Abstract
Muscle exercise induces intramuscular triglyceride (TG) accumulation and promotes mitochondrial maintenance in myotubes. However, the mechanism underlying exercise effects remains unknown. In this study, lactic acid was tested as a signaling molecule in C2C12 myotubes to understand the mechanism. Intracellular TG storage was induced in the cells by sodium lactate. The lactate activity was observed with an inhibition of the cAMP-PKA pathway as indicated by a reduction in the phosphorylation status of CREB (pCREB). Induction of pCREB signal by forskolin was blocked by pretreatment of cells with lactate. The impact of lactate on mitochondrial function was examined with a focus on the activities of two enzymes, MCAT (malonylCoA:ACP transferase) and PDH (pyruvate dehydrogenase). The enzyme activities were induced in the cells by lactate. Expression of the lactate receptor (GPR81) and lactate transporters (MCT1/4) were induced as well by lactate. The lactate activities were observed at concentrations between 4–64 mM, and were not dependent on the increase in intracellular pyruvate. Pyruvate treatment did not generate the same effects in the cells. Those results suggest that lactate may induce intramuscular TG storage and mitochondrial maintenance in myotubes through inhibition of the cAMP pathway by activation of GPR81 in a positive feedback manner.
Similar content being viewed by others
Introduction
Lactic acid is a product of glycolysis in cells and the production is enhanced under hypoxia conditions. Lactic acid exists in the sodium salt (i.e. sodium lactate) form in the body in most conditions. Lactate level is dramatically increased in the skeletal muscle by oxygen deficiency during the intensive physical exercise. Lactate concentrations may reach 10–20 mM in the circulation1,2 or even 40 mM in the skeletal muscle after the intense anaerobic exercise in humans3. In the classical biochemistry, lactate is used in ATP production in the tricarboxylic acid cycle in the presence of oxygen or used as a substrate in gluconeogenesis4. The lactate utilization is dependent on conversion into pyruvate by pyruvate dehydrogenase (PDH). Extracellular lactate is transferred into cells through its transporters, such as the proton-linked monocarboxylate transporters (MCTs). MCT1 is expressed in most cell types at a low level and MCT4 is expressed at a high level in the white skeletal muscle fibers5. The transporters moves lactate in and out of cells.
The signaling activity of lactate (termed lactormone) has been identified in adipocytes recently6, which provides a mechanism for reduction of plasma free fatty acids (FFAs) by lactate, a well-known effect of lactate. Lactate reduces FFAs by inhibition of adipocyte lipolysis. Lactate binds to the cell membrane receptor GPR81, an orphan Gi-protein-coupled receptor in adipocytes7,8,9. The receptor is activated by lactate to suppress the cAMP production by down-regulation of the adenyl cyclase activity9. In 2008, GPR81 was identified as a receptor of lactate in adipocytes in cell culture systems8. In 2009, GPR81 was found to be internalized after lactate ligation7. The highest expression of GPR81 was found in the adipose tissue followed by skeletal muscle, liver and kidney7. In 2010, the GPR81 activity was confirmed in adipose tissue in a line of global GPR81 gene knockout mice9. In the GPR81 pathway, the receptor activation leads to inhibition of the adenylyl cyclase activity for the down-regulation of the cAMP-PKA pathway. Given the role of cAMP in the induction of lipolysis and inhibition of lipogenesis10, inhibition of the cAMP-PKA pathway provides a perfect model for the lactate activity in the induction of TG accumulation in adipocytes. The studies present strong evidence for the signaling activity of lactate in adipocytes. However, the signaling activity remains to be established in skeletal muscle.
Exercise training increases intramuscular TG content, which is positively associated with the muscle endurance in athletes. The intramuscular TG provides a local source of high-dense energy to support the muscle contraction11,12. Although the exercise effect on intramuscular TG has been known for a long time, the molecular mechanism is poorly understood. Based on the adipocyte studies, we propose that lactate may induce intramuscular TG accumulation by activation of the GPR81 pathway.
