nazhat, S. N. Ultrarapid engineering of biomimetic materials and tissues: Fabrication of nano- and microstructures by plastic compression. Adv. Funct. Mater. 15, 1762–1770 (2005).
Bouligand, Y. Twisted fibrous arrangements in biological materials and cholesteric mesophases. Tissue Cell 4, 189–190; 192-217 (1972).
Giraud-Guille, M-M. Liquid crystallinity in condensed type I collagen solutions: A clue to the packing of collagen in extracellular matrices. J. Mol. Biol. 224, 861–873 (1992).
Giraud-Guille, M. M., Mosser, G. & Belamie, E. Liquid crystallinity in collagen systems in vitro and in vivo. Curr. Opin. Colloid Interf. Sci. 13, 303–313 (2008).
Besseau, L. & Giraud-Guille, M-M. Stabilization of fluid cholesteric phases of collagen to ordered gelated matrices. J. Mol. Biol. 251, 197–202 (1995).
Nassif, N. et al. Self-assembled collagen-apatite matrix with bone-like hierarchy. Chem. Mater. 22, 3307–3309 (2010).
Silver, F. H. & Landis, W. J. Deposition of apatite in mineralizing vertebrate extracellular matrices: A model of possible nucleation sites on type I collagen. Connect. Tissue Res. 52, 242–254 (2011).
Wang, Y. et al. Controlled collagen assembly to build dense tissue-like materials for tissue engineering. Soft Matter 7, 9659–9664 (2011).
Nassif, N. et al. In vivo inspired conditions to synthesize biomimetic hydroxyapatite. Chem. Mater. 22, 3653–3663 (2010).
Mkukuma, L. D. et al. Effect of the proportion of organic material in bone on thermal decomposition of bone mineral: An investigation of a variety of bones from different species using thermogravimetric analysis coupled to mass spectrometry, high-temperature X-ray diffraction, and Fourier transform infrared spectroscopy. Calcif. Tissue Int. 75, 321–328 (2004).
Rámila, A. & Vallet-Regí, M. Static and dynamic in vitro study of a sol–gel glass bioactivity. Biomaterials 22, 2301–2306 (2001).
Bohner, M. & Lemaitre, J. Can bioactivity be tested in vitro with SBF solution? Biomaterials 30, 2175–2179 (2009).
Gobeaux, F. et al. Fibrillogenesis in dense collagen solutions: A physicochemical study. J. Mol. Biol. 376, 1509–1522 (2008).
Chen, J. et al. In vitro mineralization of collagen in demineralized fish bone. Macromol. Chem. Phys. 206, 43–51 (2005).
Landis, W. J. & Glimcher, M. J. Electron diffraction and electron probe microanalysis of the mineral phase of bone tissue prepared by anhydrous techniques. J. Ultrastruct. Res. 63, 188–223 (1978).
Hodge, A. & Petruska, J. in Aspects of Protein Structure (ed. Ramachandran, G. N.) 289–300 (Academic, 1963).
Traub, W., Arad, T. & Weiner, S. Three-dimensional ordered distribution of crystals in turkey tendon collagen fibers. Proc. Natl Acad. Sci. USA 86, 9822–9826 (1989).
Zhu, P. et al. Time-resolved dehydration-induced structural changes in an intact bovine cortical bone revealed by solid-state NMR spectroscopy. J. Am. Chem. Soc. 131, 17064–17065 (2009).
Tseng, Y-H., Mou, C-Y. & Chan, J. C. C. Solid-state NMR study of the transformation of octacalcium phosphate to hydroxyapatite: A mechanistic model for central dark line formation. J. Am. Chem. Soc. 128, 6909–6918 (2006).
Cho, G., Wu, Y. & Ackerman, J. L. Detection of hydroxyl ions in bone mineral by solid-state NMR spectroscopy. Science 300, 1123–1127 (2003).
Jäger, C., Welzel, T., Meyer-Zaika, W. & Epple, M. A solid-state NMR investigation of the structure of nanocrystalline hydroxyapatite. Magn. Reson. Chem. 44, 573–580 (2006).
