Abstract
Uveal melanoma has traditionally been treated with enucleation, plaque brachytherapy, or external beam radiation. Following the results of the multicenter Collaborative Ocular Melanoma Study (COMS), which established no significant difference in mortality rates between enucleation and brachytherapy, plaque brachytherapy has become the favoured modality given its potential for preservation of vision and the eye. Among the radioisotopes that have been used, iodine-125 (I-125) has become the increasingly popular choice in the United States. However, I-125 brachytherapy is associated with complications, including keratitis, iris neovascularization, neovascular glaucoma, radiation retinopathy, and optic neuropathy. The purpose of this review is to discuss the pathogenesis, clinical presentation, and management of complications related to I-125 plaque brachytherapy for choroidal melanoma.
Similar content being viewed by others
Introduction
Choroidal melanoma has traditionally been treated with enucleation, plaque brachytherapy, or external beam radiation. In 2001, the Collaborative Ocular Melanoma Study (COMS) established that mortality rates for medium-sized melanomas did not significantly differ after treatment with iodine-125 (I-125) brachytherapy or enucleation.1, 2 Brachytherapy has since become the favoured modality given its potential for preservation of vision and the eye. Many radioisotopes have been utilized for brachytherapy, including cobalt-60,3 palladium-103,4 I-125,5 iridium-192, and ruthenium-106.6 I-125 emits relatively low energy photons, which theoretically decrease radiation-related complications. In spite of this favourable profile, I-125 brachytherapy is associated with complications, including keratitis, radiation cataract, neovascular glaucoma, retinopathy, and optic neuropathy. This paper will review the complications of I-125 brachytherapy for the treatment of choroidal melanoma as well as the management of these complications.
Cornea
Radiation-induced dry eye and keratitis
Dry eye and keratitis are complications often seen after external beam radiation therapy for choroidal melanoma. Following radiotherapy, an increase in conjunctival epithelial stratification and a reduction of goblet cell numbers both contribute to dry eye.7 Tear film instability and dysfunction may lead to a temporary keratitis, often seen as punctate epithelial erosions.8
However, in our experience, dry eye after I-125 brachytherapy is not more frequent than other procedures that involve alteration of the conjunctiva, such as scleral buckling. This may be due to the often posterior position of the plaque. Dry eye was reported in 8.3% of patients and occurs an average of 20.7 months after treatment.9 Quivey et al9 found that keratitis developed in 3.8% of their patients following I-125 brachytherapy, occurring an average of 34 months after treatment. In contrast, another study found that keratitis was present in 20.9% of patients at 2 years after treatment, and this decreased to 2.8% of patients by 5 years after treatment.10 Few other studies using I-125 brachytherapy describe this complication. Symptomatic treatment is recommended and includes topical lubricants and lacrimal punctual occlusion.8
Anterior segment
Pathogenesis and clinical features of radiation-induced iris neovascularization and neovascular glaucoma
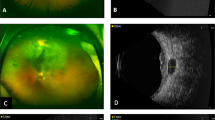
Though complications of the anterior segment are thought to occur more frequently with external beam radiation, they also occur with plaque brachytherapy.11 Untreated choroidal melanoma may cause neovascularization. In addition, ischaemia associated with radiation retinopathy or the ischaemia from a radiation-damaged iris may cause the proliferation of iris vessels. This presents clinically as rubeosis iridis, neovascularization of the anterior chamber, and angle or neovascular glaucoma among other findings (Figure 1). A careful examination of the iris and anterior chamber angle before dilation is important to detect early signs of neovascularization.
Development of radiation-induced iris neovascularization and neovascular glaucoma
Rubeosis iridis following I-125 plaque brachytherapy is reported at rates of 4–23%, occurring at a mean of 26.7 months (Table 1). Although some studies did not specify whether glaucoma in post-plaque eyes was related to neovascularization, those that did had neovascular glaucoma rates ranging from 2 to 45% (Table 1). Puusaari et al12 found a cumulative incidence for any type of glaucoma to be 60% at 5 years after treatment for large melanoma. Neovascular glaucoma was reported to occur anywhere from 2 to 58 months after treatment (Table 1).
Numerous factors may contribute to iris neovascularization. Studies using other radioisotopes, such as cobalt-60 and palladium-103, have associated increased neovascularization with an anterior tumour location.21, 22 With I-125, our group showed higher rates of iris neovascularization with anterior tumours than with posterior tumours though the difference was not statistically significant.15 In addition, we found increased iris neovascularization in cases with disinsertion of a horizontal rectus muscle.15 We attributed this to rectus muscle disinsertion and radiation damage to the long posterior ciliary arteries that run along the horizontal meridians, leading to anterior segment ischaemia.
Increased tumour thickness is associated with higher rates as well as decreased time to the development of iris neovascularization.12, 15 Larger tumours are treated with higher cumulative radiation to collateral structures that likely results in greater iris microvascular injury. Increased internal tumour vascularity was also correlated with iris neovascularization and was postulated to be related to the elevated levels of tumour-related angiogenic factors.15
Few studies have commented on associations relating to neovascular glaucoma. Puusaari et al12 found that elevated preoperative intraocular pressure and presence of preoperative exudative retinal detachment were associated with decreased time to postoperative glaucoma (defined as intraocular pressure greater than 24 mmHg).12 Of the glaucomas they observed, 84% were classified as neovascular.