Mitochondrial function is required for ATP production from fatty acids, which involves β-oxidation of long chain fatty acids to generate acetyl-CoA. The mitochondrial function is dependent on many enzymes whose activities are regulated by post-translational modification from variety of small molecules. Alpha-lipoic acid (α-lipoic acid) is one of the small molecules, which is required for protein lipoylation. Lipoylation is a protein modification by adding a lipoic acid to the lysine residue of protein, which is required for the activities of pyruvate dehydrogenase (PDH) and other enzymes (such as αKDH) in mitochondria. α-lipoic acid is synthesized from acetyl-CoA by the fatty acid synthase II system (FAS II) in mitochondria13. MCAT (malonylCoA:ACP transferase) is a major component in the FAS II complex in mitochondria14,15. Exercise training increases the mitochondrial function, but the role of MCAT is not known in the exercise effect. In this study, lactate is tested in the regulation of MCAT activity.
In this study, we tested our lactate hypothesis in mouse C2C12 myotubes. The results suggest that lactate induces TG accumulation and MCAT expression through inhibition of the cAMP-PKA pathway by activation of GPR81. Additionally, lactate induced expression of its receptor GPR81 and lactate transporters (MCT1/4). These data suggest that lactate may serves as a signaling molecule in muscle cells by activation of GPR81 in the regulation of TG accumulation and mitochondrial function.
Results
Induction of TG accumulation by lactate in C2C12 myotubes
At the physiological concentrations, lactate induces TG accumulation in adipocytes of humans, mice, and rats9. To test the lactate activity in muscle cells, we treated C2C12 myotubes with lactate in cell culture and examined the intracellular TG. The TG content was significantly induced by the lactate treatment at concentrations of 16 mM and 20 mM, respectively (Fig. 1A,B). The increase was observed under microscope with oil-red O staining, and with TG quantification in the cell lysate. The increase was in a dose-dependent manner for lactate at concentrations of 16–64 mM (Fig. 1B). A similar dose-dependent effect was observed in the induction of intracellular glycerol content by lactate, a marker of TG synthesis. In the positive control, the two parameters were induced by lactate in 3T3-L1 adipocytes (Fig. 1C). The lactate activity was observed in both types of cells. However, the muscle cells and adipocytes exhibited different sensitivities to lactate. The adipocytes had a higher sensitivity as the strongest effect was observed at 16 mM of lactate. In myotubes, the strongest effect was observed at 64 mM of lactate. The data suggests that lactate induces intracellular TG accumulation in C2C12 in a dose-dependent manner at concentrations of 16–64 mM.
(A) TG in cells with oil-red O staining. C2C12 cells were treated with lactate at 16 mM and 20 mM for 4 h. Lipid accumulation was determined by oil-red O staining. (B) TG and glycerol quantification. C2C12 cells were treated with lactate at three concentrations for 4 h. TG and glycerol contents were determined in the whole cell lysate with the lipoprotein lipase-based colorimetric assay. (C) Lactate effect on adipocytes. 3T3-L1 adipocytes were treated with increasing concentrations of lactate for 4 h. TG and glycerol contents were determined with the lipoprotein lipase-based colorimetric assay. The result represents mean ± SE. *p < 0.05, **p < 0.01 compared with the control.
Inhibition of the cAMP-PKA pathway by lactate
Activation of the cAMP-PKA pathway leads to lipolysis and suppression of lipogenesis16, which results in the reduction of intracellular TG. In the pathway, the transcription factor CREB is activated by phosphorylation on S133 to change gene expression in the lipolytic and lipogenic pathways. When the pathway is inhibited in adipocyte by lactate, the TG accumulation is increased. To test the lactate activity in muscle cells, CREB phosphorylation was examined in C2C12 myotubes. The GPR81 agonist, 3,5-DHBA and cAMP inducer forskolin were employed in the positive controls, respectively. pCREB signal was significantly reduced by lactate at 16 mM (Fig. 2A). A similar activity was observed for lactate in 293 cells (Fig. 2B). Lactate exhibited a stronger activity in 293 cells than that in C2C12 cells, which was associated with higher level of GPR81 protein in 293 cells (Fig. 2C). When GPR81 was activated with the positive control 3,5-DHBA (1 mM), a similar inhibition was observed in the pCREB signal (Fig. 2D). In contrast, pCREB signal was induced by forskolin (Fig. 2E), and the effect was blocked by lactate or 3,5-DHBA (Fig. 2F). These data suggest that lactate may activate GPR81 to inhibit the cAMP-PKA pathway in C2C12 cells. GPR81 was reported to activate the ERK signaling pathway7,17. ERK activity was examined in C2C12 cells after lactate treatment. The phosphorylation of ERK was modestly induced by lactate (Fig. 2G).
pCREB and CREB signals were determined in Western blotting. (A) Inhibition of the pathway in C2C12 cells by lactate (16 mM) treatment for 10 min. (B) Lactate activity in 293 cells. (C) The protein level of GPR81 in C2C12 and 293 cells. (D) Inhibition of pCREB signal by the GPR81 activator 3,5-DHBA. (E) Induction of pCREB signal in C2C12 cells by forskolin (20 μM) in a time course study. (F) Blockage of forskolin-induced pCREB signal with lactate (16 mM) pretreatment for 0.5 h. (G) Regulation of pERK signal by forskolin and lactate.
