Abstract
CREB3L3 is involved in fatty acid oxidation and ketogenesis in a mutual manner with PPARα. To evaluate relative contribution, a combination of knockout and transgenic mice was investigated. On a ketogenic-diet (KD) that highlights capability of hepatic ketogenesis, Creb3l3−/− mice exhibited reduction of expression of genes for fatty oxidation and ketogenesis comparable to Ppara−/− mice. Most of the genes were further suppressed in double knockout mice indicating independent contribution of hepatic CREB3L3. During fasting, dependency of ketogenesis on CREB3L3 is lesser extents than Ppara−/− mice suggesting importance of adipose PPARα for supply of FFA and hyperlipidemia in Creb3l3−/− mice. In conclusion CREB3L3 plays a crucial role in hepatic adaptation to energy starvation via two pathways: direct related gene regulation and an auto-loop activation of PPARα. Furthermore, as KD-fed Creb3l3−/− mice exhibited severe fatty liver, activating inflammation, CREB3L3 could be a therapeutic target for NAFLD.
Similar content being viewed by others
Introduction
The common characteristics of metabolic disorders, such as obesity, diabetes, cardiovascular diseases, and fatty liver, impair nutrient homeostasis, which is tightly regulated by balancing energy production (e.g. ketogenesis, gluconeogenesis, and lipid synthesis) with energy utilization (e.g. lipid oxidation). As fasting progresses, metabolic substrates stored in white adipose tissue (WAT) are released into the circulation as glycerol and free fatty acids (FFA) and transported into the liver. The liver then adapts by increasing β-oxidation, which converts fatty acids into acetyl coenzyme A (acetyl-coA), and by increasing ketogenesis, which converts the resulting acetyl-CoA into ketone bodies.
The first ketone body formed from acetyl-CoA is acetoacetate (Acac), which can generate acetone via non-enzymatic decarboxylation, as well as β-OH butyrate in a reaction catalysed by D-β-hydroxybutryate dehydrogenase (BDH1). The rate of conversion from acetyl-CoA to these ketone bodies is limited by hydroxymethylglutaryl CoA synthase 2 (HMGCS2), which converts acetoacetyl-CoA to HMG-CoA. The production of ketone bodies as an alternative energy source is crucial for maintaining energy homeostasis during fasting, as they are used as the main energy source for peripheral tissue, particularly the brain.
The fatty acid oxidation process consists of three pathways: peroxisomal β-oxidation, mitochondrial β-oxidation, and ω-oxidation in the endoplasmic reticulum (ER). Although the substrate spectra of mitochondrial and peroxisomal β-oxidation partly overlap, an important distinction is that the mitochondria catalyse the β-oxidation of the bulk of the short (<C8), medium (C8–C12), and long (C14–C20) chain fatty acids (LCFAs), whereas β-oxidation in the peroxisomes preferentially shortens very long chain fatty acids (>C20) to LCFAs, which can then be further oxidized in the mitochondria. FA transport across the mitochondrial membrane is triggered by carnitine palmitoyltransferase 1a, liver (CPT1a) and carnitine palmitoyltransferase 1b, muscle (CPT1b), which are localized in the mitochondrial membrane.
The activation of peroxisome proliferator-activated receptor α (PPARα) by fatty acids promotes hepatic fatty acid oxidation and ketogenesis. Several genes are directly regulated by PPARα in the liver, including those encoding acyl-CoA oxidase (Acox1)1, which is involved in the peroxisomal β-oxidation of fatty acids; Cpt1a2,3, which transports fatty acids across the outer mitochondrial membrane; and HMGCS22,3,4. Consequently, mice that lack PPARα accumulate copious amounts of hepatic triglycerides (TG) and become hypoketonaemic and hypoglycaemic during fasting and starvation5,6,7.
cAMP responsive element-binding protein 3-like 3 (CREB3L3) is a basic, liver-specific leucine zipper transcription factor belonging to the CREB/ATF family8. Under ER stress, CREB3L3 traffics to the Golgi apparatus where site 1 and 2 proteases cleave its amino-terminal portion to induce the expression of genes that are responsible for the systemic inflammatory response9. Creb3l3 expression is induced in fasted or insulin-resistant states, resulting in the accumulation of the nuclear form of CREB3L310, which activates hepatic gluconeogenic genes, including phosphoenolpyruvate carboxykinase 1 (Pck1) and glucose-6-phosphatase (G6pc)11. Creb3l3 deletion causes a defect in TG lipolysis in the blood, with Creb3l3−/− mice exhibiting hypertriglyceridaemia as a result of inefficient catalysis of TG clearance by lipoprotein lipase (LPL); this is due to a reduction in the expression of the LPL coactivators Apolipoprotein c2 (Apoc2), Apoa4, and Apoa5, and upregulation of the LPL inhibitor Apoc312,13. Thus, the defective expression of the enzymes that are required for lipolysis and lipid transport in the liver of Creb3l3−/− mice may explain why mice that are fed either an atherogenic high-fat diet or normal chow exhibit hypertriglyceridaemia, reduced fat mass, body-weight gain, and massive steatosis12. When fed a methionine-choline-deficient diet or ketogenic diet, the deficiency of Creb3l3 also develops hepatic steatosis14,15.
FGF21, a unique member of the FGF family with hormone-like actions16, is a key mediator of starvation that regulates lipolysis in WAT and increases fatty acid oxidation and ketogenesis in the liver17,18. There is some controversy over the effects of FGF21 on ketogenesis. Hotta et al.19 reported that FGF21 regulates lipolysis in WAT but is not required for ketogenesis and TG clearance in the liver, but Badman et al.20 showed that the adaptation to ketogenesis is impaired in Fgf21−/− mice. FGF21 has been shown to have therapeutic effects on obesity-related metabolic disturbances, such as insulin resistance, diabetes, and hypertriglyceridaemia in ob/ob mice, diet-induced obese mice, and diabetic monkeys21,22. Fgf21 expression is regulated by PPARα, which plays a key role in lipid oxidation, and is induced by fasting or ketogenic diet (KD) consumption17,18.
CREB3L3 and PPARα are activated in an auto-loop manner in response to starvation and, in turn, synergistically activate Fgf21 expression23,24. Therefore, it is believed that these molecules have overlapping functions. In this study, we examined the role of CREB3L3 in energy metabolism during fasting by comparing Creb3l3−/−, Ppara−/−, and Creb3l3−/−Ppara−/− mice.
Results
Effect of nutrient condition on the expression of Creb3l3
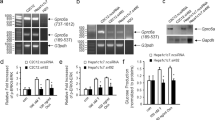
First, we examined the expression levels of Creb3l3 in the liver of wild type (WT) mice that had been fasted for 24 h (Fasted), fed ad libitum (Fed), or fed a KD for 3 days. Both the Fasted and KD groups had significantly higher expression levels of Creb3l3 than did the Fed group (Fig. 1a), which led to higher protein levels of both mature and active forms of CREB3L3 in these groups (Fig. 1b, Supplementary Fig. 1). Ppara and Fgf21 were also upregulated in the Fasted and KD groups (Fig. 1a). Thus, there was a positive correlation between the expression of Creb3l3 and that of Ppara and Fgf21 in response to the feeding conditions.