Treatment of radiation-induced iris neovascularization and neovascular glaucoma
Currently, there is no study in the Medline-indexed English language supporting any specific treatment for I-125-induced neovascular glaucoma or rubeosis iridis. Neovascular glaucoma secondary to ischaemic central retinal vein occlusion (CRVO) or diabetic retinopathy is currently treated both medically and surgically. Medical treatment to control intraocular pressure includes aqueous suppressants (β-blockers, α-adrenergics, and carbonic anhydrase inhibitors), anti-VEGF therapy, and corticosteroid therapy, whereas surgical treatment includes cyclophotocoagulation, filtering surgery, glaucoma drainage device surgery, photodynamic therapy, and pan-retinal photocoagulation.23
Detorakis et al15 treated iris neovascularization after I-125 brachytherapy with panretinal photocoagulation, although efficacy of treatment was not reported. Puusaari et al12 treated post-plaque glaucoma with topical and systemic carbonic anhydrase inhibitors, β-blockers, and latanoprost, and 12% of patients required trans-scleral contact krypton or red diode laser cyclophotocoagulation. Mydriatics and corticosteroids were used for eyes with and without light perception for symptomatic relief. Although other ocular complications following I-125 brachytherapy primarily affect only vision, neovascular glaucoma may lead to a painful and blind eye requiring enucleation. The rate of enucleation secondary to neovascular glaucoma after I-125 brachytherapy ranges from 1 to 12% (Table 1) and indicates the difficulty in managing this complication. At our centre, we have found the rate of enucleation following I-125 brachytherapy to be very low at less than 1%.
Lens
Pathogenesis and clinical features of radiation-induced cataract
Ionizing radiation is known to damage the lens equatorial fibres because of their high mitotic rate.24 The compensatory mitosis occurs with disrupted organization and leads to deposition of Wedl cells at the posterior pole. The clinical appearance of a radiation cataract is well established.25 It may initially appear as a small dot at the posterior pole of the lens and subsequently increase to a diameter of 1–2 mm. The opaque region is comprised of scattered granules and vacuoles (Figure 2). As the cataract continues to develop, the centre of the opacity clears, and the overall appearance is that of a doughnut with a total diameter of 3–4 mm. Radiation exposure may also lead to the development of cortical cataract or exacerbate existing nuclear sclerotic cataract.26
Development of radiation-induced cataract
The reported rate of cataract formation following I-125 brachytherapy for choroidal melanoma varies, from 8% to a predicted 83% by 5 years, as does the time to development, from a median of 11 months after treatment of anterior tumours to a median of 35.3 months after treatment of tumours at any intraocular location (Table 2). The development of cataract is associated with a dose-dependent increase in radiation to the lens. In the largest study to date, the COMS followed the incidence of cataract development in phakic patients over the first 5 years following brachytherapy.27 The study found that 68% (362/532) of study eyes developed vision-limiting cataract or underwent cataract surgery after I-125 brachytherapy with a greater proportion developing cataract following higher doses to the lens. With a cumulative dose to the lens of 24 Gy or more, the 5-year cumulative incidence of cataract was 92% compared with 65% in those with less than 12 Gy. Stack et al14 also found that cataract development was dose-related with a 44% incidence of cataract when doses exceeded 25 Gy.
The radiation dose to the lens is affected by both the tumour size and location. Increasing tumour height has been shown to decrease the time to cataract development and a greater tumour diameter increases the risk of cataract.10, 12 The location of the tumour is also important as treatment of an anterior tumour puts the plaque in closer proximity to the lens, therefore exposing the lens to more radiation. Fontanesi et al17 found that cataract developed much earlier with anterior tumours (median 11 months post-plaque) compared with posterior tumours (median 26 months post-plaque) with a greater proportion of cataract occurring in eyes with anterior tumours.
Treatment of radiation-induced cataract
Radiation-induced cataract may be successfully treated with standard surgical techniques. Fish et al29 studied 13 eyes with radiation-induced cataract following brachytherapy with either cobalt-60 or I-125. Of the 13 cataracts, 11 underwent extracapsular extraction with intraocular lens implantation. The average preoperative visual acuity was 20/400, which improved to 20/90 at 24 months follow-up. The average improvement in the early postoperative period was 5.5 Snellen lines with a mean improvement of 4.5 Snellen lines after an average follow-up of 24 months. Cystoid macular oedema complicated one case.
In a retrospective study, Wachtlin et al30 examined 35 patients who underwent phacoemulsification for cataract following I-125 brachytherapy. A high frequency of preoperative pathology was noted, including rubeosis iridis, secondary glaucoma, and posterior synechiae. In 19 of 35 eyes, the best-corrected postoperative visual acuity improved by 2 or more Snellen lines, and at the last recorded follow-up (mean follow-up of 16.8 months), 9 of 35 eyes maintained this improvement. Operative complications included a 12.5% rate of capsular rupture necessitating anterior vitrectomy and the use of iris retractors in many cases due to the presence of posterior synechiae.
Recently, the COMS published the outcomes of cataract surgery in the first 5 years following I-125 brachytherapy.27 Forty-nine eyes with a median preoperative visual acuity of 20/125 underwent cataract surgery. The best postoperative median visual acuity was 20/40, and the visual acuity improved by more than 2 Snellen lines in 66% (32/48) of patients. Patients whose vision failed to improve had comorbidities, including radiation retinopathy, vitreous haemorrhage, retinal detachment, or optic neuropathy. We have found that eyes developing cataract following I-125 plaque bracytherapy tolerate standard phacoemulsification with lens implantation well.
