Abstract
NF-κB transcription factors have a critical role in regulating cell survival and apoptosis. We have previously shown that 4-(3-Cl-(1-adamantyl)-4-hydroxyphenyl)-3-chlorocinnamic acid (3-Cl-AHPC), an adamantyl-substituted retinoid molecule, induced apoptosis and required NF-κB activation in prostate and breast carcinoma cells. Here, we show that 3-Cl-AHPC activated both IκB kinase (IKK)α and IKKβ with subsequent activation of the canonical and noncanonical NF-κB pathways in the human breast carcinoma and leukemia cell lines. 3-Cl-AHPC-mediated activation of the NF-κB canonical pathway occurred within 6 h, whereas maximal activation of the NF-κB noncanonical pathway required 48 h. Knockout of IKKα or IKKβ expression in mouse embryonic fibroblast cells and knockdown of IKKα or IKKβ in MDA-MB-468 cells resulted in the inhibition of 3-Cl-AHPC-mediated apoptosis, indicating that activation of canonical and noncanonical pathways are required for maximal 3-Cl-AHPC-mediated apoptosis. 3-Cl-AHPC activation of the noncanonical pathway was preceded by caspase-mediated decrease in the E3-ligase c-IAP1 with subsequent stabilization of NF-κB-inducing kinase (NIK) expression, increased binding of NIK by TRAF3, activation of IKKα, and the resultant increased levels of RelB and p52. Increased expression of c-IAP1 blocked 3-Cl-AHPC-mediated stabilization of NIK levels and 3-Cl-AHPC-mediated apoptosis. Cdc37 expression was required for activation of IKKα and IKKβ by 3-Cl-AHPC. These findings suggest that NF-κB pathways have an important role in 3-Cl-AHPC-mediated apoptosis.
Similar content being viewed by others
Main
The ability of a novel class of compounds, termed adamantyl-substituted retinoid-related molecules (ARRs) by several investigators to induce apoptosis in malignant cells has been well documented.1, 2 While inducing death of malignant cells, several studies have documented that these compounds have no detrimental effects on normal cells.3, 4 In addition, ST1926, an analog of the parent compound CD437, is now undergoing Phase I clinical trials.3 We have previously shown the ability of the retinoid-related molecule 4-(3-Cl-(1-adamantyl)-4-hydroxyphenyl)-3-chlorocinnamic acid (3-Cl-AHPC) to induce apoptosis in a number of malignant cell types.5 Furthermore, we and others have also reported that apoptosis induction by 3-Cl-AHPC requires NF-κB activation.1, 6
NF-κB represents a complex family of proteins that have key roles in a variety of cellular processes, including oncogenesis, proliferation, inflammatory and stress responses as well as apoptosis.7, 8 The NF-κB family consists of p50, p52, RelA(p65), c-Rel and RelB.7, 8 Recent investigations have documented the presence of the following three major NF-κB-activating pathways.7, 8 (1) A canonical pathway in which external stimuli through a number of intracellular cascades activate the IκB kinase (IKK) complex that in turn phosphorylates IκB and p105 followed by their polyubiquitination by the SCFβTrCP E3 ligase complex and their subsequent destruction. These events in turn result in the release of p50, p65 and c-Rel; their localization in the nucleus; formation of heterodimers and modulation of gene transcription. (2) A noncanonical pathway involving the processing of NF-κB2 p100 to generate p52-containing complexes preceded by NF-κB-inducing kinase (NIK) activation of a unique IKK signalosome consisting of IKKα homodimers.7, 8 (3) DNA damage or oxygen stress induced NF-κB activation using an IKK-independent process.9 Numerous studies using in vitro as well as in vivo model systems have documented a role for NF-κB in cell survival and the inhibition of cellular apoptosis.10, 11, 12 Knockout of RelA resulted in an embryonic lethal phenotype in transgenic mice because of RelA's failure to inhibit tumor necrosis factor (TNF)-induced hepatocyte apoptosis.10 Moreover, NF-κB was shown to block TNF-mediated apoptosis in a number of malignant and nonmalignant cell lines.11
More recent studies have strongly suggested that NF-κB activation under specific situations may have an important role in the induction of apoptosis in certain cell types. Many of the studies examined the effects of inhibition of NF-κB activation on apoptosis induction and have shown that NF-κB inhibition blocked cellular apoptosis.1 Other studies have shown that NF-κB activation by a number of therapeutic agents promoted cell death.13 NF-κB activation by doxorubicin and daunorubicin in U2OS osteosarcoma cells promoted cell death.13 Similar observations were made in colorectal carcinoma cell lines where activation of NF-κB was required for doxorubicin induction of apoptosis in these cells.13 Although some observations have suggested that NF-κB-mediated induction of apoptosis may be related to the activation of specific family members, other studies have suggested that this is not the case and that family members such as c-Rel and RelA can function as both pro-apoptotic or anti-apoptotic agents depending upon the context in which NF-κB is activated.14 Here, we delineated the pathways by which 3-Cl-AHPC activates NF-κB. We also show that maximum 3-Cl-AHPC-mediated apoptosis and NF-κB activation require the combined activation of both IKKα in the noncanonical and IKKβ in the canonical pathways. 3-Cl-AHPC mediates the enhanced expression of NIK, which then activates IKKα. Activated IKKα results in the phosphorylation and processing of NF-κB2p100 and the subsequent generation and nuclear translocation of p52.