Effect of fasting and ketogenic diet on Creb3l3 mRNA and the nuclear form of the CREB3L3 protein.
Eight-week-old male wild type (WT) mice were fasted for 24 h, fed ad libitum, and fed a ketogenic diet (KD) for 3 days (n = 4–6 per group). (a) Gene expression in the liver of mice, as estimated using qPCR. Data are represented as mean ± SEM. Significant differences were determined by one-way ANOVA followed by Tukey-Kramer test and shown by *P < 0.05, **P < 0.01. (b) Immunoblotting of total liver lysates against the indicated antibodies. Full-length blots are presented in Supplementary Fig. 1.
KD-induced hepatic ketogenesis
Mice that are fed on KD, a low-carbohydrate high-fat diet, have enhanced lipid oxidation and ketogenesis in the liver25. WT mice that had been fed KD for a short term (3 days) exhibited significantly increased levels of plasma ketone bodies. In both Creb3l3−/− and Ppara−/− mice, these KD-induced plasma ketone body levels were suppressed by 50% compared with WT mice (Fig. 2a). Plasma glucose levels were identical in all three types of mice when fed a moderate-fat (MF) diet. In contrast, when they were fed KD, Ppara−/− mice showed significantly low levels compared with WT mice, while Creb3l3−/− mice showed a trend of increase (Fig. 2a). Plasma TG, total cholesterol (TC), and FFA levels were markedly higher in Creb3l3−/− KD-fed mice than in any other group, and thus increased in Creb3l3−/−Ppara−/− mice (Fig. 2a). These findings indicate that the deficiency of Creb3l3 mainly contributes to these changes. In contrast, liver TG and TC levels increased in Creb3l3−/−, Ppara−/−, and Creb3l3−/−Ppara−/− mice (Fig. 2a). KD-fed induction emphasized the contribution of CREB3L3 along with PPARα and discriminated the effects of these factors on plasma glucose.
Metabolic parameters of wild type (WT), Creb3l3−/−, Ppara−/−, and Creb3l3−/−Ppara−/− mice fed a ketogenic diet (KD) for 3 days.
Eight-week-old male WT, Creb3l3−/−, Ppara−/−, and Creb3l3−/−Ppara−/− mice were fed a medium-fat (MF) diet or KD for 3 days. (a) Plasma levels of glucose, insulin, β-OH butyrate, FGF21, triglycerides (TG), total cholesterol (TC), and free fatty acids (FFA). Liver TG and TC contents. (b) Gene expression in the liver of these mice, as determined using qPCR. All data are represented as mean ± SEM. Significant differences were determined by one-way ANOVA followed by Tukey-Kramer test and shown by *P < 0.05, **P < 0.01. n = 5–16 per group.
The expression of Creb3l3 and Ppara was significantly downregulated in Ppara−/− mice and in Creb3l3−/− mice, respectively, that were fed both MF diet and KD (Fig. 2b). In response to carbohydrate depletion, expression of Fgf21 significantly upregulated in KD-fed WT mice compared with normal chow-fed mice, but it completely blunted in Creb3l3−/−, Ppara−/−, and Creb3l3−/−Ppara−/− mice (Fig. 2b). The expression of Acox1 and Hmgcs2, which encode the rate-limiting enzymes for fatty acid oxidation and ketogenesis, were blunted in KD-fed Ppara−/− and Creb3l3−/−Ppara−/− mice. In addition, the expression of Cpt1a was significantly downregulated in Creb3l3−/− KD-fed mice but unaffected in Ppara−/− KD-fed mice. The expression of both Hmgcl and Bdh1 was additionally downregulated in Creb3l3−/−Ppara−/− mice compared with that in Ppara−/− mice, indicating that CREB3L3 regulates these independent of PPARα. Thus, we speculated that Cpt1a, Hmgcl, and Bdh1 are specifically candidate target genes of CREB3L3 in KD-fed mice. Sirt3, which is an activator for HMGCS2 to deacetylate it26, was downregulated in KD-fed Creb3l3−/− and Ppara−/− mice and further downregulated in Creb3l3−/−Ppara−/− mice (Fig. 2b). These results indicate that CREB3L3 might regulate HMGCS2 enzymatic activity by Sirt3-mediated deacetylation.
Metabolic parameters in WT, Creb3l3−/−, Ppara−/−, and Creb3l3−/−Ppara−/− mice
To determine the exact functions of CREB3L3 in comparison with PPARα, we evaluated the metabolic parameters of WT, Creb3l3−/−, Ppara−/−, and Creb3l3−/−Ppara−/− mice in a fasted state. There was no difference in body weight between the four groups of mice. However, liver weight was the highest in Ppara−/− mice, whereas WAT weight was significantly low in Creb3l3−/−Ppara−/− mice (Fig. 3a). Plasma glucose levels were higher solely in Creb3l3−/− mice, but there was no obvious difference in plasma insulin levels among the groups (Fig. 3b). Plasma β-OH butyrate levels were significantly lower in the three types of knockout (KO) mice than in WT mice, especially with further decrease in Ppara−/− and Creb3l3−/−Ppara−/− mice (Fig. 3b). Plasma FGF21 levels were also markedly lower in Creb3l3−/− and Ppara−/− mice than in WT mice and completely blunted in Creb3l3−/−Ppara−/− mice (Fig. 3b), suggesting that Fgf21 expression in a fasted state is entirely controlled by both CREB3L3 and PPARα.
Metabolic parameters of wild type (WT), Creb3l3−/−, Ppara−/−, and Creb3l3−/−Ppara−/− mice fasted for 24 h.
Eight-week-old male WT, Creb3l3−/−, Ppara−/−, and Creb3l3−/−Ppara−/− mice were fasted for 24 h. (a) Body, liver, and white adipose tissue (WAT) weights. (b) Plasma levels of glucose, insulin, FGF21, β-OH butyrate, triglycerides (TG), total cholesterol (TC), and free fatty acids (FFA). (c) Liver contents of glycogen, TG, and TC. Data are represented as mean ± SEM. Significant differences were determined by one-way ANOVA followed by Tukey-Kramer test and shown by *P < 0.05, **P < 0.01. n = 4–11 per group.
Creb3l3−/−, but not Ppara−/− mice, exhibited a marked increase in plasma TG levels in a fasted state, but there were no differences in the plasma TC and FFA levels between the groups of mice (Fig. 3b). There were also no differences in liver glycogen contents between WT, Creb3l3−/−, and Ppara−/− mice; however, these were strongly higher in Creb3l3−/−Ppara−/− mice (Fig. 3c). The KO mice had higher liver lipid contents (including TG and TC) than WT mice, but there was no difference between the KO mouse genotypes (Fig. 3c).