Huang, S-J., Tsai, Y-L., Lee, Y-L., Lin, C-P. & Chan, J. C. C. Structural model of rat dentin revisited. Chem. Mater. 21, 2583–2585 (2009).
Wu, Y. et al. Nuclear magnetic resonance spin–spin relaxation of the crystals of bone, dental enamel, and synthetic hydroxyapatites. J. Bone Miner. Res. 17, 472–480 (2002).
Fantner, G. E. et al. Sacrificial bonds and hidden length dissipate energy as mineralized fibrils separate during bone fracture. Nature Mater. 4, 612–616 (2005).
He, G. & George, A. Dentin matrix protein 1 immobilized on type I collagen fibrils facilitates apatite deposition in vitro. J. Biol. Chem. 279, 11649–11656 (2004).
Bradt, J-H., Mertig, M., Teresiak, A. & Pompe, W. Biomimetic mineralization of collagen by combined fibril assembly and calcium phosphate formation. Chem. Mater. 11, 2694–2701 (1999).
Mahamid, J. et al. Mapping amorphous calcium phosphate transformation into crystalline mineral from the cell to the bone in zebrafish fin rays. Proc. Natl Acad. Sci. USA 107, 6316–6321 (2010).
Bauer, T. W. & Muschler, G. F. Bone graft materials—an overview of the basic science. Clin. Orthop. Relat. R 371, 10–27 (2000).
Giraud-Guille, M. M. Twisted plywood architecture of collagen fibrils in human compact bone osteons. Calcif. Tissue Int. 42, 167–180 (1988).
Fratzl, P., Fratzl-Zelman, N. & Klaushofer, K. Collagen packing and mineralization. An X-ray scattering investigation of turkey leg tendon. Biophys. J. 64, 260–266 (1993).
Maxwell, C. A., Wess, T. J. & Kennedy, C. J. X-ray diffraction study into the effects of liming on the structure of collagen. Biomacromolecules 7, 2321–2326 (2006).
Dorozhkin, S. V. & Epple, M. Biological and medical significance of calcium phosphates. Angew. Chem. Int. Ed. 41, 3130–3146 (2002).
Acknowledgements
We dedicate this work to the memory of Y. Bouligand (1935–2011). This work was supported by the Agence Nationale de la Recherche (ANR) through the ANR-09-BLAN-0120-01 ‘NanoShap’ program and the DRITT-SAIC (UPMC). We thank G. Laurent and M. Selmane for technical assistance with the NMR spectrometer and WAXD experiments respectively; A. Anglo, C. Illoul and E. Jallot for preparation of TEM samples; S. Mann, J. Peron, F. Michaux and Ö. Sel for insightful discussions and critical suggestions; A. Galtayries for X-ray photoelectron spectroscopy experiments and IMM Recherche, especially L. Behr, for providing the fresh bone samples.
Author information
Authors and Affiliations
Contributions
Y.W., T.A., M.R., C.C., P.L., G.P-A. and N.N. performed the research; F.B. looked for financial support for the project; Y.W., T.A., A.V., G.P-A., F.B., M-M.G-G. and N.N. analysed data; Y.W., T.A., C.C., A.V., M-M.G-G. and N.N. wrote the paper; N.N. designed the research.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
Supplementary Information (PDF 2769 kb)
Rights and permissions
About this article
Cite this article
Wang, Y., Azaïs, T., Robin, M. et al. The predominant role of collagen in the nucleation, growth, structure and orientation of bone apatite. Nature Mater 11, 724–733 (2012). https://doi.org/10.1038/nmat3362
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nmat3362
This article is cited by
-
Hierarchical helical carbon nanotube fibre as a bone-integrating anterior cruciate ligament replacement
Nature Nanotechnology (2023)
-
Cartilage calcification in osteoarthritis: mechanisms and clinical relevance
Nature Reviews Rheumatology (2023)
-
Solid-state NMR studies on the organic matrix of bone
Nano Research (2023)
-
Multiscale Effects of Collagen Damage in Cortical Bone and Dentin
JOM (2023)
-
Functionalized Coatings on Degradable Magnesium Alloys for Orthopedic Implants: A Review
Transactions of the Indian Institute of Metals (2023)