Results
3-Cl-AHPC activates the canonical and noncanonical NF-κB pathways
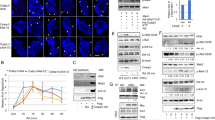
We found that inhibition of 3-Cl-AHPC-mediated NF-κB activation in human DU145 prostate and MDA-MB-468 breast cancer cells blocked 3-Cl-AHPC induction of apoptosis.1 We examined the role of NF-κB activation in 3-Cl-AHPC apoptosis induction in KG-1 human leukemia cells using the NF-κB inhibitor JSH-23 that selectively blocks nuclear translocation of the NF-κB p65/RelA subunit and its transcriptional activity.15 Treatment with 3-Cl-AHPC resulted in a twofold NF-κB activation at 24 h in KG-1 cells (Figure 1a). Inhibition of this activation by JSH-23 resulted in a 35% inhibition of 3-Cl-AHPC-mediated apoptosis (Figure 1b). JSH-23 only blocks the NF-κB canonical pathway and not the noncanonical pathway that is still activated in the JSH-23- and 3-Cl-AHPC-treated cells; both pathways contribute to maximal apoptosis (see below), thus the noncanonical pathway still enhances apoptosis even in the presence of JSH-23. We have previously shown that 3-Cl-AHPC treatment of DU145 and MDA-MB-468 cells resulted in the IKKα kinase activation at 24 h as indicated by the increased phosphorylation of GST-IκBα.1 3-Cl-AHPC activation of IKKβ kinase was not noted at 24 h. Phosphorylation of the activation loops of IKKα and IKKβ has been associated with their conformational change and IKK kinase activation.7, 16 We examined 3-Cl-AHPC activation of IKKα and IKKβ by assessing 3-Cl-AHPC-mediated phosphorylation of their activation loops at both early and late time points. 3-Cl-AHPC treatment of MDA-MB-468 cells resulted in rapid IKKβ activation with maximum phosphorylation 7.5-fold noted at 6 h and its decrease to 3-fold at 24 h and total loss at 48 h (Figure 1c and Supplementary Figure S1a). 3-Cl-AHPC enhanced IKKα phosphorylation in MDA-MB-468 cells by 1.5-fold at 6 and 24 h with maximum IKKα phosphorylation 3.2-fold occurring at 48 h after the addition of 3-Cl-AHPC (Figure 1c and Supplementary Figure S1a). 3-Cl-AHPC-mediated IKKβ phosphorylation in KG-1 cells displayed a similar pattern to that noted in MDA-MB-468 cells with a 5.2-fold increase noted at 6 h and 2.9-fold increase noted at 24 h after 3-Cl-AHPC exposure (Figure 1c and Supplementary Figure S1a). Basal IKKα activation loop phosphorylation was observed before 3-Cl-AHPC exposure in KG-1 cells. Enhanced IKKα phosphorylation was noted at 6 h (1.4-fold), 24 h (1.2-fold) and 48 h (1.2-fold) after the addition of 3-Cl-AHPC (Figure 1c and Supplementary Figure S1a).
3-Cl-AHPC-mediated phosphorylation of IKKα and IKKβ, and activation of the NF-κB canonical and noncanonical pathways. (a) 3-Cl-AHPC activates NF-κB in KG-1 cells; cells were transduced with lentiviral NF-κB reporter (GFP) particles transiently for 48 h and then treated with 1 μM 3-Cl-AHPC for 24 h. (b) NF-κB activation inhibitor JSH-23 blocks 3-Cl-AHPC-mediated apoptosis. Induction of apoptosis and cell death was assessed using Annexin V-FITC labeling with propidium iodide (PI) staining; the percentage of apoptotic cells corresponds to the sum of percent noted in upper right (late apoptotic cells, annexin V and PI-positive cells) and lower right (early apoptotic cells, annexin V positive, PI-negative) quadrants. KG-1 cells were exposed to 1 μM 3-Cl-AHPC for 24 h. (c) 3-Cl-AHPC enhances phosphorylation of IKKα and IKKβ. (d) 3-Cl-AHPC induces phosphorylation of NF-κBp65/RelA at Ser276. (e) 3-Cl-AHPC enhances phosphorylation of NF-κB2p100 followed by processing to p52 in both cell lines. (f) 3-Cl-AHPC induces increased RelB expression and the RelB binding with NF-κB2p100/p52. Cells were grown and exposed to vehicle or 3-Cl-AHPC (1.0 μM) for various times as described in Materials and Methods section. Columns represent mean of two independent experiments. Error bars indicate standard deviations. * and ** significantly different from control cells; and JSH-23+3-Cl-AHPC from 3-Cl-AHPC treated cells, respectively (P-value is <0.05 and <0.01 as determined by t-test)
3-Cl-AHPC activation of the canonical pathway was documented by increased phosphorylation of the NF-κB p65/RelA at Ser276, whereas its activation of the noncanonical pathway was documented by phosphorylation of NF-κB2p100 and generation of p52 (Figures 1d and e). Exposure of MDA-MB-468 to 3-Cl-AHPC was accompanied by increased phosphorylation of NF-κB2p100 with maximal phosphorylation noted at 24 and 6 h in MDA-MB-468 and KG-1 cells, respectively (Figure 1e). In both cell lines, there were associated and correlated increases in the NF-κB2p100 levels and the p52 levels (Figure 1e). Activation of the noncanonical pathway has been shown to generate an increase in RelB/p52 heterodimer levels with the binding to unique consensus sequences.17 3-Cl-AHPC-mediated activation of the noncanonical pathway also resulted in the markedly increased RelB levels and the binding of RelB to NF-κB2p100/p52 (Figure 1f).
Inhibition of the 3-Cl-AHPC-mediated activation of either the canonical or noncanonical pathways blocks 3-Cl-AHPC-mediated apoptosis
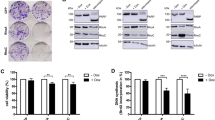
Ablation of IKKα, IKKβ or IKKγ in mouse embryonic fibroblast (MEF) cells resulted in decreases of 99, 95 and 85%, respectively, in the 3-Cl-AHPC-mediated apoptosis at 48 h (Figures 2a and b). Knockout of IKKβ and IKKγ of MEF control cells showed 30 and 35% apoptosis, respectively; 3-Cl-AHPC enhanced only 2 and 7% apoptosis in IKKβ−/− and IKKγ−/− cells, respectively, whereas it enhanced 52% apoptosis in wild-type cells at 48 h. Thus, activation of both IKKα and IKKβ were required for maximal 3-Cl-AHPC-mediated apoptosis in the MEF cells (Figures 2a and b). We also examined the effect of IKKα and IKKβ knockdown on 3-Cl-AHPC-mediated apoptosis in MDA-MB-468 cells. Knockdown of IKKα and IKKβ was accomplished in MDA-MB-468 cells using small-hairpin (sh)RNA-IKKα and shRNA-IKKβ as described in Materials and Methods section (Supplementary Figure S1b). We found that knockdown of IKKα or IKKβ resulted in an approximately 50% reduction in 3-Cl-AHPC-mediated apoptosis in the MDA-MB-468 cells (Figures 2c and d). Thus activation of both IKKα and IKKβ is required for maximal 3-Cl-AHPC-mediated apoptosis in both MEF and MDA-MB-468 cells. As expected, knockdown in IKKα and IKKβ levels resulted in decreased phosphorylated IKKα and IKKβ levels in MDA-MB-468 cells after exposure to 3-Cl-AHPC (Figure 3a). We next examined the effect of knockdown of IKKα and IKKβ on 3-Cl-AHPC-mediated NF-κB2p100 phosphorylation in MDA-MB-468 cells. Knockdown of IKKβ had no effect on 3-Cl-AHPC-mediated NF-κB2p100 phosphorylation, whereas knockdown of IKKα delayed NF-κB2p100 phosphorylation to 48 h and decreased the levels of phosphorylated NF-κB2p100 (Figure 3b). Total inhibition of NF-κB2p100 phosphorylation would not be expected because activated IKKα was still present but at reduced levels.