Phacoemulsification for radiation-induced cataracts is thus beneficial and safe for many patients, particularly in the short-term, although visual improvement is often limited by other radiation complications, such as retinopathy and optic neuropathy. However, the high incidence of coexisting synechiae and increased need for iris retractors should be considered when planning such procedures.
Retina
Pathogenesis and clinical features of radiation-induced retinopathy
Radiation retinopathy was first described in 1933 and includes microaneurysms, telangiectases, neovascularization, vitreous haemorrhage, hard exudates, cotton wool spots, and macular oedema (Figure 3a). The pathogenesis of radiation retinopathy begins after radiation exposure with the preferential loss of vascular endothelial cells and relative sparing of pericytes.31 It has been hypothesized that the differential sensitivity between retinal endothelial cells and pericytes may be related to the direct exposure of the endothelial cells to high ambient oxygen and iron from blood that generates free radicals and damages cell membranes.32 Areas of focal capillary occlusion are developed and dilated capillary collaterals are formed. In acellular, poorly supported capillaries, microaneurysms emerge and telangiectatic-like channels appear, straddling regions of non-perfusion. Ultimately, the inner retinal ischaemia leads to neovascularization, vitreous haemorrhage, tractional retinal detachment, and macular oedema. On fluorescein angiography, the earliest changes that appear are focal capillary closure with neighbouring areas of irregular capillary dilation and microaneurysms (Figure 3b).31, 33
Development of radiation-induced retinopathy and maculopathy
The rate at which radiation-induced retinopathy and maculopathy develop ranges from 10 to 63% and 13 to 52%, respectively (Table 3). The mean time to develop maculopathy was found to be 25.6 months after treatment.9 The risk of radiation retinopathy and maculopathy after plaque therapy is related to radiation dose and factors affecting radiation dose, such as the height and location of tumour. Higher radiation dose and tumours with thickness greater than 4 mm increase the risk for radiation maculopathy.14 Stack et al14 found a 63% risk for radiation maculopathy if the dose to the macula exceeded 90 Gy. A decreased distance to the foveola as well as male gender have also been associated with a decreased time to maculopathy.12 It is our experience that tumours located nasal to the optic nerve develop relatively little retinopathy.
Treatment of radiation-induced retinopathy and maculopathy
Numerous treatment modalities have been utilized in the management of radiation retinopathy and maculopathy. Recent studies and their results are summarized in Table 4, and include intravitreal injections of triamcinolone and bevacizumab, laser photocoagulation, and hyperbaric oxygen treatment.
Intravitreal triamcinolone acetonide is used to treat macular oedema secondary to other retinal vascular diseases. Although the mechanism is poorly understood, triamcinolone may help to restore a compromised inner blood–retinal barrier.45 Triamcinolone acetonide is thought to modulate cytokines and regulate capillary permeability.46 In 31 patients with I-125 radiation-induced maculopathy, a single intravitreal triamcinolone acetonide (4 mg/1 ml) injection was given and visual acuity improved by 2 Snellen lines in 68% at 1 month and 26% at 6 months.43 Optical coherence tomography showed a preinjection foveal thickness averaging 417 μm, which subsequently averaged 207 μm at 1 month postinjection and 292 μm at 6 months postinjection. Noted complications included transiently increased intraocular pressure in 16% (five eyes), persistent glaucoma requiring topical medication in 10% (three eyes), and cataract in 10% (three eyes).
Bevacizumab has been used to treat exudative macular degeneration, diabetic retinopathy, and CRVO among other diseases related to retinal ischaemia. Bevacizumab is a humanized monoclonal antibody to vascular endothelial growth factor (VEGF), and blocking VEGF is thought to decrease vascular permeability and inhibit abnormal neovascularization.47 Twenty-one patients with radiation retinopathy following palladium-103 brachytherapy were treated with intravitreal bevacizumab (1.25 mg/0.05 ml) every 6–12 weeks. After a mean follow-up of 7.8 months, 86% (18/21) had stable or improved visual acuity, with 14% (3/21) regaining 2 or more Snellen lines.36
However, some studies utilizing bevacizumab suggest that the improvement may only be temporary. Ten patients with radiation-induced macular oedema were treated with one intravitreal bevacizumab injection.39 Mean visual acuity at the time of injection was 20/100, which improved to 20/86 at 6 weeks and 20/95 at 4 months. Before injection, mean foveal thickness by OCT was 482 μm, which decreased to 284 μm at 6 weeks postinjection, but increased to 449 μm at 4 months postinjection. It is our experience that intravitreal bevacizumab in patients with recent onset visual decrease secondary to radiation maculopathy have only a transient subjective response. We feel that the role of bevacizumab following I-125 plaque surgery is limited.
Laser photocoagulation has also been used to treat or prevent radiation retinopathy and macular oedema. Pan-retinal photocoagulation has been shown to successfully treat proliferative radiation retinopathy, whereas focal photocoagulation has been used to treat or prevent macular oedema with variable degrees of visual acuity improvement.42, 48, 49 In 19 patients with radiation-induced macular oedema, focal laser therapy led to resolution of oedema in 26% (5/19) at 6 months compared with 4% (1/19) in the untreated group.42 However, after 2 years, there was no significant difference in visual acuity between treated and untreated eyes.