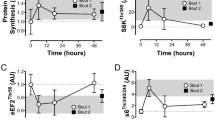
Induction of mitochondrial MCAT activity by lactate
MCAT is a mitochondrial protein that is required for the maintenance of mitochondrial function. MCAT promotes synthesis of α-lipoic acid in the control of lipoylation of PDH and αKDH in mitochondria18. MCAT was examined to investigate the potential lactate impact on mitochondrial function. The level of MCAT protein was induced by lactate (16 mM) in C2C12 cells in the time course study (Fig. 3A). The induction was observed at 30 minutes and maintained for 4 h. An increase in lipoylation was observed in PDH and αKDH in the lactate-treated cells, suggesting that the enzyme activity of MCAT was enhanced by the protein elevation. In the dose-dependent study, lactate induced MCAT protein at the concentrations of 2–64 mM in a 4 h treatment (Fig. 3B). Interestingly, MCAT mRNA was not increased by lactate in the time- and dose-dependent studies (Fig. 3C,D), suggesting a mechanism of post-translational modification in the control of MCAT protein level. As an indicator of mitochondrial function, the enzyme activity of PDH was enhanced together with the lipoylation by lactate in C2C12 cells (Fig. 3E). These data suggest that lactate may use an mRNA-independent mechanism to up-regulate MCAT activity in the control of mitochondrial function.
. (A) MCAT activity. MCAT protein and lipoylation of PDH were determined in C2C12 cells after lactate treatment (16 mM) in Western blot. (B) Induction of MCAT protein by different dosages of lactate in C2C12 cells at 4 h. (C) Time-dependent study of lactate effect on MCAT. mRNA was determined by qRT-PCR at different time points of lactate treatment (16 mM) in C2C12 cells. (D) Dose-dependent study of lactate effect on MCAT. mRNA was determined in C2C12 cells at 40 min with lactate treatment at different dosages. (E) Catalytic activity of PDH in C2C12 cells after lactate treatment. The test was conducted with lactate at 8 mM and 16 mM for 2 h. In the bar figure, the result represents mean ± SE (n = 3). Statistical analysis was performed with student’s test. *p < 0.05 versus control group.
Inhibition of MCAT protein by cAMP
MCAT is important in the maintenance of mitochondrial function, but the signaling pathway remains unknown in the regulation of MCAT activity. To address this issue, the cAMP-PKA pathway was investigated in the regulation of MCAT using the forskolin model (Fig. 4A). MCAT protein was reduced by forskolin activation of the cAMP pathway at 1 h, and the effect was maintained up to 8 h in the study. In contrast, the protein level was increased by down-regulation of the pathway from GPR81 activation by 3,5-DHBA (Fig. 4B). The increase was observed at 10 min with the treatment by GPR81 activator in the 60 min study (Fig. 4B). The peak increase was observed at 20 min. The MCAT inhibition by forskolin was significantly attenuated by pre-treatment of C2C12 cells with lactate (Fig. 4C). These data suggest that MCAT protein is reduced by the cAMP pathway, and is up-regulated upon inhibition of the pathway by lactate or 3,5-DHBA.
. (A) Reduction of MCAT protein by forskolin treatment (20 μM). MCAT protein was determined in C2C12 cells after forskolin treatment in Western blot. (B) Induction of MCAT protein by GPR81 activator. The test was performed in C2C12 cells after 3,5-DHBA treatment (1 mM). (C) Inhibition of forskolin activity by lactate. MCAT protein was determined in C2C12 cells after lactate pretreatment for 2 h followed by 4 h forskolin treatment.