Knockout (KO) and knockdown (KD) of IKKs attenuates 3-Cl-AHPC-mediated apoptosis in MEF and MDA-MB-468 cells. (a and b) Knockout of IKKα, IKKβ and IKKγ inhibited 3-Cl-AHPC-mediated apoptosis in MEF cells. (c and d) Knockdown of IKKα and IKKβ inhibited 3-Cl-AHPC-mediated apoptosis in IKKα-KD and IKKβ-KD MDA-MB-468 stably transfected cells for 24 h. Cells were grown and exposed to vehicle or 3-Cl-AHPC (1 μM) for the indicated time. Apoptosis of cells was assessed by flow cytometry. Columns represent mean of three independent experiments, and error bars indicate standard deviations. ** significantly different from sh-vector and MEF wild-type 3-Cl-AHPC treated cells (P-value is <0.01 respectively, as determined by t-test)
Knockdown of IKKα and IKKβ decreases IKK and NF-κB phosphorylation levels, 3-Cl-AHPC-mediated NIK stabilization and binding of TRAF3 with NIK in the NF-κB noncanonical pathway. (a) phosphorylation of IKKα, IKKβ in knockdown (IKKα-KD and IKKβ-KD) MDA-MB-468 cells and (b) loss of IKKα but not of IKKβ expression inhibited the phosphorylation of NF-κB2p100 and generation of p52 in IKKα-KD cells. (c) 3-Cl-AHPC increases the expression of NIK at 24 h and the densitometric analysis of NIK levels (right panel). (d) Proteasome inhibitor MG132 (20 μM) increased NIK expression more than the increase noted with 3-Cl-AHPC. Cells were grown and pre-incubated with MG132 for 2 h then exposed to vehicle or 3-Cl-AHPC (1 μM) for 24 h. (e) 3-Cl-AHPC-mediated TRAF2 and TRAF3 expression in cells. (f) TRAF3 increased binding with NIK in 3-Cl-AHPC-treated cells. Cells were grown and exposed to vehicle or 3-Cl-AHPC (1 μM) for various indicated times
The role of the NIK in the activation of the NF-κB noncanonical pathway has been well demonstrated.17, 18 We therefore assessed the effect of 3-Cl-AHPC exposure on NIK levels. We found that incubation of MDA-MB-468 cells with 3-Cl-AHPC resulted in a sixfold increase in the NIK levels (Figure 3c). NIK expression in cells has been found to be tightly regulated by c-IAP1 and c-IAP2.18 The E-3 ligases c-IAP1 and c-IAP2 have been found to inhibit NIK expression through its destruction by means of the proteasome pathway and thus inhibit activation of the NF-κB noncanonical pathway.19 As expected, inhibition of the proteasome pathway resulted in a marked increase in NIK levels in the absence and presence of 3-Cl-AHPC (Figure 3d).
Recent reports suggest that c-IAP1 and c-IAP2 degradation of NIK requires the formation of a regulatory complex containing c-IAP1, c-IAP2, TNF receptor-associated factors (TRAFs) TRAF2 and TRAF3 with subsequent binding of NIK.20, 21, 22 TRAF2 and TRAF3 appear to have separate and unique roles.20, 22 Exposure of MDA-MB-468 cells to 3-Cl-AHPC resulted in increased expression of TRAF2 and TRAF3 but only an increase in TRAF3 levels was noted in KG-1 cells after the addition of 3-Cl-AHPC (Figure 3e), which was accompanied by decreased binding of NIK by TRAF2 (Figure 3e). Interestingly, a significant decrease in binding of c-IAP1 by TRAF3 but not by TRAF2 in MDA-MB-468 cells occurred after 3-Cl-AHPC exposure (Figure 4a). Increase in NIK levels in the cells after exposure to 3-Cl-AHPC was preceded by a decrease in c-IAP1 and XIAP levels within 6 h (Figure 4b). We therefore hypothesized that the 3-Cl-AHPC-mediated decrease of c-IAP1 levels had a major role in the associated increase in NIK levels. We assessed the effect of c-IAP1 overexpression on 3-Cl-AHPC-mediated activation of the noncanonical pathway and apoptosis induction in MDA-MB-468 cells. Elevated c-IAP1 levels inhibited 3-Cl-AHPC-mediated apoptosis as well as 3-Cl-AHPC-mediated increase in NIK levels (Figures 4c–e; Supplementary Figure S2a). We examined the potential mechanisms by which 3-Cl-AHPC inhibited c-IAP1 levels using inhibitors to the proteasome (MG-132), lysosomal (CA074Me) and caspase (ZVAD-fmk) pathways. Inhibition of the proteasome pathway did not block 3-Cl-AHPC-mediated decrease of c-IAP1 levels (Supplementary Figure S2b) and apoptosis (Supplementary Figure S2c) in MDA-MB-468 cells. In addition, there was no evidence of c-IAP1 ubiquitination after 3-Cl-AHPC exposure (data not shown). Use of an inhibitor of the lysosomal pathway, CA074Me, also had no effect on 3-Cl-AHPC-mediated decrease in c-IAP1 levels (Supplementary Figure S2d). Treatment of the cells with the proteasome or lysosomal inhibitors resulted in the degradation of c-IAP1, whereas the addition of the caspase pathway inhibitor ZVAD-fmk completely blocked 3-Cl-AHPC-mediated decrease of c-IAP1 levels (Supplementary Figure S2d).