Optic nerve
Pathogenesis and clinical features of radiation-induced optic neuropathy
Though not fully understood, ionizing radiation is thought to damage the optic nerve through injury to both glial and endothelial cells. Somatic mutations in the glial cells render them metabolically deficient. Over time, these injured cells accumulate and lead to demyelination and neuronal degeneration. Damage to the vascular endothelial cells leads to vascular occlusion and necrosis. Pathology specimens show a decreased number of endothelial cells and endothelial cell-lined vessels as well as fibrosis of vessel walls, reactive gliosis, ischaemic demyelination, and perivascular inflammation.50, 51 The slow cellular turnover rate of endothelial and glial cells is consistent with the delayed onset of radiation-induced optic neuropathy.52
Clinically, radiation-induced optic neuropathy typically presents with sudden, painless, monocular vision loss. Plaque brachytherapy may lead to ischaemic insult anterior to the lamina cribrosa, which causes swelling of the optic nerve head.53 Post-plaque optic neuropathy may also appear as peripapillary hard exudates, haemorrhages, and subretinal fluid (Figure 4).54 Swelling of the optic nerve head may persist for weeks to months at which point optic pallor develops.54
Development of radiation-induced optic neuropathy
With the exception of one study, in which none of the 58 patients developed optic neuropathy after I-125 brachytherapy, other studies found that between 8 and 16% of patients developed post-plaque optic neuropathy (Table 5).19With large tumours, a much higher cumulative incidence of 39% at 3 years and 46% at 5 years has been calculated.12 Fontanesi et al17 reported that optic neuropathy developed after a median of 22 months post-plaque, whereas Quivey et al9 reported a mean time of 16 months.
Important risk factors for developing post-plaque optic neuropathy include close proximity of the tumour to the optic disc, greater dose to the optic disc, and large tumour size.12, 14, 28 Stack et al14 reported a 50% risk of developing optic neuropathy if the tumour is less than 4 mm from the disc margin.14
Treatment of radiation-induced optic neuropathy
There are some reports of spontaneous improvement of anterior radiation-induced optic neuropathy. However, most cases progress to severe monocular vision loss and optic atrophy. Although there are studies examining treatment for optic neuropathy secondary to external beam radiation, few reports describe treatment for optic neuropathy after plaque brachytherapy.
Shields et al55 treated nine patients who had developed radiation papillopathy after plaque brachytherapy with an intravitreal triamcinolone acetonide (4 mg/0.1 ml) injection. Radiation papillopathy was seen as optic disk hyperaemia, oedema, and circumpapillary haemorrhage. Within the first week after injection, seven patients had improved visual acuity. At 11 months postinjection, seven of the patients had stable or improved visual acuity and resolution of optic disk hyperaemia and oedema was seen in all patients.
Hyperbaric oxygen treatment has also been used to treat radiation-induced optic neuropathy. The treatment theoretically counteracts the ischaemia of radiation neuropathy by improving oxygenation. In a case report on a patient with post-plaque radiation retinopathy and optic neuropathy, hyperbaric oxygen treatment was given (20 sessions over an unspecified period of time, 2 h each, 100% oxygen at 2 atm), and at 2 months follow-up, the patient's visual field studies showed marked improvement.44 However, the results of most studies of hyperbaric oxygen treatment in the setting of external beam related optic neuropathy have been poor.52 A recent review on the efficacy of hyperbaric oxygen treatment found that only a few cases of radiation optic neuropathy had improved with treatment.56 Response to treatment was better in patients who were treated shortly after the onset of visual loss (within 72 h) and in patients without optic pallor. Significant visual recovery has not been seen in patients whose treatment was initiated 2 or more weeks after the onset of symptoms.53
Of all the complications associated with I-125 plaque brachytherapy, optic neuropathy is the most devastating to visual function. It is our experience that most cases of severe visual loss following treatment are the results of this unavoidable and untreatable complication.