Hepatic gene expression in WT, Creb3l3−/−, Ppara−/−, and Creb3l3−/−Ppara−/− mice
We determined the effect of CREB3L3 on the expression of genes involved in gluconeogenesis, β-oxidation, and ketogenesis in the liver by comparing gene expression in WT, Creb3l3−/−, Ppara−/−, and Creb3l3−/−Ppara−/− mice in a fasted state (Fig. 4a). We found that Creb3l3 mRNA decreased by approximately 50% in Ppara−/− mice, and Ppara mRNA decreased by approximately 50% in Creb3l3−/− mice presumably due to the mutual auto-loop system of the two factors23,24. Furthermore, the PPARα coactivator PPAR gamma coactivator 1α (Ppargc1a) decreased by 40% in both single KO and further in double KO mice, and Fgf21 expression was completely blunted in all KO mice compared with WT mice, indicating that both factors are crucial for Fgf21 expression.
Gene expression in wild type (WT), Creb3l3−/−, Ppara−/−, and Creb3l3−/−Ppara−/− mice fasted for 24 h.
(a) Gene expression in the liver of 8-week-old male WT, Creb3l3−/−, Ppara−/−, and Creb3l3−/−Ppara−/− mice fasted for 24 h, as determined using qPCR. Data are represented as mean ± SEM. Significant differences were determined by one-way ANOVA followed by Tukey-Kramer test and shown by *P < 0.05, **P < 0.01. n = 4–9 per group. (b) Ketogenic activity in 8-week-old male WT, Creb3l3−/−, Ppara−/−, and Creb3l3−/−Ppara−/− mice fasted for 18 h and injected with sodium octanoate. Data are represented as mean ± SEM. Significant differences were determined by repeated-measures two-way ANOVA with Bonferroni post hoc t-test and shown by *P < 0.05, **P < 0.01. n = 4–7 per group. (c) A schematic diagram of how CREB3L3 affects Cpt1a in β-oxidation and Bdh1 in ketogenesis, leading to the regulation of ketogenesis.
Cpt1a and Bdh1 demonstrated stronger and comparable suppression in Creb3l3−/−, respectively, with a further decrease in Creb3l3−/−Ppara−/− mice (Fig. 4a), suggesting that Cpt1a and Bdh1 are direct targets for CREB3L3 (Fig. 4b). Most of the genes related to peroxisomal β-oxidation, microsomal β-oxidation, and microsomal ω-oxidation were significantly downregulated in Creb3l3−/− mice, but more markedly in Ppara−/− mice with further suppression in both KO mice (Fig. 4a), suggesting that both factors are involved, but Ppara is more important in these gene expressions. Cyp4a10 was not regulated by CREB3L3 but by PPARα. The gluconeogenic genes, including Pck1 and G6pc, were downregulated in Creb3l3−/− mice, which is consistent with previous reports8,11 as being a direct target, whereas only G6pc was downregulated in Ppara−/− mice. Fatty acid synthase (Fasn) was significantly higher in Creb3l3−/− mice.
In terms of ketogenic enzymes, the expression of Hmgcs2 was blunted in Ppara−/− and Creb3l3−/−Ppara−/− mice and not in Creb3l3−/−mice, indicating that PPARα governs Hmgcs2 expression. Hmgcl wass similarly decreased in all KO mice. There is the possibility that both factors co-operate its expression. The expression of Bdh1 decreased in Creb3l3−/− and Ppara−/− mice and further in Creb3l3−/−Ppara−/− mice, suggesting that this is a target for CREB3L3 as well as PPARα.
These findings indicated that CREB3L3 may play a role in regulating ketogenesis, although the contribution was less as compared to PPARα. Therefore, to confirm this, we directly estimated ketogenesis in mice by fasting the mice overnight and then intraperitoneally injecting them with sodium octanoate, a mitochondrial-permeable ketogenic substrate27. Creb3l3−/− mice had significantly low β-OH butyrate levels compared with WT mice during this test (Fig. 4b), showing the impairment of hepatic ketogenesis activity in Creb3l3−/− mice. Ppara−/− and Creb3l3−/−Ppara−/− mice showed a further decrease in β-OH butyrate levels during its test (Fig. 4b), indicating PPARα is more effective for ketogenesis.
In vivo expression of Bdh1 and Cpt1a
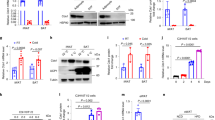
The Creb3l3 knockout mouse studies outlined above identified Cpt1a, Hmgcl, and Bdh1 as candidates of target genes for CREB3L3. To determine whether CREB3L3 directly regulates the expression of these genes, Ad-CREB3L3 was introduced into the mouse hepatoma cell line AML12.2 and quantitative polymerase chain reaction (qPCR) was performed. Ad-CREB3L3 significantly activated the expression of Bdh1 and Cpt1a but did not affect the expression of any other β-oxidation and ketogenesis genes, such as Acox1, Hmgcs2, and Hmgcl (Fig. 5a).
Effect of CREB3L3 on the expression of Cpt1 and Bdh1.
(a) Gene expression in AML12.2 mouse hepatoma cells 24 h after infection with an adenovirus encoding the active form of CREB3L3, as estimated using qPCR. Data are represented as mean ± SEM. Significant differences were determined by unpaired Student’s t test and shown by *P < 0.05, **P < 0.01. n = 3 per group. (b) Hepatic gene expression in 8-week-old male wild type (WT), Ppara−/−, and Ppara−/− crossed with CREB3L3 Tg (Ppara−/− × CREB3L3 Tg) mice in a fed state. Data are represented as mean ± SEM. Significant differences were determined by one-way ANOVA followed by Tukey-Kramer test and shown by *P < 0.05, **P < 0.01. n = 4 per group.
To test our hypothesis that if Cpt1a and Bdh1 are direct targets for CREB3L3, the reduction of them in Ppara−/− mice might be dependent on CREB3L3. To determine the effects of CREB3L3 on the expression of both Cpt1a and Bdh1, we crossed Ppara−/− mice with mice overexpressing the active form of human CREB3L3 in the liver (CREB3L3 Tg) to generate CREB3L3 Tg × Ppara−/− mice, and then performed qPCR. The expression levels of the PPARα target genes Acox1, Hmgcs2, Hmgcl, and Bdh1 were significantly downregulated in the liver of Ppara−/− mice compared with WT mice (Fig. 5b). The expression of Bdh1 and Cpt1a was significantly increased in CREB3L3 Tg × Ppara−/− mice compared with that in Ppara−/− mice (Fig. 5b). In contrast, there was no difference in the expression levels of the other genes, such as Hmgcs2, and Acox1, between Ppara−/− mice and CREB3L3 Tg × Ppara−/− mice (Fig. 5b). These results functionally confirmed that CREB3L3 could directly upregulate Bdh1 and Cpt1a expression in the liver.