3-Cl-AHPC apoptosis induction requires degradation of c-IAP1. (a) 3-Cl-AHPC decreases TRAF3 binding with c-IAP1. (b) 3-Cl-AHPC induces loss of c-IAP1 and XIAP expression in MDA-MB-468 and KG-1 cells. (c) Overexpression of c-IAP1 inhibited 3-Cl-AHPC-mediated apoptosis in pcDNA3-Flag-c-IAP1 expression vector stably transfected MDA-MB-468 cells. Cells were grown and exposed to vehicle or 3-Cl-AHPC (1 μM) for 24 h as described in Materials and Methods section. Apoptosis was assessed by flow cytometry. ** significantly different from 3-Cl-AHPC-treated vector cells (P-value is <0.01 as determined by t-test). (d) Overexpression of c-IAP1 blocked 3-Cl-AHPC-mediated NIK stabilization and (e) densitometric analysis of NIK protein expression
Role of Cdc37 in 3-Cl-AHPC-mediated IKK phosphorylation
We have previously shown that 3-Cl-AHPC-mediated IKK activation is associated with the enhanced binding of HSP90 binding to IKKα.1 The association of chaperones Cdc37 and HSP90 with the IKK complex is required for ligand-mediated IKK activation, including TNFα-mediated IKK activation.8, 23 It has been shown that Cdc37 recruits HSP90 to the IKK complex through the direct interaction between Cdc37 and the catalytic region of IKKα/β.23 Exposure of MDA-MB-468 cells to 3-Cl-AHPC resulted in the increased binding of Cdc37 to IKKα at 6 h (Figure 5a); this was accompanied by an enhanced association between Cdc37 to HSP90 (Figure 5a). We therefore examined whether Cdc37 has a role in 3-Cl-AHPC-mediated IKKα/β activation and induction of apoptosis using shRNA to decrease Cdc37 expression in MDA-MB-468 cells (Supplementary Figure S2e). Knockdown of Cdc37 inhibited 3-Cl-AHPC-mediated phosphorylation of both IKKα and IKKβ (Figure 5b) and inhibited both 3-Cl-AHPC- and TNFα-mediated NF-κB activation in cells (Figure 5c) as well as 3-Cl-AHPC-mediated apoptosis in MDA-MB-468 cells (Figure 5d). In addition, knockdown of Cdc37 levels in MDA-MB-468 cells markedly inhibited 3-Cl-AHPC-mediated enhanced HSP90 binding to IKKα (Figure 5e). Thus, the recruitment by Cdc37 of HSP90 to the IKKs is essential for 3-Cl-AHPC-mediated NF-κB activation and the induction of apoptosis.
Interactions of Cdc37 with HSP90 and IKKα are essential for 3-Cl-AHPC-mediated NF-κB activation and apoptosis. (a) 3-Cl-AHPC-mediated Cdc37 increased binding with HSP90 and IKKα and 3-Cl-AHPC induction of Cdc37 protein expression after 3-Cl-AHPC exposure. (b) Phosphorylation of IKKα and IKKβ in Cdc37-KD cells. (c) Loss of Cdc37 expression inhibited 3-Cl-AHPC- and TNF-mediated NF-κB activation. (d) Knockdown of Cdc37 expression inhibited 3-Cl-AHPC-mediated apoptosis, and apoptosis was analyzed by flow cytometry. (e) Inhibition of Cdc37 expression in cells (Cdc37-KD) blocked HSP90 binding with IKKα. Cells were grown and exposed to vehicle or 3-Cl-AHPC (1 μM) for various time; immunoprecipitations and immunoblots were performed as described in Material and Methods section. Columns represent mean of two independent experiments, and error bars indicate standard deviations. * and ** significantly different from 3-Cl-AHPC-treated sh-vector cells (P-value is <0.05 and <0.01, respectively, as determined by t-test)
TNFα-mediates IKK phosphorylation in MDA-MB-468 and KG-1 cells
TNFα exposure resulted in IKKβ phosphorylation and activation at 6 h in MDA-MB-468 and KG-1 cells with the absence of IKKβ phosphorylation noted at 24 h (Figure 6a). No activation of IKKα was noted in the TNFα-exposed MDA-MB-468 cells. KG-1 cells express constitutively phosphorylated IKKα that was not modulated by the presence of TNFα in the KG-1 cells (Figure 6a); however, there was a significant increase in IKKβ phosphorylation/activation. Exposure to TNFα did not result in modulation of XIAP, c-IAP1 or phospho-Bad levels as noted after 3-Cl-AHPC exposure in either the MDA-MB-468 or KG-1 cells (Figure 6b). In addition, TNFα exposure did not result in significant apoptosis induction in either MDA-MB-468 or KG-1 cells (Figures 6c and d). Thus, despite TNFα-mediated increase in IKKβ phosphorylation and activation and the presence of constitutively phosphorylated/activated IKKα in KG-1, neither was there induction of apoptosis (Figures 6a and c) nor was there a decrease in c-IAP1, XIAP or phospho-Bad levels in these cells (Figure 6b). These results imply that in addition to 3-Cl-AHPC-enhanced IKKα and IKKβ phosphorylation/activation, additional 3-Cl-AHPC-mediated events must occur for the induction of apoptosis.