Difference of pyruvate and lactate
The GPR81 receptor represents a receptor-dependent pathway for lactate in the regulation of TG accumulation and mitochondrial function. The receptor-independent pathway was investigated with a focus on pyruvate. Intracellular pyruvate was increased in C2C12 cells by the lactate treatment (Fig. 5A). The increase may mediate the lactate activity as well. To test this possibility, we treated C2C12 cells with pyruvate at a concentration (16 mM) with different duration (0.5–8 h), which mimic in the condition in the study of lactate activities. TG and MCAT were examined to determine the pyruvate effects. The TG level was not increased by pyruvate in the time course study (Fig. 5B). The protein level of MCAT and lipoylation signals (PDH and αKDH) were not significantly induced by pyruvate (Fig. 5C). The lipoylation signals were even reduced by pyruvate at 8 h in the study. No effect was observed for pyruvate in the regulation of MCAT protein and the lipoylation signals in C2C12 cells in the dose-dependent study (Fig. 5D). A mild reduction was observed in MCAT protein upon pyruvate treatment at 4 mM concentration. These data suggest that pyruvate may not mediate the lactate activity in the induction of TG accumulation and MCAT protein.
(A) Induction of intracellular pyruvate by lactate. Pyruvate was examined in C2C12 cells treated with 16 mM lactate for different times. (B) TG in whole cell lysate. Total triglyceride was determined in C2C12 cells after pyruvate treatment (16 mM) at different times. (C) Time course of MCAT activity. MCAT protein and lipoylation signals were determined in pyruvate-treated cells by Western blot. (D) Does-dependent study of pyruvate. MCAT protein and lipoylation signals were determined after pyruvate treatment at different concentrations (2–64 mM) for 4 h. The data are presented as mean ± SD (n = 3) in the bar figure *p <0.05, **p <0.01.
Induction of GPR81 by lactate
The factors that regulate GPR81 receptor expression remain largely unknown. To address this issue, protein and mRNA of GPR81 were examined in C2C12 cells following the lactate treatment. In the time course studies, the cells were treated with 16 mM lactate for different times. GPR81 protein was induced at 1 h of lactate treatment (Fig. 6A). The increase was associated with elevated expression of GPR81 mRNA. The mRNA was induced by lactate as early as 0.5 h in the treatment, and the lactate activity was observed at all of time points in the 4 h study (Fig. 6B). In the dose-dependent study, the lactate activity was observed at 4 mM with the highest activity at 8 mM (Fig. 6C). GPR81 mRNA was induced by lactate at concentrations of 16–64 mM in the 40 min study (Fig. 6D). No effect was observed for lactate at concentrations of 4–8 mM. These data suggest that lactate induces GPR81 expression in C2C12 cells.
(A) Lactate induction of GPR81 protein. GPR81 protein was determined in the whole cell lysate of C2C12 by Western blot after lactate treatment (16 mM) at different times. (B) mRNA of GPR81. mRNA was quantified in cells at 0.5 h and 4 h after lactate treatment. (C) GPR81 protein in a dose-dependent study of lactate. The protein was determined in C2C12 cells after lactate treatment at concentrations of 4–64 mM for 2 h. (D) mRNA in a dose-dependent study of lactate. The cells were treated with lactate for 40 min at different concentrations (4–64 mM). The data are expressed as mean ± SD (n = 3). Statistical analysis was performed with Student’s test. *p < 0.05 and **p < 0.01 versus control group.
Induction of MCT1 and MCT4 expression by lactate
MCT1 and MCT4 are proton-coupled transporters of lactate, which are required for cross-membrane transport of lactate in cells. MCT1 involves in lactate uptakes by cells, and MCT4 is for export of lactate from cells19. To determine the role of lactate in the regulation of the transporters, we examined mRNA of MCT1 and MCT4 in C2C12 cells following the lactate treatment. MCT1 expression was induced by lactate (Fig. 7A) and the peak induction was observed at 4 h. MCT4 expression was induced as well, but the peak was observed at 0.5–1 h (Fig. 7B). These data suggest that lactate induces expression of MCT1 and MCT4, and the dynamics are different in the expression of two transporters upon lactate stimulation.
mRNA of MCT1 and MCT4 was determined in C2C12 cells after lactate treatment. (A) Time course study of MCT1 mRNA. The level was determined by qRT-PCR at time points as indicated after 16 mM lactate treatment. (B) Time course study of MCT4 mRNA. Relative mRNA level of MCT4 was determined after 16 mM lactate treatment. The data are expressed as mean ± SD (n = 3). Statistical comparisons are performed with Student’s test. *p < 0.05 and **p < 0.01 versus control group.