Visual acuity
Although globe preservation is usually achieved with I-125 plaque brachytherapy, most patients experience a decline in visual function secondary to the radiation complications described above. In the COMS Report No. 16, 623 patients were followed for 3 years after receiving I-125 plaque brachytherapy.57 Life-table estimates from this study predicted that at 3 years, 49% of patients would have a loss of 6 or more Snellen lines, whereas 43% of patients would have a visual acuity of 20/200 or less.57
With varying durations of follow-up, smaller studies find that the percentage of post-plaque patients who retained visual acuity within 2 Snellen lines of pre-plaque visual acuity ranges from 26 to 62% (Table 6). The percentage of patients retaining a visual acuity of 20/200 or better post-plaque ranges from 41 to 58%.9, 17, 20 However, this number may be as low as 5.5% in patients with large tumours (defined as tumour height exceeding 7.5 mm).16
Many risk factors contribute to an increased risk for decline in post-plaque visual acuity. Predictably, they encompass the various risk factors that contribute to the development of cataract, retinopathy, maculopathy, and optic neuropathy. In the COMS, the characteristics, including greater tumour apical height (>5.0 mm) and decreased distance between the tumour and the foveal avascular zone (FAZ) (<2.0 mm), were most strongly associated with losing 6 Snellen lines or more.57 Comorbid diabetes, tumour-associated retinal detachment, and non-dome-shaped tumours were associated with poor visual outcome as well.57
Other studies have also supported factors, such as radiation dose, greater tumour size, and close proximity to macula and optic disc, increase the risk of visual decline.5, 9, 10, 16, 19, 20, 34 Quivey et al9 found that a tumour thickness greater than 6 mm was correlated with decreased visual acuity. Specific thresholds for locations correlating with an increased risk included tumour located in less than 3 mm from or within 3 disc diameters of the fovea or optic nerve.9, 20
Jones et al34 reported that a dose rate of 111±11.1 cGy/h to the macula predicted a 50% risk of visual decline. Systemic conditions, such as diabetes and coronary artery disease, were also correlated with an increased risk of visual decline.5, 10, 28, 57
Episclera and sclera
Pathogenesis and clinical features of episcleral deposits
Toivonen and Kivela described the development of pigmented episcleral deposits following either ruthenium or iodine plaque brachytherapy.59 Immunohistochemically, they found that the deposits stained positive for macrophage-related marker, CD68, but were negative for melanoma-related HMB-45, suggesting that the deposits were composed of migrating macrophages and their ingested debris. Clinically, the deposits appeared as black or brown spots ranging from 8 μm to 3 mm, first appearing between 1 and 6 months with increasing numbers until 7 years after treatment. In their patients, they found that 85% developed deposits within 1 year. The deposits often appeared near the tumour centre, and a larger plaque size was associated with a greater number of deposits. Fewer deposits were seen with iodine plaques.
Treatment of episcleral deposits
As the deposits are thought to represent migrating macrophages rather than extrascleral extension of the melanoma, the authors caution against unnecessary enucleation.59
Scleral necrosis
Scleral necrosis following plaque brachytherapy has been reported, often in association with postoperative brachytherapy after pterygium excision.60, 61, 62 Petrovich et al63 described the histologic appearance of enucleated eyes with choroidal melanoma that had been treated with plaque brachytherapy. Scleral atrophy was seen in 33% of post-plaque eyes. However, in the studies with I-125 brachytherapy, few reports mention scleral atrophy or necrosis as a complication. Stack et al14 documented that none of their 84 patients developed scleral necrosis after I-125 brachytherapy.
Adnexa
Pathogenesis and clinical features of extraocular muscle alterations
Extraocular muscles are theoretically shielded from most of the I-125 radiation as only 0.1% of radiation passes through a 0.5 mm thick gold foil.64 However, with the current plaque design, extraocular muscles may actually be exposed to a significant amount of laterally directed and uncollimated radiation. Kiratli et al65 compared biopsy specimens from radiation-exposed extraocular muscles with non-irradiated, extraocular muscles from enucleated controls, and found that the radiation-exposed muscles had a focal decrease in muscular tissue with increased fibroblasts and collagen. Furthermore, on electron microscopy, a loss of sarcoplasmic reticulum with mitochondrial swelling was noted. The authors argued that the sarcoplasmic reticulum loss, vascular wall thickening, and focal muscle tissue loss suggested radiation injury rather than pure mechanical injury due to stretching and ischaemia. Although these may simply represent non-specific ultrastructural changes, they may affect extraocular muscle function.
Development of extraocular muscle alterations
Sener et al66 observed that 60% of their patients (12/20) had ocular alignment and motility problems following plaque brachytherapy. However, only 10% (2/20) of patients complained of diplopia. Dawson et al67 found that 1.7% (16/929) of their patients developed persistent diplopia or strabismus following plaque brachytherapy during an 8-year follow-up. For 69% (11/16) of these patients, the onset occurred within the first year. Whether these findings are attributable to radiation or simply mechanical injury from muscle manipulation, patients and physicians should be aware of this potential complication.
It is our experience that a significant proportion of patients develop transient diplopia following I-125 plaque brachytherapy that resolves within the first postoperative month, and we found that disinsertion and repositioning of a rectus muscle does not appear to influence this phenomenon.
Treatment of extraocular muscle alterations
At this point, there is no study in the Medline-indexed English literature comparing management methods of diplopia following plaque brachytherapy. Sener et al66 suggest using either prism correction or botulinum toxin A injections in the early postoperative period, and recommend waiting at least 6 months before pursuing surgical correction of strabismus as radiation effects may be variable for some time.66
In our experience, patients may require strabismus surgery in the long term to correct exotropia secondary to the loss of fixation in a poor seeing eye. The only two cases in our experience where strabismus surgery was required in the short term was to correct a secondary Brown Syndrome, and to correct a wide-angle exotropia caused by an inadvertently sutured lateral rectus muscle.
Conclusion
Although I-125 plaque brachytherapy has become the treatment of choice for medium-sized choroidal melanoma, there are numerous post-treatment complications of relevance to the ocular oncologist and referring ophthalmologist. Anterior segment pathology occurs in 4–23% of treated patients with enucleation rates for neovascular glaucoma found to be as high as 12% after treatment. Radiation-induced cataract develops in 8–83% by 5 years post-plaque, and radiation-induced retinopathy occurs in 10–63% of treated eyes. Optic neuropathy has been reported in up to 16% of patients. All of these complications affect overall visual acuity, and 26–62% of treated eyes experience a loss of 2 Snellen lines or more. Although cataract surgery for radiation-induced cataract may be effective in improving visual acuity, other treatment modalities, such as intravitreal triamcinolone or bevacizumab injections, hyperbaric oxygen treatments, and laser photocoagulation, for radiation-induced retinopathy, maculopathy, and optic neuropathy appear to be far less effective.