TNFα mediates IKKβ phosphorylation/activation. (a) TNFα (10 ng/ml) induces phosphorylation of IKKβ but not of IKKα. (b) TNFα has no effect on cIAP1, XIAP and p-Bad protein levels. (c and d) TNFα and 3-Cl-AHPC-mediated apoptosis in MDA-MB-468 and KG-1 cell lines at 24 h. The cells were treated with TNFα (10 ng/ml) 2 h before adding 3-Cl-AHPC (1 μM). Apoptosis was analyzed by flow cytometry as described in Materials and Methods section. Error bars indicate standard deviations
Discussion
NF-κB is well known to function in an anti-apoptotic manner, but we have previously reported that 3-Cl-AHPC-mediated apoptosis requires NF-κB activation.1 Here, we showed that 3-Cl-AHPC-mediated phosphorylation and activation of both IKKα and IKKβ are required for maximal 3-Cl-AHPC apoptosis induction. Knockout and knockdown of either IKKα or IKKβ inhibited 3-Cl-AHPC-mediated apoptosis in MEF as well as in MDA-MB-468 cells. We found that 3-Cl-AHPC induction of IKKα and IKKβ phosphorylation displayed significantly different time courses. Furthermore, 3-Cl-AHPC-mediated IKKα and IKKβ phosphorylation/activation were independent of each other; knockdown of IKKβ had no effect on 3-Cl-AHPC-mediated IKKα phosphorylation/activation. We have previously shown that HSP90 is required for 3-Cl-AHPC activation of IKKα and IKKβ.1 We have now shown that Cdc37 is also essential for 3-Cl-AHPC-mediated IKKα and IKKβ activation; inhibition of Cdc37 expression resulted in decreased association of IKKα with HSP90 as well as inhibition of 3-Cl-AHPC-mediated apoptosis.
3-Cl-AHPC enhanced NIK expression resulted in the increased binding of NIK to TRAF3. We speculate that the complex of NIK and TRAF3 resulted in the activation of IKKα through NIK phosphorylation of IKKα. A number of investigators have documented that IKKβ-mediated NF-κB activation occurs through the canonical pathway, whereas IKKα-mediated NF-κB activation occurs primarily through the noncanonical pathway.18, 24, 25 NIK is usually expressed at extremely low levels in cells.25 TRAF3 that possesses ubiquitin E3 ligase activity targets NIK degradation through the proteasome pathway, and the induction of the noncanonical pathway involves TRAF3 degradation with the associated enhancement of NIK expression.24, 25, 26 We have found that MDA-MB-468 exposure to 3-Cl-AHPC resulted in increased NIK expression, which was accompanied by increases in TRAF2 and TRAF3 levels. NIK was found to phosphorylate the T-loop serine of IKKα resulting in IKKα activation with the subsequent IKKα-mediated phosphorylation of NF-κB2p100 at the C-terminal serines to trigger ubiquitination and proteasomal processing of NF-κB2p100.18, 24 3-Cl-AHPC-enhanced NIK expression resulted in IKKα activation with concomitant NF-κB2p100 phosphorylation, which was accompanied by increased p52 levels. Interestingly, we found an increase in NF-κB2p100 levels rather than a decrease during 3-Cl-AHPC-enhanced phosphorylation/degradation of NF-κB2p100. A similar observation was made by Varfolomeev et al.26 who found there was a progressive increase in NF-κB2p100 and p52 levels after treatment of EVSA-T human breast carcinoma cells with an IAP antagonist. Lombardi et al.27 found that the NF-κBp65 subunit enhances the activation of the NF-κB2 promoter. We have previously reported that 3-Cl-AHPC enhances IκBα degradation through the proteasome pathway with the release of the NF-κBp65 subunit and its translocation to the nucleus. Thus, we speculate that 3-Cl-AHPC activation of p65 may result in enhanced NF-κB2p100 expression.
TRAF3 has been found to be associated with NIK, which allows it to recruit TRAF2 along with associated c-IAP1 or c-IAP2. The recruited c-IAP1 or c-IAP2 can then catalyze NIK ubiquitination and degradation.20, 21, 26 Thus, the 3-Cl-AHPC-mediated decrease in c-IAP1 levels may result in enhanced NIK levels and IKKα activation. c-IAP1 and c-IAP2 as well as XIAP appear to have essential roles in genotoxic stress-induced NF-κB activation.28 XIAP regulates the activation of the upstream kinase TAK1 and couples activated TAK1 to the IKK complex. XIAP expression is essential for camptothecin- and etoposide-mediated NF-κB activation. c-IAP1 mediated NEMO ubiquitination in the same pathway, whereas c-IAP2 regulated a downstream event and was also essential for camptothecin- and etoposide-mediated as well as doxorubicin-mediated NF-κB activation.28 Valli et al.3 have shown that ST1926 and CD437, analogs of 3-Cl-AHPC, induce DNA double-strand breaks in the acute myelogenous cell line NB4; these investigators speculated that this may be the mechanism by which ST1926 and CD437 induce cell death. The fact that 3-Cl-AHPC inhibits the expression of c-IAP1 as well as of XIAP and yet requires NF-κB activation for apoptosis induction suggests that genotoxic stress-induced NF-κB activation is not involved in 3-Cl-AHPC-mediated apoptosis. The 3-Cl-AHPC-mediated decrease in c-IAP1 levels was blocked by the addition of the caspase inhibitor ZVAD-fmk. This is somewhat perplexing because we did not find evidence of activation of the caspase pathway at 6 h when the decrease in c-IAP1 levels was noted in KG-1 cells.
A number of investigators have shown that although IKKα and IKKβ activation are both involved in NF-κB activation, the IKKβ and NEMO complex transmits distinct signals and regulates the expression of different genes than noted with IKKα.29, 30 This is further supported by the observation that IKKα and IKKβ knockout mice display markedly different phenotypes.31 In addition to NF-κB activation, IKKα regulates a number of NF-κB-independent developmental processes. It has been shown that nuclear IKKα may have a role in the transcriptional activation of a number of genes at the histone level through its ability to phosphorylate and modify histone structure.31
A number of diverse mechanisms have been proposed through which adamantyl-substituted retinoids induce apoptosis in malignant cells;32, 33, 34 whether these are cell-type specific remains to be determined. These pathways have included activation of the c-JunNH2-terminal kinase, DNA adduct formation and TR3 translocation to mitochondria, and its binding to Bcl-2. Interestingly, several investigators have found that adamantyl arotinoids possessing an internal chalcone group inhibit the IKKβ kinase.34, 35 Whether the presence of the chalcone group is entirely responsible for this difference in biological activity remains to be determined. We found that 3-Cl-AHPC as well as the parent compound AHPN and its analogs bind to the orphan receptor SHP.36 Although SHP has been shown to heterodimerize to a number of nuclear receptors resulting in the inhibition of their ability to induce the transcriptional activity of a number of genes, SHP has been found to bind to the NF-κBp65 subunit and activate the NF-κB nuclear receptor.37 Inhibition of SHP expression blocks ARR-mediated NF-κB induction and apoptosis.38 Thus, SHP and NF-κB both appear to be interlinked and essential for ARR-apoptosis induction.