Confirmation of Cpt1 and Bdh1 as CREB3L3-target genes using promoter analysis
To investigate whether CREB3L3 directly increases the expression of Cpt1 and Bdh1, we performed a luciferase analysis using the Cpt1a and Bdh1 promoters. CREB3L3 can bind to the cAMP response element (CRE) consensus sequence, which is a specific binding site of the CREB/ATF family of proteins8. We identified the CRE site at approximately −3 kb from the transcriptional initiation site of the mouse Cpt1a promoter. When the Cpt1a promoter luciferase vector containing this region was used in the luciferase assay, we found that it was CREB3L3 rather than PPARα that significantly activated the Cpt1a luciferase activity in the AML12.2 cells (Fig. 6a). We were unable to find the CRE sequence in the Bdh1 promoter; however, we successfully cloned the −750-bp region of its promoter. As in the Cpt1 promoter analysis, we found that CREB3L3, not PPARα, significantly activated Bdh1 luciferase activity in the AML12.2 cells (Fig. 6a). These results indicate that CREB3L3 activates these promoters by regulating these regions independent of PPARα.
Promoter analysis of CREB3L3-induced Cpt1a and Bdh1 expression.
(a) Effects of CREB3L3 and PPARα on the Cpt1a and Bdh1 promoter activity in mouse AML12.2 cells, as estimated using the luciferase promoter assay. Data are represented as mean ± SEM. Significant differences were determined by one-way ANOVA followed by Tukey-Kramer test and shown by *P < 0.05, **P < 0.01. n = 3 per group. (b) The effect of CREB3L3 on the Cpt1a promoter, as estimated using cAMP response element (CRE)-deleted and mutated vectors using the luciferase assay; the electrophoretic mobility shift assay (EMSA) showed that CREB3L3 directly bound to the CRE sequence. Data are represented as mean ± SEM. Significant differences were determined by unpaired Student’s t test and shown by **P < 0.01. n = 3 per group. (c) The effects of CREB3L3 on the Bdh1 promoter, as estimated with a luciferase analysis using deletion constructs of the Bdh1 promoter; the EMSA assay indicated that CREB3L3 directly bound to the region from −128 to −98 bp of the Bdh1 promoter. The effect of CREB3L3 on the region from −128 to −98 bp of the Bdh1 promoter, as estimated using the luciferase assay with 4-tandem region from −128 to −98 bp of the Bdh1 promoter vector Data are represented as mean ± SEM. Significant differences were determined by one-way ANOVA followed by Tukey-Kramer test and shown by *P < 0.05, **P < 0.01. n = 3 per group. (d) Results of the chromatin immunoprecipitation (ChIP) assay demonstrating that CREB3L3 directly binds to the Cpt1a and Bdh1 promoters in vivo in fasted and fed mice (n = 4 per group), and in vitro in AML12.2 cells infected with adenoviral GFP or the active form of CREB3L3 (n = 3 per group). Data are represented as mean ± SEM. Significant differences were determined by unpaired Student’s t test and shown by **P < 0.01.
The CRE-deleted and mutated Cpt1a promoter luciferase vectors were completely blunted to activation by CREB3L3 (Fig. 6b). The electrophoretic mobility shift assay (EMSA) showed that CREB3L3 could bind to the CRE sequence in the Cpt1a promoter (Fig. 6b). We also constructed a series of deletion mutants of the Bdh1 luciferase vectors, which showed that CREBL3 activated −200 bp of the Bdh1 luciferase vector, but the −100-bp region was blunted to activation by CREB3L3. The EMSA assay showed that CREB3L3 bound to the region between −126 bp and −98 bp in the Bdh1 promoter, but no other regions (Fig. 6c). CREB3L3 activated the luciferase vector containing 4x the region between −126 bp and −98 bp in the Bdh1 promoter (Fig. 6c). The chromatin immunoprecipitation (ChIP) assay using the liver of mice showed that CREB3L3 directly bound to the Bdh1 and Cpt1a promoter region more efficiently in mice that were in a fasted state than those that were in a fed state (Fig. 6d), which is consistent with the finding that the active form of the CREB3L3 protein is upregulated in fasted mice. Moreover, the ChIP assay using AML12.2 cells infected with adenoviral CREB3L3 showed that CREB3L3 directly bound to the Bdh1 and Cpt1a promoter region (Fig. 6d). Thus, we confirmed Bdh1 and Cpt1a as new target genes of CREB3L3.
Effect of Creb3l3 deficiency on KD-induced hepatosteatosis
It has previously been shown that KD-fed mice exhibit suppressed lipogenic gene expression and increased expression of genes involved in fatty acid oxidation, ketogenesis, and inflammation of the liver28,29. Furthermore, long-term KD causes an injury pattern in WT mice similar to nonalcoholic fatty liver disease (NAFLD) phenotypes28,30. To clarify the effects of CREB3L3 on ketogenesis-related hepatosteatosis, WT and Creb3l3−/− mice were fed MF or KD for 4 weeks, when WT mice does not show steatohepatitis. Histological analysis showed severe lipid accumulation and inflammation in the liver of Creb3l3−/− mice compared with WT mice (Fig. 7a). Consistently, liver TG and TC levels were also significantly higher in Creb3l3−/− mice than in WT mice (Fig. 7a). Immunohistochemistry using the anti-CD11b antibody as the macrophage marker showed that the liver sections from Creb3l3−/− mice exhibited increased infiltration by inflammatory cells compared with WT mice (Fig. 7a), which resulted in increased levels of both plasma aspartate aminotransferase (AST) and alanine aminotransferase (ALT), which are markers of liver injury (Fig. 7b).
Effect of Creb3l3 deficiency in the liver on ketogenic diet (KD)-induced fatty liver.
Eight-week-old male wild type (WT) and Creb3l3−/− mice were fed a medium-fat (MF) diet or KD for 4 weeks. (a) Histological analysis of liver sections from WT and Creb3l3−/− mice that had been fed KD for 4 weeks, using haematoxylin and eosin staining and immunohistochemistry using the anti-CD11b antibody. Liver contents of triglycerides (TG) and total cholesterol (TC). Data are represented as mean ± SEM. Significant differences were determined by unpaired Student’s t test and shown by *P < 0.05. n = 5 per group. (b) Plasma aspartate aminotransferase (AST), alanine aminotransferase (ALT), and FGF21 levels, and (c) hepatic gene expression profiles of WT and Creb3l3−/− mice that had been fed KD for 4 weeks. Data are represented as mean ± SEM. Significant differences were determined by one-way ANOVA followed by Tukey-Kramer test (b,c) and shown by *P < 0.05, **P < 0.01. n = 5–10 per group.
The hepatic expression of inflammatory marker genes, such as chemokine (C–C motif) ligand 2 (Ccl2), interleukin 6 (Il6), and tumour necrosis factor (Tnf), and fibrosis marker genes, such as alpha 2, smooth muscle, aorta (Acta2), and collagen, type I, alpha 1 (Col1a1), was significantly upregulated in KD-fed Creb3l3−/− mice compared with that in MF diet-fed WT mice and KD-fed WT mice (Fig. 7c). These results confirmed that inflammation was enhanced in Creb3l3−/− KD-fed mice. The expression of fatty acid oxidation genes Ppara and Cpt1a, and the ketogenesis genes Hmgcs2, Bdh1, Fgf21, and Creb3l3 were also significantly upregulated in KD-fed WT mice compared with that in MF-fed WT mice (Fig. 7c) but significantly downregulated in KD-fed Creb3l3−/− mice compared with that in KD-fed WT mice. Thus, the Creb3l3 deficiency suppressed fatty acid oxidation- and ketogenesis-related gene expression in the liver, even in mice that had been fed KD for a long time. Collectively, these data indicate that the dysfunction of fatty acid oxidation and ketogenesis in the liver of Creb3l3−/− mice exacerbated the development of KD-induced hepatosteatosis.