Complications associated with I-125 plaque brachytherapy are well known, and this review shows that the incidence of complications is highly variable. Complications not only depend on tumour size and location, but also may be related to planning and surgical technique that may vary between treatment centres. Further study in this area with an emphasis on visual function parameters in addition to prospective evaluation of clinical characteristics will help provide a framework for studying potential preventive strategies for reducing the complications of radiation.
References
Diener-West M, Earle JD, Fine SL, Hawkins BS, Moy CS, Reynolds SM et al. The COMS randomized trial of iodine 125 brachytherapy for choroidal melanoma, III: initial mortality findings. COMS Report No. 18. Arch Ophthalmol 2001; 119 (7): 969–982.
Collaborative Ocular Melanoma Study Group. The COMS randomized trial of iodine 125 brachytherapy for choroidal melanoma: V. Twelve-year mortality rates and prognostic factors: COMS report No. 28. Arch Ophthalmol 2006; 124 (12): 1684–1693.
Shields JA, Augsburger JJ, Brady LW, Day JL . Cobalt plaque therapy of posterior uveal melanomas. Ophthalmology 1982; 89 (10): 1201–1207.
Finger PT, Moshfeghi DM, Ho TK . Palladium 103 ophthalmic plaque radiotherapy. Arch Ophthalmol 1991; 109 (11): 1610–1613.
Packer S, Rotman M . Radiotherapy of choroidal melanoma with iodine 125. Int Ophthalmol Clin 1980; 20 (2): 135–142.
Lommatzsch P . Treatment of choroidal melanomas with 106Ru/106Rh beta-ray applicators. Surv Ophthalmol 1974; 19 (2): 85–100.
Heimann H, Coupland SE, Gochman R, Hellmich M, Foerster MH . Alterations in expression of mucin, tenascin-c and syndecan-1 in the conjunctiva following retinal surgery and plaque radiotherapy. Graefes Arch Clin Exp Ophthalmol 2001; 239 (7): 488–495.
Durkin SR, Roos D, Higgs B, Casson RJ, Selva D . Ophthalmic and adnexal complications of radiotherapy. Acta Ophthalmol Scand 2007; 85 (3): 240–250.
Quivey JM, Char DH, Phillips TL, Weaver KA, Castro JR, Kroll SM . High intensity 125-iodine (125I) plaque treatment of uveal melanoma. Int J Radiat Oncol Biol Phys 1993; 26 (4): 613–618.
Lumbroso-Le Rouic L, Charif Chefchaouni M, Levy C, Plancher C, Dendale R, Asselain B et al. 125I plaque brachytherapy for anterior uveal melanomas. Eye 2004; 18 (9): 911–916.
Finger PT . Radiation therapy for choroidal melanoma. Surv Ophthalmol 1997; 42 (3): 215–232.
Puusaari I, Heikkonen J, Kivela T . Ocular complications after iodine brachytherapy for large uveal melanomas. Ophthalmology 2004; 111 (9): 1768–1777.
Krohn J, Monge OR, Skorpen TN, Mørk SJ, Dahl O . Posterior uveal melanoma treated with I-125 brachytherapy or primary enucleation. Eye 2008; 22 (11): 1398–1403.
Stack R, Elder M, Abdelaal A, Hidajat R, Clemett R et al. New Zealand experience of I125 brachytherapy for choroidal melanoma. Clin Experiment Ophthalmol 2005; 33 (5): 490–494.
Detorakis ET, Engstrom Jr RE, Wallace R, Straatsma BR . Iris and anterior chamber angle neovascularization after iodine 125 brachytherapy for uveal melanoma. Ophthalmology 2005; 112 (3): 505–510.
Bechrakis NE, Bornfeld N, Zoller I, Foerster MH . Iodine 125 plaque brachytherapy vs transscleral tumor resection in the treatment of large uveal melanomas. Ophthalmology 2002; 109 (10): 1855–1861.
Fontanesi J, Meyer D, Xu S, Tai D . Treatment of choroidal melanoma with I-125 plaque. Int J Radiat Oncol Biol Phys 1993; 26 (4): 619–623.
Mameghan H, Karolis C, Fisher R, Mameghan J, Billson FA, Donaldson EJ et al. Iodine-125 irradiation of choroidal melanoma: clinical experience from the Prince of Wales and Sydney Eye Hospitals. Australas Radiol 1992; 36 (3): 249–252.
Bosworth JL, Packer S, Rotman M, Ho T, Finger PT . Choroidal melanoma: I-125 plaque therapy. Radiology 1988; 169 (1): 249–251.
Garretson BR, Robertson DM, Earle JD . Choroidal melanoma treatment with iodine 125 brachytherapy. Arch Ophthalmol 1987; 105 (10): 1394–1397.
Finger PT . Tumour location affects the incidence of cataract and retinopathy after ophthalmic plaque radiation therapy. Br J Ophthalmol 2000; 84 (9): 1068–1070.