On the basis of our results, we proposed a model to illustrate 3-Cl-AHPC-mediated NF-κB activation in canonical and noncanonical pathways (Figure 7). In this model, we have found that exposure of cells to 3-Cl-AHPC resulted in enhanced binding of Cdc37/HSP90 to IKKα and the activation of both the NF-κB canonical and noncanonical pathways (Figure 7). Activation of the NF-κB canonical pathway resulted in the proteasomal degradation of IκBα, translocation of p50 and p65 to the nucleus and NF-κB activation (Figure 7).1 Activation of the noncanonical pathway resulted in the stabilization of NIK, phosphorylation and processing of NF-κB2p100 and the enhanced expression of p52 and RelB with their subsequent DNA binding and gene modulation. Whether the RelB/p52 heterodimer regulates the expression of unique genes whose products are pro-apoptotic is under study. The initial steps by which 3-Cl-AHPC activated the canonical and noncanonical pathways have not been delineated. The involvement of signaling complexes including TRAF2, TANK and TBK1 are also under study.39 Our results reveal that NF-κB activation contributes a role in ARR-mediated apoptotic pathway and activation of both NF-κB pathways was required for maximal 3-Cl-AHPC-mediated apoptosis.
3-Cl-AHPC-mediated activation of NF-κB canonical and noncanonical pathways. 3-Cl-AHPC enhanced binding of HSP90 to Cdc37 and the binding of this complex to IKKα and activation of IKKα and IKKβ in NF-κB canonical pathway, and is summarized in the text. In the NF-κB noncanonical pathway, 3-Cl-AHPC exposure resulted in the rapid decrease in c-IAP1 and activation of NIK. Enhanced NIK expression occurred within 6 h of 3-Cl-AHPC exposure with the increased binding of NIK to TRAF3, activation of IKKα, phosphorylation of NF-κB2p100, its subsequent processing through the proteasome pathway and the increase in p52 levels
Materials and Methods
Reagents
3-Cl-AHPC was synthesized as described.5 DMEM-F12 and RPMI 1640 medium, and fetal bovine serum (FBS) were purchased from Invitrogen (Carlsbad, CA, USA). Antibodies and their sources were as follows: antibodies for phospho-IKKα/β (2681), and phospho-NF-κB2p100-p52 (4810), non-phospho-NF-κB2p100-p52 (3017), phospho-NF-κBp65 (3037), phospho-Bad (9291); IKKγ (2695), NIK (4994), RelB (4922), XIAP (2045) and the NF-κB noncanonical pathway antibody sampler kit (Cell Signaling Technology, Boston, MA, USA); anti-NIK, IKKα/β (sc-7609), TRAF2 (sc-877), TRAF3 (sc-6933), Cdc37 (sc-17758), NF-κBp65 (sc-7151) and HSP90 (sc-7949) antibodies (Santa Cruz Biotechnology, Santa Cruz, CA, USA); anti-c-IAP1 antibody from (R&D Systems Inc., Minneapolis, MN, USA); and anti-α-tubulin antibody (Oncogene Research Products, Boston, MA, USA). TNFα and caspase inhibitor ZVAD-fmk were from BIOMOL International (Plymouth Meeting, PA, USA). NF-κB activation inhibitor II JSH-23, proteasome inhibitor MG132 and lysosomal inhibitor CA-074Me from EMD Biosciences (Gibbstown, NJ, USA); and Cdc37 shRNA expression vector from Open Biosystems (Frederick, MD, USA). Wild-type and IKKα−/−, IKKβ−/− and IKKγ−/− knockout MEFs were generously provided by Dr. Michael Karin (University of California, San Diego, CA, USA). The pcDNA3-Flag-c-IAP1 expression vector was a kind gift from Dr. John C Reed (Burnham Institute for Medical Research, La Jolla, CA, USA).
Cell culture
KG-1 human leukemia and MDA-MB-468 breast carcinoma cell lines, and MEFs, were maintained in RPMI 1640 and DMEM-F12 medium containing 5 and 10% FBS, respectively, and gentamicin.
shRNA plasmid and expression vector construction
shRNA-IKKα and shRNA-IKKβ expression vectors were constructed by directionally cloning 5′-BamHI and 3 EcoRI overhang nucleotides in a pSIREN-RetroQ vector according to the manufacturer's instructions (Clontech, Mountain View, CA, USA). IKKα and IKKβ target sequences were obtained from the coding sequence for PubMed accession numbers NM_001278 and NM_001556, respectively, and synthesized by Integrated DNA Technology Inc. (Coralville, IA, USA). shRNA regions in the plasmid backbone were confirmed by sequencing. shRNA-IKKα and shRNA-IKKβ plasmids were stably transfected into MDA-MB-468 cell lines, the standard calcium phosphate method. Stable cell lines were selected with puromycin. The sh-vector containing scrambled sequences, 5′-GTTATTACTGTTCGATCGC-3′ and 5′-CTTAAGATGACAGCCGAGATCCA-3′, in pSIREN-RetroQ vector was used as a control. Cdc37 shRNA pLK01 expression vector clone ID RCN0000116635 knocked down Cdc37 more effectively in MDA-MB-468 cells than other clones from a set of five clones purchased from Open Biosystems (Huntsville, AL, USA).