Discussion
CREB3L3 and PPARα have previously been shown to reciprocally activate each other and have overlapping functions such as changes of gene expression in the liver during fasting23,24. However, the functional differences between these proteins remained unclear. In this study, we investigated the direct effects of CREB3L3 on ketogenesis under various feeding conditions by comparing the phenotypes of WT, Creb3l3−/−, Ppara−/−, and Creb3l3−/−Ppara−/− mice. We found that the deletion of either Creb3l3 or Ppara resulted in considerable defects in the adaptation to energy starvation in mice. It should be noted that in either of the single KO mice, expression of the other gene was also decreased and thus additional effects of double deletion were further severe. Roughly, the deletion of Ppara largely blunted the hepatic gene expression of fatty acid oxidation and ketogenesis. It is conceivable that the synergistic interaction between CREB3L3 and PPARα should contribute to defected ketogenesis in Creb3l3−/− mice as well as Ppara−/− mice. We also found that CREB3L3 directly transactivates Cpt1a and Bdh1, thereby regulating ketogenesis independent of PPARα. Creb3l3−/− mice, especially on a KD, accelerated KD-induced hepatosteatosis.
PPARα is ubiquitously expressed in not only the liver but some peripheral tissues. In fasting, PPARα induces lipolysis in WAT by activating ATGL and HSL31,32. In turn, fatty acids derived from lipolysis are important activators of PPARα33,34 and the substrates of fatty acid oxidation and ketogenesis in liver. Hepatic PPARα governs fatty acid oxidation and ketogenesis gene expression. Therefore, it is thought that the deficiency of adipose Ppara aggravates the defects of hepatic fatty acid oxidation and ketogenesis due to insufficiency of energy source supply from peripheral tissues and metabolic activity in the liver. On the other hand, as CREB3L3 is expressed in only the liver and small intestine, lipolysis in WAT in Creb3l3−/− mice were comparative with that in WT mice. This could explain that reduced ketosis in Creb3l3−/− mice was limited in fasting.
KD is a high-fat, low-carbohydrate diet containing approximately 80% fat. Although both fasting and a KD feeding induce ketogenesis, a KD feeding supplies energy, the sources of which are mainly from dietary fat, and not lipolysis in adipose tissue. Therefore, a KD feeding study can reflect on the functions in hepatic fatty acid oxidation and ketogenesis. The changes of fatty acid oxidation and ketogenesis in Creb3l3−/− mice were comparable with those in Ppara−/− mice, indicating both hepatic factors are equally important in this metabolism.
In this study, we identified that CREB3L3 directly regulates Cpt1a, the rate limiting molecule for FA transport into the mitochondria, and Bdh1, a component gene in the ketogenesis pathway. Moreover, CREB3L3 upregulates PPARα target genes via the regulation of both the gene expression and transcriptional activity of PPARα. The expression of Cpt1a was solely regulated by CREB3L3 in a KD feeding (Fig. 2), whereas it was regulated by both CREB3L3 and PPARα during fasting (Fig. 4a). These finding indicates that the PPARα-CREB3L3 interaction in a KD feeding is more important for the PPARα-mediated Cpt1a expression than in fasting.
CPT1a is one of the rate-limiting enzymes involved in mitochondrial β-oxidation, catalysing the esterification of long-chain acyl-CoAs to L-carnitine for transport into the mitochondria for fatty acid oxidation. Therefore, the mitochondrial carnitine system plays a crucial role in β-oxidation. The reduction in Cpt1a expression in the liver of Creb3l3−/− mice inhibit the transport of fatty acids to the mitochondria and suppress fatty acid oxidation. KD contains approximately 80% fat, which mainly consists of long-chain acyl-CoAs. Thus, Creb3l3−/− mice cannot catalyse the excess long-chain acyl-CoAs.
Ketogenesis is catalysed by the enzymes HMGCS2, HMGCL, and BDH1, which converts acetyl-CoA into β-OH butyrate. BDH1 is involved in the final step of this process, catalysing Acac to β-OH butyrate. In this study, we demonstrated that CREB3L3 directly regulates Bdh1 expression. To increase the enzymatic activity of HMGCS2, deacetylation by SIRT3 is required26. The deficiency of Creb3l3 reduced the expression of Sirt3 in feeding a KD diet, indicating the reduction of Hmgcs2 enzymatic activity in Creb3l3−/− mice. Taken together, these results demonstrate that CREB3L3 plays a pivotal role in the ketogenic process that converts acetyl-CoA into β-OH butyrate in the liver.
These findings could propose that CREB3L3 contributes to ketogenesis; however, in the ketogenesis activity test, the deficiency of Creb3l3 did not show the predicted loss of its activity. Because its test uses sodium octanoate as a substrate, octanoate can cross the mitochondrial membranes independently of CPT1a35. Therefore, the differences of Cpt1a expression between Creb3l3−/− mice and Ppara−/− mice could not reflected a change in the ketogenesis activity (Fig. 4b). The deficiency of Ppara had the further decrease of other fatty acid oxidation and ketogenesis gene expression compared with the deficiency of Creb3l3, thereby further decreasing the ketogenesis activity.
It has previously been shown that Fgf21−/− mice exhibit impaired adaptation to ketosis20. In the present study, we found that the expression of hepatic Fgf21 was blunted in both Creb3l3−/− and Ppara−/− mice, which could at least partially explain why the Creb3l3 deficiency led to impaired ketogenesis.
It is well established that hepatic fatty acid oxidation disorders, including a deficiency in PPARα, lead to NAFLD5,6,7,36. High fat feeding of mice with insufficiency of ketogenesis results in NAFLD37. Consistently, the deficiency of Creb3l3 developed severe hepatic steatosis.
In summary, our findings indicate that CREB3L3 regulates ketogenesis via two pathways: (1) by directly activating Ppara expression to activate PPARα-induced genes related to fatty acid oxidation and ketogenesis and (2) by directly activating Cpt1a and Bdh1 expression. We propose that CREB3L3 co-operates with PPARα by directly and indirectly regulating the expression of genes involved in fatty acid oxidation and ketogenesis. In particular, CREB3L3 plays a role in exogenous (dietary) fatty acid homeostasis, while PPARα plays dual roles in exogenous and endogenous (released from lipolysis in adipose tissues) fatty acid homeostasis. Individuals with NASH have been shown to have lower levels of ketone bodies than individuals with simple steatosis38, and studies on humans have revealed that the levels of plasma ketone bodies is negatively correlated with the pathology of NASH, suggesting a reduction in ketone body metabolism in individuals with NASH38. Thus, as ketogenesis prevents diet-induced fatty liver injury and hyperglycaemia37, CREB3L3 may represent a new therapeutic target for NAFLD.