Char DH, Lonn LI, Margolis LW . Complications of cobalt plaque therapy of choroidal malanomas. Am J Ophthalmol 1977; 84 (4): 536–541.
Hayreh SS . Neovascular glaucoma. Prog Retin Eye Res 2007; 26 (5): 470–485.
Yanoff M, Duker JS, Augsburger JJ . Ophthalmology, 2nd edn. Mosby: St Louis, 2004.
Cogan DG, Donaldson DD, Reese AB . Clinical and pathological characteristics of radiation cataract. AMA Arch Ophthalmol 1952; 47 (1): 55–70.
Ferrufino-Ponce ZK, Henderson BA . Radiotherapy and cataract formation. Semin Ophthalmol 2006; 21 (3): 171–180.
Collaborative Ocular Melanoma Study Group. Incidence of cataract and outcomes after cataract surgery in the First 5 Years after iodine 125 brachytherapy in the Collaborative Ocular Melanoma Study COMS Report No. 27. Ophthalmology 2007; 117 (7): 1363–1371.
Jensen AW, Petersen IA, Kline RW, Stafford SL, Schomberg PJ, Robertson DM . Radiation complications and tumor control after 125I plaque brachytherapy for ocular melanoma. Int J Radiat Oncol Biol Phys 2005; 63 (1): 101–108.
Fish GE, Jost BF, Snyder WI, Fuller DG, Birch DG . Cataract extraction after brachytherapy for malignant melanoma of the choroid. Ophthalmology 1991; 98 (5): 619–622.
Wachtlin J, Bechrakis NE, Schueler AO, Helbig H, Bornfeld N, Foerster MH . Phacoemulsification following treatment of choroidal melanoma. Graefes Arch Clin Exp Ophthalmol 2000; 238 (12): 942–948.
Archer DB, Amoaku WM, Gardiner TA . Radiation retinopathy—clinical, histopathological, ultrastructural and experimental correlations. Eye 1991; 5 ( Pt 2): 239–251.
Archer DB, Gardiner TA . Ionizing radiation and the retina. Curr Opin Ophthalmol 1994; 5 (3): 59–65.
Amoaku WM, Archer DB . Fluorescein angiographic features, natural course and treatment of radiation retinopathy. Eye 1990; 4 (partt 5): 657–667.
Jones R, Gore E, Mieler W, Murray K, Gillin M, Albano K et al. Posttreatment visual acuity in patients treated with episcleral plaque therapy for choroidal melanomas: dose and dose rate effects. Int J Radiat Oncol Biol Phys 2002; 52 (4): 989–995.
Sia S, Harper C, McAllister I, Perry A . Iodine-I25 episcleral plaque therapy in uveal melanoma. Clin Experiment Ophthalmol 2000; 28 (6): 409–413.
Finger PT . Radiation retinopathy is treatable with anti-vascular endothelial growth factor bevacizumab (Avastin). Int J Radiat Oncol Biol Phys 2008; 70 (4): 974–977.
Arriola-Villalobos P, Donate-Lopez J, Calvo-Gonzalez C, Reche-Frutos J, Alejandre-Alba N, Díaz-Valle D . Intravitreal bevacizumab (Avastin) for radiation retinopathy neovascularization. Acta Ophthalmol Scand 2008; 86 (1): 115–116.
Ziemssen F, Voelker M, Altpeter E, Bartz-Schmidt KU, Gelisken F . Intravitreal bevacizumab treatment of radiation maculopathy due to brachytherapy in choroidal melanoma. Acta Ophthalmol Scand 2007; 85 (5): 579–580.
Mason III JO, Albert Jr MA, Persaud TO, Vail RS . Intravitreal bevacizumab treatment for radiation macular edema after plaque radiotherapy for choroidal melanoma. Retina 2007; 27 (7): 903–907.
Finger PT, Chin K . Anti-vascular endothelial growth factor bevacizumab (avastin) for radiation retinopathy. Arch Ophthalmol 2007; 125 (6): 751–756.
Finger PT, Kurli M . Laser photocoagulation for radiation retinopathy after ophthalmic plaque radiation therapy. Br J Ophthalmol 2005; 89 (6): 730–738.
Hykin PG, Shields CL, Shields JA, Arevalo JF . The efficacy of focal laser therapy in radiation-induced macular edema. Ophthalmology 1998; 105 (8): 1425–1429.
Shields CL, Demirci H, Dai V, Marr BP, Mashayekhi A, Materin MA et al. Intravitreal triamcinolone acetonide for radiation maculopathy after plaque radiotherapy for choroidal melanoma. Retina 2005; 25 (7): 868–874.
Gall N, Leiba H, Handzel R, Pe′er J . Severe radiation retinopathy and optic neuropathy after brachytherapy for choroidal melanoma, treated by hyperbaric oxygen. Eye 2007; 21 (7): 1010–1012.
Gillies MC . Regulators of vascular permeability: potential sites for intervention in the treatment of macular edema. Doc Ophthalmol 1999; 97 (3-4): 251–260.
Jermak CM, Dellacroce JT, Heffez J, Peyman GA . Triamcinolone acetonide in ocular therapeutics. Surv Ophthalmol 2007; 52 (5): 503–522.
Rosenfeld PJ, Schwartz SD, Blumenkranz MS, Miller JW, Haller JA, Reimann JD et al. Maximum tolerated dose of a humanized anti-vascular endothelial growth factor antibody fragment for treating neovascular age-related macular degeneration. Ophthalmology 2005; 112 (6): 1048–1053.