Apoptosis
IKKα-knockdown (KD), IKKβ-KD and Cdc37-KD stable cell lines derived from MDA-MB-468 cells were treated with 3-Cl-AHPC for 24 h before proliferation and apoptosis was assessed by flow cytometry. Apoptosis of wild-type MEFs and MEF IKKα−/−, IKKβ−/− and IKKγ−/−-knockout stable cell lines was determined by flow cytometry using Annexin V-FITC labeling with propidium iodide staining (Annexin V-FITC apoptosis Detection Kit 1; BD Biosciences, San Diego, CA, USA). The fluorochrome-coupled Annexin V binds to phosphatidylserine in apoptotic cells, which is exposed on the outer leaflet of the plasma membrane;40 the results were analyzed following the guidelines of Galluzzi et al.40 Data acquisition was carried out on a FACS Calibur flow cytometer (BD Biosciences) and analyzed with CellQuest software (BD Biosciences).
Western blots and immunoprecipitation
Cells were extracted with lysis buffer containing 25 mM Tris-Cl buffer (pH 8.0), 150 mM NaCl, 0.2% nonidet P-40, 10% glycerol, 10 mM NaF, 8 mM β-glycerophosphate, 0.2 mM Na3VO4, 1 mM DTT and 10 μl/ml protease inhibitor cocktail (Sigma-Aldrich, St Louis, MO, USA) for the detection of phospho-protein. Immunoprecipitations and western blots were performed as we previously described.1
Transfection
Transfection of MDA-MB-468 cells for NF-κB activation was performed using the calcium phosphate method.1 Cells were treated with 3-Cl-AHPC after 36 h of post-transfection of NF-κB reporter plasmid and incubated for 24 h before luciferase and β-galactosidase assays were performed. Cignal lentiviral NF-κB reporter (GFP) particles were used to transduce KG-1 cells either transiently using the manufacturer's instructions (SABiosciences, Frederick, MD, USA), and luciferase assay and GFP fluorescence reporter protein assays were performed using the BioTek Synergy HT (BioTek, Winooski, VT, USA).
Abbreviations
- CD437:
-
6-[3′-(1-adamantyl)-4′-hydroxyphenyl]-2-naphthalenecarboxylic acid
- IKK:
-
Inhibitor of nuclear factor-κB kinase complex
- SCF:
-
Skp1-Cullins-F-box proteins
- c-IAP:
-
cellular inhibitor of apoptosis protein
- XIAP:
-
X-linked inhibitor of apoptosis protein
- HSP90:
-
heat shock protein 90
- ZVAD-fmk:
-
Z-Val-Ala-Asp (OMe)-fluoromethyl ketone
- NEMO:
-
Nuclear factor-κB essential modulator
- SHP:
-
small heterodimer partner
- TANK:
-
TRAF-associated NF-κB activator
- TBK1:
-
TANK-binding Kinase 1
References
Farhana L, Dawson MI, Fontana JA . Apoptosis induction by a novel retinoid-related molecule requires nuclear factor-κB activation. Cancer Res 2005; 65: 4909–4917.
Cincinelli R, Dallavalle S, Nannei R, De Zani D, Merlini L, Penco S et al. Synthesis and structure-activity relationships of a new series of retinoid related biphenyl-4-ylacrylic acids endowed with anti-proliferative and proapoptotic activity. J Med Chem 2005; 48: 4931–4946.
Valli C, Paroni G, Di Francesco AM, Riccardi R, Tavecchio M, Erba E et al. Atypical retinoids ST1926 and CD437 are S-phase-specific agents causing DNA double-strand breaks: significance for the cytotoxic and antiproliferative activity. Mol Cancer Ther 2008; 7: 2941–2954.
Sun SY, Yue P, Chen X, Hong WK, Lotan R . The synthetic retinoid CD437 selectively induces apoptosis in human lung cancer cells while sparing normal human lung epithelial cells. Cancer Res 2002; 62: 2430–2436.
Zhang Y, Dawson MI, Ning Y, Polin L, Parchment RE, Corbett T et al. Induction of apoptosis in retinoid-refractory acute myelogenous leukemia by a novel AHPN analog. Blood 2003; 102: 3743–3752.
Jin F, Liu X, Zhou Z, Yue P, Lotan R, Khuri FR et al. Activation of nuclear factor-kappaB contributes to induction of death receptors and apoptosis by the synthetic retinoid CD437 in DU145 human prostate cancer cells. Cancer Res 2005; 65: 6354–6363.
Perkins ND . Integrating cell-signalling pathways with NF-kappaB and IKK function. Nat Rev Mol Cell Biol 2007; 8: 49–62.
Hinz M, Broemer M, Arslan SC, Otto A, Mueller E-C, Dettmer R et al. Signal responsiveness of IB kinases is determined by Cdc37-assisted transient interaction with HSP90. J Biol Chem 2007; 282: 32311–32319.
Janssens S, Tschopp J . Signals from within: the DNA-damage-induced NF-kappaB response. Cell Death Differ 2006; 13: 773–784.
Beg AA, Sha WC, Bronson RT, Ghosh S, Baltimore D . Embryonic lethality and liver degeneration in mice lacking the RelA component of the NF-κB. Nature 1995; 376: 167–170.
Liu ZG, Hsu H, Goeddel DV, Karin M . Dissection of the TNF receptor 1 effector functions: JNK activation is not linked to apoptosis while NF-kappaB activation prevents cell death. Cell 1996; 87: 565–576.
Fan Y, Dutta J, Gupta N, Fan G, Gelinas C . Regulation of programmed cell death by NF-κappaB and its role in tumorigenesis and therapy. Adv Exp Med Biol 2008; 615: 223–250.
Kim HJ, Hawke N, Baldwin AS . NF-κB and IKK as the therapeutic targets in cancer. Cell Death Differentiation 2006; 13: 238–247.
Chen X, Kandasamy K, Srivastava RK . Differential roles of RelA (p65) and c-Rel subunits of nuclear factor kappaB in tumor necrosis factor-related apoptosis-inducing ligand signaling. Cancer Res 2003; 3: 1059–1066.
Shin HM, Kim MH, Kim BH, Jung SH, Kim YS, Park HJ et al. Inhibitory action of novel aromatic diamine compound on lipopolysaccharide-induced nuclear translocation of NF-kappaB without affecting IkappaB degradation. FEBS Lett 2004; 571: 50–54.
Delhase M, Hayakawa M, Chen Y, Karin M . Positive and negative regulation of IkappaB kinase activity through IKKbeta subunit phosphorylation. Science 1999; 284: 309–313.