Experimental Procedures
Animals
Eight-week-old male C57/BL6J (WT) mice were obtained from CLEA Japan, and B6;129S4-Pparatm1G°nz/J (Ppara−/−)39 and Creb3l3tm1.1Sad/J (Creb3l3−/−)40 mice were purchased from Jackson Laboratory. To generate the active form of human CREB3L3 transgenic (CREB3L3 Tg) mice, cDNA encoding the rat Pck1 promoter41, human CREB3L3 (1–320 aa), and the 3′ polyadenylation signal of human growth hormone were microinjected into C57BL6J eggs24. To generate Creb3l3−/−Ppara−/− mice, Creb3l3−/− mice were crossed with Ppara−/− mice; and to generate Ppara−/− × CREB3L3 Tg (Ppara−/−CREB3L3 Tg) mice, Ppara−/− mice were crossed with CREB3L3 Tg mice.
For KD analysis, 8-week-old male mice were fed for 3 days or 4 weeks and sacrificed in a fed state. KD consisted 76% fat, 8.8% protein, and 0.74% carbohydrates (no sucrose) (w/w)24. For adenoviral infection, 8-week-old male mice were infected with the indicated adenovirus at 5.0 × 108 pfu/g body weight, following which samples were collected 6 days after infection from mice in a fed state. For the fasting and re-feeding experiments, mice were fasted for 24 h and then fed a high-sucrose/fat-free diet for 12 h, as previously described42. All animal husbandry procedures and animal experiments were performed in accordance with the Regulation of Animal Experiments of the University of Tsukuba and were approved by the Animal Experiment Committee, University of Tsukuba.
Histological analysis
Mouse livers were fixed, embedded in paraffin, sectioned, and stained with haematoxylin and eosin, and Masson trichrome.
Immunohistochemistry
Mouse livers were fixed in 10% formalin and embedded in paraffin blocks. The liver sections were then deparaffinized, and the antigens were retrieved by heating at 80 °C for 20 min. Following blocking, the sections were incubated with the primary antibodies for CD11b (1:600) (abcam, ab75476). After washing the sections with phosphate buffered saline (PBS), Histostar™ mouse (Medical & Biological Laboratories Co. Ltd. Japan) was applied at room temperature for 30 min, following which the samples were again washed with PBS. The reaction product was visualized by applying diaminobezidine (Dako Japan) for 5 min and then counterstaining the sections with haematoxylin.
Cell culture
Mouse AML12.2 hepatoma cells were maintained in Dulbecco’s Modified Eagle Medium/Ham’s F12 media supplemented with ITS Liquid Media Supplement (SIGMA) and 10% fetal calf serum. Cells were infected with adenoviral green fluorescent protein (GFP) or the active form of CREB3L3 at a multiplicity of infection of 100 and then incubated for 24 h.
Ketogenesis assay
To measure ketogenic activities in vivo, 8-week-old male mice were administered 0.5 M sodium octanoate at a dose of 6 μl per gram of body weight after 18 h of fasting27. Blood samples were collected 0, 1, 2, and 4 h after the injection, and plasma β-OH butyrate levels were determined using the β-OH butyrate colorimetric assay (Sanwa kagaku).
Plasmids
The active form of human CREB3L3 (1–320 aa) (AB0050902) was cloned using PCR into the pcDNA3 vector. The mouse Cpt1a promoter from −2200 bp to −1480 bp was cloned using PCR and subcloned into the pGL3-promoter luciferase vector (Promega). The mouse Bdh1 promoter from −720 bp to +130 bp and the mouse Ppara promoter from −480 bp to +100 bp were cloned using PCR and subcloned into the pGL3-basic luciferase vector (Promega).
Preparation of recombinant adenovirus
The cDNAs coding the active form (1–320 aa) of human CREB3L3 and GFP were cloned into pShuttle vectors (Clontech). In addition, the recombinant adenovirus vectors were recombined with pAdeno-X vectors (Clontech). The recombinant adenoviruses were produced in 293A cells (Invitrogen) and purified using CsCl gradient centrifugation, as previously described43.
Metabolic measurements
The levels of glucose, insulin, TG, FFA, TC, AST, ALT, and β-OH butyrate in the plasma, and the levels of glycogen, TG, and TC in the liver were measured as previously described43. Plasma FGF21 levels were measured using mouse FGF21 Quantikine® ELISA kits (R&D systems).
Immunoblotting
Total cell lysates were immunoblotted as previously described43, using antibodies to α-tubulin (Millipore) and an anti-mouse CREB3L3 polyclonal antibody, which was generated as previously described24.
Analysis of gene expression
Total RNA from cells and tissues was prepared using TRIzol® Reagent (Invitrogen). Real-time PCR analysis was performed using total RNA for cDNA synthesis (Invitrogen) with the ABI Prism 7300 system (ABI) and SYBR® Green Master Mix (Roche)44. Primer sequences are available upon request.
Promoter analysis
Mouse AML12.2 hepatoma cells were transfected with the indicated luciferase reporter, expression plasmids, and a pRL-SV40 plasmid as a reference (Promega) using FuGENE6 (Roche). After a 24-h incubation, the firefly luciferase activity was measured and normalized to the Renilla luciferase activity. We generated CREB3L3 from an expression vector using an in vitro reticulocyte transcription–translation system (Promega). We used the following sequences in the EMSAs: 5′-gaaaacctggtgacgttggctgagcaaata-3′ for the WT of the Cpt1a promoter; 5′-gaaaacctggtaaccttggctgagcaaata-3′ for the Mut of the Cpt1a promoter; 5′-gcttactttcctggccttgctcagggttctctgg-3′ for approximately −200 to −166 of the Bdh1 promoter; 5′-gttctctggctgtgtgtgtgtgtgtgtgtgtcc-3′ for −175 to −141 of the Bdh1 promoter; 5′-gtgtgtgtcccttagctgcagcgtctacccttgat-3′ for −151 to −117 of the Bdh1 promoter; and 5′-gtctacccttgatcttctgcttgccagagggt-3′ for −126 to −98 of the Bdh1 promoter. We incubated the in vitro-translated protein lysate in a reaction mixture as previously described43 and resolved the DNA-protein complexes on a 4% polyacrylamide gel.
Chromatin immunoprecipitation (ChIP) assay
ChIP assays were performed as previously described with some modifications43. In brief, we collected the livers of normal mice in fasted for a 24 h and fed states. AML12.2 cells were infected with GFP or the active form of CREB3L3 tagged with HA in the N-terminus at a multiplicity of infection of 100 and then incubated for 48 h. Minced liver tissues and AML12.2 cells were fixed in 1% formaldehyde for 15 min at room temperature. The soluble chromatin was subjected to immunoprecipitation with anti-mouse CREB3L3 polyclonal antibody24, anti-HA (Y-11, Santa Cruz), or with control IgG, and rotated overnight at 4 °C. Immune complexes were washed and then incubated overnight at 65 °C for reverse crosslinking. Chromatin DNA was extracted with phenol-chloroform, precipitated with ethanol, resuspended in water, and subjected to real-time PCR analysis. The primers used for real-time PCR were as follows: Cpt1a promoter region containing the CREB3L3 binding site, 5′-cgttggcagccttgggtttg-3′ and 5′-acacgttttgagtcaatatcggaggtag-3′; Cpt1a distal region, 5′-tgctctgtgaaagatgcttatg-3′ and 5′-cacactggccccaagcca-3′; Bdh1 promoter region containing the CREB3L3 binding site for mouse liver, 5′-gcttactttcctggccttgctcagggtt-3′ and 5′-accctctggcaagcagaagatcaagggt-3′; Bdh1 promoter region containing the CREB3L3 binding site for AML12.2 cells, 5′-gttcccagcatgccagaca-3′ and 5′-gaatttgaccctctggcaag-3′; Bdh1 negative region, 5′-ccgtgtaacctcgaaactgc-3′ and 5′-gtgcatgctgagcgagcac-3′.