Kinyoun JL, Zamber RW, Lawrence BS, Barlow WE, Arnold AM . Photocoagulation treatment for clinically significant radiation macular oedema. Br J Ophthalmol 1995; 79 (2): 144–149.
Kinyoun JL, Chittum ME, Wells CG . Photocoagulation treatment of radiation retinopathy. Am J Ophthalmol 1988; 105 (5): 470–478.
Levin LA, Gragoudas ES, Lessell S . Endothelial cell loss in irradiated optic nerves. Ophthalmology 2000; 107 (2): 370–374.
Kline LB, Kim JY, Ceballos R . Radiation optic neuropathy. Ophthalmology 1985; 92 (8): 1118–1126.
Miller NR . Radiation-induced optic neuropathy: still no treatment. Clin Experiment Ophthalmol 2004; 32 (3): 233–235.
Danesh-Meyer HV . Radiation-induced optic neuropathy. J Clin Neurosci 2008; 15 (2): 95–100.
Brown GC, Shields JA, Sanborn G, Augsburger JJ, Savino PJ, Schatz NJ . Radiation optic neuropathy. Ophthalmology 1982; 89 (12): 1489–1493.
Shields CL, Demirci H, Marr BP, Mashayekhi A, Dai VV, Materin MA et al. Intravitreal triamcinolone acetonide for acute radiation papillopathy. Retina 2006; 26 (5): 537–544.
Levy RL, Miller NR . Hyperbaric oxygen therapy for radiation-induced optic neuropathy. Ann Acad Med Singapore 2006; 35 (3): 151–157.
Melia BM, Abramson DH, Albert DM, Boldt HC, Earle JD, Hanson WF et al. Collaborative ocular melanoma study (COMS) randomized trial of I-125 brachytherapy for medium choroidal melanoma. I. Visual acuity after 3 years COMS report no. 16. Ophthalmology 2001; 108 (2): 348–366.
Brady LW, Hernandez JC . Brachytherapy of choroidal melanomas. Strahlenther Onkol 1992; 168 (2): 61–65.
Toivonen P, Kivela T . Pigmented episcleral deposits after brachytherapy of uveal melanoma. Ophthalmology 2006; 113 (5): 865–873.
Fukushima S, Inoue T, Inoue T, Ozeki S . Postoperative irradiation of pterygium with 90Sr eye applicator. Int J Radiat Oncol Biol Phys 1999; 43 (3): 597–600.
MacKenzie FD, Hirst LW, Kynaston B, Bain C . Recurrence rate and complications after beta irradiation for pterygia. Ophthalmology 1991; 98 (12): 1776–1780, discussion 1781).
Monteiro-Grillo I, Gaspar L, Monteiro-Grillo M, Pires F, Ribeiro da Silva JM . Postoperative irradiation of primary or recurrent pterygium: results and sequelae. Int J Radiat Oncol Biol Phys 2000; 48 (3): 865–869.
Petrovich Z, McDonnell JM, Palmer D, Langholz BM, Liggett PE . Histopathologic changes following irradiation for uveal tract melanoma. Am J Clin Oncol 1994; 17 (4): 298–306.
Earle J, Kline RW, Robertson DM . Selection of iodine 125 for the Collaborative Ocular Melanoma Study. Arch Ophthalmol 1987; 105 (6): 763–764.
Kiratli H, Yilmaz PT, Sargon M . Ultrastructural alterations in extraocular muscles following iodine-125 brachytherapy for uveal melanoma. Strabismus 2007; 15 (2): 103–109.
Sener EC, Kiratli H, Gedik S, Sanac AS . Ocular motility disturbances after episcleral plaque brachytherapy for uveal melanoma. J AAPOS 2004; 8 (1): 38–45.
Dawson E, Sagoo MS, Mehta JS, Comer R, Hungerford J, Lee J et al. Strabismus in adults with uveal melanoma following episcleral plaque brachytherapy. J AAPOS 2007; 11 (6): 584–588.
Acknowledgements
We thank Dr Bradley R Straatsma for his thoughtful comments on the manuscript. This study was supported by unrestricted grants from Research to Prevent Blindness Inc. and by the Frederic G Rappaport Fellowship Award to Scott CN Oliver, MD.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wen, J., Oliver, S. & McCannel, T. Ocular complications following I-125 brachytherapy for choroidal melanoma. Eye 23, 1254–1268 (2009). https://doi.org/10.1038/eye.2009.43
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/eye.2009.43
Keywords
This article is cited by
-
Early anti-VEGF treatment for radiation maculopathy and optic neuropathy: lessons learned
Eye (2023)
-
Central subfield thickness predicts visual acuity outcomes in plaque-irradiated eyes with choroidal melanoma
Graefe's Archive for Clinical and Experimental Ophthalmology (2023)
-
Diagnostic methods and therapeutic options of uveal melanoma with emphasis on MR imaging—Part II: treatment indications and complications
Insights into Imaging (2021)
-
Occupational radiation exposure and glaucoma and macular degeneration in the US radiologic technologists
Scientific Reports (2018)
-
Transscleral resection without hypotensive anaesthesia vs iodine-125 plaque brachytherapy in the treatment of choroidal melanoma
Eye (2016)