Bonizzi G, Bebien M, Otero DC, Johnson-Vroom KE, Cao Y, Vu D et al. Activation of IKKalpha target genes depends on recognition of specific kappaB binding sites by RelB:p52 dimers. EMBO J 2004; 23: 4202–4210.
Senftleben U, Cao Y, Xiao G, Greten FR, Krahn G, Bonizzi G et al. Activation of IKKalpha of a second, evolutionary conserved, NF-kappa B signaling pathway. Science 2001; 293: 1495–1499.
Xiao G, Harhaj EW, Sun SC . NF-kappaB-inducing kinase regulates the processing of NF-κB2 p100. Mol Cell 2001; 7: 401–409.
Zarnegar BJ, Wang Y, Mahoney DJ, Dempsey PW, Cheung HH, He J et al. Noncanonical NF-kappaB activation requires coordinated assembly of a regulatory complex of the adaptors cIAP1, cIAP2, TRAF2 and TRAF3 and the kinase NIK. Nat Immunol 2008; 12: 1371–1378.
Varfolomeev E, Vucic D . (Un)expected roles of c-IAPs in apoptotic and NF-κappaB signaling pathways. Cell Cycle 2008; 7: 1511–1521.
Vallabhapurapu S, Matsuzawa A, Zhang W, Tseng PH, Keats JJ, Wang H et al. Nonredundant and complimentary functions of TRAF2 and TRAF3 in a ubiquitination cascade that activates NIK-dependent alternative NF-kappaB signaling. Nat Immunol 2008; 9: 1364–1370.
Chen G, Cao P, Goeddel DV . TNF-induced recruitment and activation of the IKK complex require Cdc37 and Hsp90. Mol Cell 2002; 9: 401–410.
Qing G, Qu Z, Xiao G . Stabilization of basally translated NF-κB-inducing kinase (NIK) protein functions as a molecular switch of processing of NF-kappaB2 p100. J Biol Chem 2005; 280: 40578–40582.
Liao G, Zhang M, Harhaj EW, Sun SC . Regulation of the NF-kappaB-inducing kinase by tumor necrosis receptor-associated factor 3-induced degradation. J Biol Chem 2004; 279: 6243–6250.
Varfolomeev E, Blankenship JW, Wayson SM, Fedorova AV, Kayagaki N, Garg P et al. IAP antagonists induce autoubiquitination of c-IAPs, NF-kappaB activation and TNFalpha-dependent apoptosis. Cell 2007; 131: 669–681.
Lombardi L, Ciana P, Cappellini C, Trecca D, Guerrini L, Migliazza A et al. Structural and functional characterization of the promoter regions of the NFKB2 gene. Nucleic Acids Res 1995; 23: 2328–2336.
Jin HS, Lee DH, Kim DH, Chung JH, Lee SJ, Lee TH et al. cIAP1, cIAP2 and XIAP act cooperatively via nonredundant pathways to regulate genotoxic stress-induced nuclear factor-kappaB activation. Cancer Res 2009; 69: 1782–1791.
Hatada EN, Krappmann D, Scheidereit C . NF-kappaB and the innate immune response. Curr Opin Immunol 2000; 12: 52–58.
Gerondakis S, Grumont R, Gugasyan R, Wong L, Isomura I, Ho W et al. Unravelling the complexities of the NF-kappaB signaling pathway using mouse knockout and transgenic models. Oncogene 2006; 25: 6781–6799.
Scheidereit C . IkappaB kinase complexes: gateways to NF-kappaB activation and transcription. Oncogene 2006; 25: 6685–6705.
Zhao X, Spanjaard RA . The apoptotic action of the retinoid CD437/AHPN: diverse effects, common basis. J Biomed Sci 2003; 10: 44–49.
Pfahl M, Piedrafita FJ . Retinoid targets for apoptosis induction. Oncogene 2003; 22: 9058–9062.
Bayon Y, Ortiz MA, Lopez-Hernandez FJ, Gao F, Karin M, Pfahl M et al. Inhibition of IkappaB kinase by a new class of retinoid-related anticancer agents that induce apoptosis. Mol Cell Biol 2003; 23: 1061–1074.
Lorenzo P, Alvarez R, Ortiz MA, Alvarez S, Piedrafita FJ, de Lera AR . Inhibition IkappaB kinase-beta and anticancer activities of novel chalcone adamantyl arotinoids. J Med Chem 2008; 51: 5431–5440.
Farhana L, Dawson MI, Leid M, Wang L, Moore DD, Liu G et al. Adamantyl-substituted retinoid related molecules bind small heterodimer partner and modulate the Sin3A repressor. Cancer Res 2007; 67: 318–325.
Kim YS, Han CY, Kim SW, Kim JH, Lee SK, Jung DJ et al. The orphan nuclear receptor small heterodimer partner as a novel coregulator of nuclear receptor-B in oxidized low density lipoprotein-treated macrophage cell line RAW 264.7. J Biol Chem 2001; 276: 33736–33740.
Farhana L, Dawson MI, Dannenberg JH, Xu L, Fontana JA . SHP and Sin3A expression are essential for adamantyl-substituted retinoid related molecule-mediated nuclear factor-B activation, c-Fos/c-Jun expression and cellular apoptosis. Mol Cancer Ther 2009; 8: 1625–1635.
Pomerantz JL, Baltimore D . NF-κappaB activation by a signaling complex containing TRAF2, TANK and TBK1, a novel IKK-related kinase. EMBO J 1999; 18: 6694–6704.
Galluzzi L, Aaronson SA, Abrams J, Alnemri ES, Andrews DW, Baehrecke EH et al. Guidelines for the use and interpretation of assays for monitoring cell death in higher eukaryotes. Cell Death and Differ 2009; 16: 1093–1107.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Edited by C Borner
Supplementary Information accompanies the paper on Cell Death and Differentiation website
Rights and permissions
About this article
Cite this article
Farhana, L., Dawson, M., Murshed, F. et al. Maximal adamantyl-substituted retinoid-related molecule-induced apoptosis requires NF-κB noncanonical and canonical pathway activation. Cell Death Differ 18, 164–173 (2011). https://doi.org/10.1038/cdd.2010.84
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/cdd.2010.84