Statistical analyses
Statistical significance was calculated using unpaired Student’s t-tests, one-way ANOVA followed by Tukey-Kramer test, or repeated-measures two-way ANOVA with Bonferroni post hoc t-test with a significance level of P < 0.05. All data are expressed as mean ± SEM.
Additional Information
How to cite this article: Nakagawa, Y. et al. CREB3L3 controls fatty acid oxidation and ketogenesis in synergy with PPARα. Sci. Rep. 6, 39182; doi: 10.1038/srep39182 (2016).
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
Tugwood, J. D. et al. The mouse peroxisome proliferator activated receptor recognizes a response element in the 5′ flanking sequence of the rat acyl CoA oxidase gene. The EMBO journal 11, 433–439 (1992).
Erol, E. et al. Liver fatty acid binding protein is required for high rates of hepatic fatty acid oxidation but not for the action of PPARalpha in fasting mice. FASEB journal : official publication of the Federation of American Societies for Experimental Biology 18, 347–349, doi: 10.1096/fj.03-0330fje (2004).
Hsu, M. H., Savas, U., Griffin, K. J. & Johnson, E. F. Identification of peroxisome proliferator-responsive human genes by elevated expression of the peroxisome proliferator-activated receptor alpha in HepG2 cells. The Journal of biological chemistry 276, 27950–27958, doi: 10.1074/jbc.M100258200 (2001).
Rodriguez, J. C., Gil-Gomez, G., Hegardt, F. G. & Haro, D. Peroxisome proliferator-activated receptor mediates induction of the mitochondrial 3-hydroxy-3-methylglutaryl-CoA synthase gene by fatty acids. The Journal of biological chemistry 269, 18767–18772 (1994).
Hashimoto, T. et al. Defect in peroxisome proliferator-activated receptor alpha-inducible fatty acid oxidation determines the severity of hepatic steatosis in response to fasting. The Journal of biological chemistry 275, 28918–28928, doi: 10.1074/jbc.M910350199 (2000).
Kersten, S. et al. Peroxisome proliferator-activated receptor alpha mediates the adaptive response to fasting. The Journal of clinical investigation 103, 1489–1498, doi: 10.1172/JCI6223 (1999).
Leone, T. C., Weinheimer, C. J. & Kelly, D. P. A critical role for the peroxisome proliferator-activated receptor alpha (PPARalpha) in the cellular fasting response: the PPARalpha-null mouse as a model of fatty acid oxidation disorders. Proceedings of the National Academy of Sciences of the United States of America 96, 7473–7478 (1999).
Omori, Y. et al. CREB-H: a novel mammalian transcription factor belonging to the CREB/ATF family and functioning via the box-B element with a liver-specific expression. Nucleic Acids Res 29, 2154–2162 (2001).
Zhang, K. et al. Endoplasmic reticulum stress activates cleavage of CREBH to induce a systemic inflammatory response. Cell 124, 587–599, doi: 10.1016/j.cell.2005.11.040 (2006).
Danno, H. et al. The liver-enriched transcription factor CREBH is nutritionally regulated and activated by fatty acids and PPARalpha. Biochem Biophys Res Commun 391, 1222–1227, doi: 10.1016/j.bbrc.2009.12.046 (2010).
Lee, M. W. et al. Regulation of hepatic gluconeogenesis by an ER-bound transcription factor, CREBH. Cell metabolism 11, 331–339, doi: 10.1016/j.cmet.2010.02.016 (2010).
Zhang, C. et al. Endoplasmic reticulum-tethered transcription factor cAMP responsive element-binding protein, hepatocyte specific, regulates hepatic lipogenesis, fatty acid oxidation, and lipolysis upon metabolic stress in mice. Hepatology 55, 1070–1082, doi: 10.1002/hep.24783 (2012).
Lee, J. H. et al. The transcription factor cyclic AMP-responsive element-binding protein H regulates triglyceride metabolism. Nature medicine 17, 812–815, doi: 10.1038/nm.2347 (2011).
Nakagawa, Y. et al. Hyperlipidemia and hepatitis in liver-specific CREB3L3 knockout mice generated using a one-step CRISPR/Cas9 system. Scientific reports 6, 27857, doi: 10.1038/srep27857 (2016).
Park, J. G. et al. CREBH-FGF21 axis improves hepatic steatosis by suppressing adipose tissue lipolysis. Scientific reports 6, 27938, doi: 10.1038/srep27938 (2016).
Kharitonenkov, A. FGFs and metabolism. Curr Opin Pharmacol 9, 805–810, doi: 10.1016/j.coph.2009.07.001 (2009).
Inagaki, T. et al. Endocrine regulation of the fasting response by PPARalpha-mediated induction of fibroblast growth factor 21. Cell metabolism 5, 415–425, doi: 10.1016/j.cmet.2007.05.003 (2007).
Badman, M. K. et al. Hepatic fibroblast growth factor 21 is regulated by PPARalpha and is a key mediator of hepatic lipid metabolism in ketotic states. Cell metabolism 5, 426–437, doi: 10.1016/j.cmet.2007.05.002 (2007).
Hotta, Y. et al. Fibroblast growth factor 21 regulates lipolysis in white adipose tissue but is not required for ketogenesis and triglyceride clearance in liver. Endocrinology 150, 4625–4633, doi: 10.1210/en.2009-0119 (2009).
Badman, M. K., Koester, A., Flier, J. S., Kharitonenkov, A. & Maratos-Flier, E. Fibroblast growth factor 21-deficient mice demonstrate impaired adaptation to ketosis. Endocrinology 150, 4931–4940, doi: 10.1210/en.2009-0532 (2009).
Xu, J. et al. Fibroblast growth factor 21 reverses hepatic steatosis, increases energy expenditure, and improves insulin sensitivity in diet-induced obese mice. Diabetes 58, 250–259, doi: 10.2337/db08-0392 (2009).
Kharitonenkov, A. et al. The metabolic state of diabetic monkeys is regulated by fibroblast growth factor-21. Endocrinology 148, 774–781, doi: 10.1210/en.2006-1168 (2007).
Kim, H. et al. Liver-enriched transcription factor CREBH interacts with peroxisome proliferator-activated receptor alpha to regulate metabolic hormone FGF21. Endocrinology 155, 769–782, doi: 10.1210/en.2013-1490 (2014).