Discussion
Our data suggests that lactate increases TG accumulation and mitochondrial function in myotubes in cell culture, which was observed at the physiological concentrations of lactate. TG accumulation is increased and mitochondrial capacity is enhanced in muscle by exercise20,21. However, the mechanism of the two events remains unknown. In this study, we tested lactate in the regulation of both events in mouse C2C12 myotubes, a cellular model. The data suggest that lactate has a signaling activity in the regulation of two events.
Our data suggest that lactate may activate the receptor GPR81 to induce the two events. Lactate activates GPR81 in adipocytes to induce TG accumulation by suppression of the cAMP-PKA pathway7,8,9,22. In this study, the pathway was tested in muscle cells. The data suggest that the same signaling pathway acts in muscle cells. In this study, expression of GPR81 was observed in C2C12 cells, and the cAMP-PKA pathway was inhibited at the downstream of GPR81. Lactate acted on GPR81 to inhibit the cAMP pathway as suggested by the activity of positive ligand of GPR81 (3,5-dihydroxybenzoic acid). Lactate blocked the forskolin activity in the activation of cAMP-PKA pathway. These data suggest that the GRP81 receptor may mediate the lactate activity in the down-regulation of the cAMP-PKA pathway in muscle cells. The pathway provides a mechanism for induction of TG accumulation by lactate in C2C12 myotubes. GPR81 was reported to induce ERK phosphorylation in adipocytes in some studies7,17. However, this effect was not strong in C2C12 cells, which may reflect a difference between myotubes and adipocytes.
Our data provide a mechanism for regulation of mitochondrial function by lactate. The lactate activity was investigated with a focus on MCAT, which is required for the maintenance of mitochondrial function13,18,23. Although the MCAT activity is known in mitochondria, it is not known how MCAT activity is regulated. Our data suggest that MCAT protein is induced by lactate through a mRNA-independent mechanism since MCAT mRNA was not up-regulated by lactate. In addition, our data suggests that the MCAT activity is inhibited by the cAMP pathway as suggested by the forskolin activity. Lactate may induce MCAT protein by blocking the cAMP activity. The change in MCAT activity was observed in protein, but not in mRNA expression. The enzyme activity of MCAT was induced by lactate as indicated by the alteration in lipoylation of PDH as well as αKDH, which are indicators of mitochondrial function.
Induction of GPR81 by lactate provides a mechanism of a positive feedback of lactate pathway. GPR81 mediates lactate signal to inhibit the cAMP-PKA pathway, but regulation of GPR81 expression has not been documented in the literature. Kashan, et al. found that lactate activated GPR81 with EC50 value of 1.5 mM in both human and mouse using GTPγS binding methods9. Changlu, et al. reported that lactate stimulated the internalization of GPR81 and GTPγS binding with EC50 value of about 5 mM7. EC50 value of lactate in the activation of GPR81 is 1.5–3 mM in adipocytes. Our results suggest that GPR81 expression is induced in protein and mRNA by lactate at 4–64 mM. The induction was consistent in the time course and dose-dependent studies of lactate. The GPR81 responses suggest a positive feedback regulation of the pathway by lactate in muscle cells.
Our data suggests that the lactate effect is not dependent on pyruvate. Lactate is converted into pyruvate for aerobic oxidation via the citric acid cycle in the presence of oxygen4,6. The effect of pyruvic acid was tested in this study. Pyruvate didn’t exhibit the same activity as lactate in the induction of TG accumulation and MCAT activity. Pyruvate enters mitochondria through two mitochondrial pyruvate carriers (MPCs)24,25, which serves as pyruvate transporters in mitochondria. In mitochondria, pyruvate is oxidized by the pyruvate dehydrogenase complex (PDH) to form acetyl-CoA26. Our data suggest that pyruvate may not involve in the signaling activity of lactate in muscle cells.
Lactate induced the mRNA expression of lactate transporters, MCT1 and MCT4. Proton-linked monocarboxylate transporters (MCTs)27 transport lactate and other intermediates metabolites such as pyruvate and ketone bodies across the cell membranes in mouse28,29 and human cells30. MCT1 mainly involves in cellular import of lactate in skeletal muscle31. While, MCT4 is highly expressed in glycolytic tissues (e.g. white muscle) for cellular export of lactate32. Regulation of MCT expression by a variety of stimuli has been reported, especially for MCT1 and MCT4 in skeletal muscle33. Mohammed S., et al. found that MCT4 expression was induced by lactate in hypoxia conditions to export lactate from the cells34. Audrey, et al. found that lactate was transported into cells through MCT1 in white adipocytes to stimulate Ucp1 expression35. In our study, lactate induced the mRNA level of both MCT1 and MCT4 although the dynamics were different. The data suggest that lactate may enhance shuttling of lactate and other metabolites (pyruvate and ketone bodies) in cells.
In conclusion, the current study suggests that lactate may sever as a signaling molecule in muscle cells to induce intramuscular triglyceride storage and maintenance of mitochondrial function. The study provides an outline of the signaling pathway of lactate, in which lactate activates the cell surface receptor GPR81 leading to inhibition of the cAMP pathway (Fig. 8). Inhibition of the cAMP pathway represents a mechanism of the lactate activities in the up-regulation TG accumulation and maintenance of mitochondrial function. The cAMP inhibition also provides a mechanism of the lactate activities in the up-regulation of GPR81, MCAT, MCT1 and MCT4. The GPR81 response suggests a positive feedback regulation of the lactate activities. These activities of lactate are likely independent of pyruvate.
Research design and methods
Cell Culture
The mouse myoblast cell line C2C12 (CRL-1772), human embryonic kidney (HEK) 293 cells (CRL-1573) and mouse fibroblast cell line 3T3-L1 (CL-173) were purchased from the American Type Culture Collection (Manassas, VA). Cells were maintained in a high glucose DMEM (GIBCO 12800-017) supplemented with 10% fetal bovine serum (FBS) at 37 °C in a 5% CO2 incubator, and routinely examined for mycoplasma contamination. At 90-95% confluence in the 6 wells plate, C2C12 cells were differentiated into myotubes with 2% horse serum in DMEM. 3T3-L1 cells were differentiated into adipocytes using the adipogenic cocktail (10 μg/ml insulin, 0.5 mM isobutyl methylxanthine, and 10 μM dexamethasone) in 10% FBS DMEM after confluence. The cells were treated with lactate or pyruvate in serum-free and pyruvate-free DMEM (GIBCO 11960) containing 0.25% fatty acid–free bovine serum albumin (BSA).
Cell treatments
After differentiation, myotubes were serum-starved overnight in pyruvate-free medium, and then treated with sodium lactate (L7022, Sigma), forskolin (F6886, Sigma) or sodium pyruvate (P2256, Sigma-Aldrich). Sodium lactate and sodium pyruvate were prepared in H2O at a stock concentration of 1 M. Forskolin was prepared in DMSO at a stock concentration of 100 mM. The GPR81 ligand 3,5-dihydroxybenzoic acid (3,5-DHBA, D110000 Aldrich) was prepared in H2O at a stock concentration of 1 M. 293 cells and adipocytes were serum-starved overnight in 0.25% BSA medium before the treatments.
Oil Red O Staining
Intracellular lipid droplets were detected with oil-red O staining using a protocol as previously reported36. Myotubes were treated with sodium-lactate (16 mM and 20 mM) for 4 h. After incubation, cells were fixed with 10% paraformaldehyde for 30 min, and stained with a freshly-prepared working solution of oil-red O (3 mg/ml) for 15 min at the room temperature. After several times of washes, stained cells were observed under a microscope, and images were taken using a CellSens standard system (OLYPUS IX51, Tokyo, Japan).
Triglyceride assay
The total triglyceride and glycerol were determined in myotubes using the triglyceride determination kit (TR0100, Sigma) according to the manufacturer’s instruction. Briefly, the cells were harvested and sonicated in the whole lysate buffer. In the TG assay, samples was mixed with 40 μl of triglyceride reagent in the well, incubated for 15 min at 37 °C, and then measured for the light absorbance. In the glycerol assay, samples (10 μl) were added into the 160 μl of free glycerol reagent, incubated for 15 min at 37 °C, then subjected to the absorbance (OD540 nm) measurement using a microplate reader (Benchmark Plus Microplate Spectrophotometer, BIO-RAD, California, USA). The relative triglyceride and glycerol concentrations were normalized by the protein concentration. For all measurements, at least four biological replicates were used.