Nakagawa, Y. et al. Hepatic CREB3L3 Controls Whole-Body Energy Homeostasis and Improves Obesity and Diabetes. Endocrinology 155, 4706–4719, doi: 10.1210/en.2014-1113 (2014).
Bough, K. J. & Eagles, D. A. A ketogenic diet increases the resistance to pentylenetetrazole-induced seizures in the rat. Epilepsia 40, 138–143 (1999).
Shimazu, T. et al. SIRT3 deacetylates mitochondrial 3-hydroxy-3-methylglutaryl CoA synthase 2 and regulates ketone body production. Cell metabolism 12, 654–661, doi: 10.1016/j.cmet.2010.11.003 (2010).
Sengupta, S., Peterson, T. R., Laplante, M., Oh, S. & Sabatini, D. M. mTORC1 controls fasting-induced ketogenesis and its modulation by ageing. Nature 468, 1100–1104, doi: 10.1038/nature09584 (2010).
Pissios, P. et al. Methionine and choline regulate the metabolic phenotype of a ketogenic diet. Molecular metabolism 2, 306–313, doi: 10.1016/j.molmet.2013.07.003 (2013).
Jornayvaz, F. R. et al. A high-fat, ketogenic diet causes hepatic insulin resistance in mice, despite increasing energy expenditure and preventing weight gain. Am J Physiol Endocrinol Metab 299, E808–815, doi: 10.1152/ajpendo.00361.2010 (2010).
Garbow, J. R. et al. Hepatic steatosis, inflammation, and ER stress in mice maintained long term on a very low-carbohydrate ketogenic diet. American journal of physiology. Gastrointestinal and liver physiology 300, G956–967, doi: 10.1152/ajpgi.00539.2010 (2011).
Poulsen, L., Siersbaek, M. & Mandrup, S. PPARs: fatty acid sensors controlling metabolism. Seminars in cell & developmental biology 23, 631–639, doi: 10.1016/j.semcdb.2012.01.003 (2012).
Bolsoni-Lopes, A. et al. Palmitoleic acid (n-7) increases white adipocyte lipolysis and lipase content in a PPARalpha-dependent manner. Am J Physiol Endocrinol Metab 305, E1093–1102, doi: 10.1152/ajpendo.00082.2013 (2013).
Zechner, R. et al. FAT SIGNALS–lipases and lipolysis in lipid metabolism and signaling. Cell metabolism 15, 279–291, doi: 10.1016/j.cmet.2011.12.018 (2012).
Liu, S. et al. A diurnal serum lipid integrates hepatic lipogenesis and peripheral fatty acid use. Nature 502, 550–554, doi: 10.1038/nature12710 (2013).
McGarry, J. D. & Foster, D. W. Regulation of hepatic fatty acid oxidation and ketone body production. Annual review of biochemistry 49, 395–420, doi: 10.1146/annurev.bi.49.070180.002143 (1980).
Montagner, A. et al. Liver PPARalpha is crucial for whole-body fatty acid homeostasis and is protective against NAFLD. Gut, doi: 10.1136/gutjnl-2015-310798 (2016).
Cotter, D. G. et al. Ketogenesis prevents diet-induced fatty liver injury and hyperglycemia. The Journal of clinical investigation 124, 5175–5190, doi: 10.1172/JCI76388 (2014).
Mannisto, V. T. et al. Ketone body production is differentially altered in steatosis and non-alcoholic steatohepatitis in obese humans. Liver international : official journal of the International Association for the Study of the Liver 35, 1853–1861, doi: 10.1111/liv.12769 (2015).
Lee, S. S. et al. Targeted disruption of the alpha isoform of the peroxisome proliferator-activated receptor gene in mice results in abolishment of the pleiotropic effects of peroxisome proliferators. Mol Cell Biol 15, 3012–3022 (1995).
Luebke-Wheeler, J. et al. Hepatocyte nuclear factor 4alpha is implicated in endoplasmic reticulum stress-induced acute phase response by regulating expression of cyclic adenosine monophosphate responsive element binding protein H. Hepatology 48, 1242–1250, doi: 10.1002/hep.22439 (2008).
Shimano, H. et al. Overproduction of cholesterol and fatty acids causes massive liver enlargement in transgenic mice expressing truncated SREBP-1a. The Journal of clinical investigation 98, 1575–1584, doi: 10.1172/JCI118951 (1996).
Ide, T. et al. SREBPs suppress IRS-2-mediated insulin signalling in the liver. Nat Cell Biol 6, 351–357, doi: 10.1038/ncb1111 (2004).
Nakagawa, Y. et al. TFE3 transcriptionally activates hepatic IRS-2, participates in insulin signaling and ameliorates diabetes. Nature medicine 12, 107–113, doi: 10.1038/nm1334 (2006).
Fujimoto, Y. et al. TFE3 controls lipid metabolism in adipose tissue of male mice by suppressing lipolysis and thermogenesis. Endocrinology 154, 3577–3588, doi: 10.1210/en.2013-1203 (2013).
Acknowledgements
This work was supported by Grants-in-Aid from the Ministry of Science, Education, Culture and Technology of Japan (to Y.N.), Suzuken Memorial Foundation (to Y.N.), Uehara Memorial Foundation (to Y.N. and H. Shimano), Kanae Foundation for The Promotion of Medical Science (to Y.N.), Astellas Foundation for Research on Metabolic Disorders (to Y.N.), Ono Medical Research Foundation (to Y.N.), Japan Diabetes Foundation (to Y.N.), Mochida Memorial Foundation for Medical and Pharmaceutical Research (to Y.N.), Takeda Science Foundation (to Y.N.), and Japan Foundation for Applied Enzymology (to Y.N.). This manuscript was edited and proofread by Enago.
Author information
Authors and Affiliations
Contributions
Y.N. and H. Shimano designed the experiments and wrote the manuscript. Y.N., A.S., H.T., S.-I.H., K.T., and T.M. performed the experiments. H.I., S.Y., N. Yahagi, Y.I., H. Suzuki, and H. Sone were involved in project planning. N. Yamada supervised this study and contributed crucial ideas to the project.
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Electronic supplementary material
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Nakagawa, Y., Satoh, A., Tezuka, H. et al. CREB3L3 controls fatty acid oxidation and ketogenesis in synergy with PPARα. Sci Rep 6, 39182 (2016). https://doi.org/10.1038/srep39182
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep39182
This article is cited by
-
Network-level analysis of ageing and its relationship with diseases and tissue regeneration in the mouse liver
Scientific Reports (2023)
-
Transcription factor CREB3 is a potent regulator of high-fat diet-induced obesity and energy metabolism
International Journal of Obesity (2022)
-
Cellular, mitochondrial and molecular alterations associate with early left ventricular diastolic dysfunction in a porcine model of diabetic metabolic derangement
Scientific Reports (2020)
-
Analysis of hepatic transcriptome demonstrates altered lipid metabolism following Lactobacillus johnsonii BS15 prevention in chickens with subclinical necrotic enteritis
Lipids in Health and Disease (2018)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.