Protein extracts
Cells were harvested and suspended in the whole cell lysis buffer (50 mM KCl, 1% NP-40, 25 mM HEPES pH 7.8, 10 μg/ml leupeptin, 20 μg/ml aprotinin, 125 μM DTT, 1 mM PMSF, 1 mM Na3VO4). The whole cell lysate was sonicated in the lysis buffer, and centrifuged at 12000 g for 15 mins at 4 °C. The supernatant was collected and saved as the whole cell extracts. The concentration of protein was measured using the PierceTM BCA Protein Assay Kit (Prod# 23225, Life technologies, Eugene, Oregon) with BSA as a protein standard.
Western blot analysis
The cell protein extracts (50–100 μg/lane) were used in Western blotting according to a protocol described elsewhere37. Briefly, protein samples were separated in an 8% SDS-PAGE gel and transferred to the nitrocellulose membrane. After blocking in 5% nonfat milk buffer containing 0.05% Tween-20, the membrane was incubated with the primary antibody. The primary antibody to lipoylation (437695) was purchased from Calbiochem® (Darmstadt, Germany); the antibody to phospho-p44/42 MAPK (pErk1/2, Thr202/Tyr204) was purchased from the Cell Signaling Technology (#9106, Danvers, MA, USA); the antibody to pDHE2 (ab66511) and CREB (ab7540) were obtained from Abcam (Cambridge, MA, USA); the antibody to pCREB-1 (Ser 133, sc-101663), GPR81 (T-16, sc-32647), β-actin (C4, sc-47778), MCAT (H-146, sc-366137) and ERK2 (D-2, sc-1647) were obtained from the Santa Cruz Biotechnology (Dallas, TX). β-actin were used as a protein loading control. The signal was visualized in a film after exposure to chemiluminescence and analyzed for optical densities at each band using an image-processing and analysis system.
Quantitative real-time RT-PCR
Total RNA was isolated from cells using the TRIzol reagent (Invitrogen, Carlsbad, CA) according to the manufacturer’s protocol. mRNA (100 ng/μl) was reverse transcribed using the High-Capacity cDNA Reverse Transcription Kit (4368814, Life Technologies/Applied Biosystems). TaqMan Universal PCR Master Mix (4304437; Applied Biosystems, Carlsbad, CA) was used to quantify mRNA using the ABI 7900 machine. MCAT mRNA was examined using the TaqMan protocol and normalized to ribosomal 18S1 RNA (Mm04277571-s1). The primer of MCAT (Mm01299438_m1) was purchased from the Applied Biosystems. mRNA of GPR81, MCT1 and MCT4 was determined using the SYBR green qRT-PCR protocol. The primers were ordered from Sigma-Aldrich and master mix reagent was from Applied Biosystems (4309155, Applied Biosystems). The mRNA signal was normalized to GADPH. The primer sequences are listed in table 1 below. The relative gene expression of target mRNA was calculated by ΔΔCT method.
PDH activity assay
The catalytic activity of PDH was determined in myoblasts using the Pyruvate Dehydrogenase (PDH) Enzyme Activity Microplate Assay Kit (ab109902, Abcam) according to the manufacturer’s instruction. Briefly, the cell lysate was diluted to a final concentration of 2.5 μg/μl. The sample was added into each well in microplate at 200 μl and was incubated for 3 h at room temperature. The reaction then was stabilized and incubated with the assay buffer. The absorbance of each well was determined at 450 nm at room temperature for 60 min with 10s interval using a kinetic program. The slopes of the curves were generated to express the PDH activity.
Pyruvate assay
The pyruvate concentration in myotubes was determined using the Pyruvate Colorimetric/Fluorometric Assay Kit (K609-100, BioVision, Milpitas CA, USA) according to the manufacturer’s instruction. Briefly, the standard curve was made with pyruvate at 0, 2, 4, 6, 8, and 10 nM, and the correlation coefficient was 0.995 or higher. Pyruvate was extracted from cells with 4 volumes of the Pyruvate Assay Buffer. Supernatant was collected and added into the reaction mix at 10 μl/well in the colorimetric assay. The reading of absorbance (OD570 nm) was taken using a microplate reader (Benchmark Plus Microplate Spectrophotometer, BIO-RAD, California, USA). The relative pyruvate concentration was normalized by the sample’s protein concentration.
Statistical Analysis
Two-tailed, unpaired Student t test was used to calculate p value. p < 0.05 was considered significant, P < 0.01 was considered very significant. All the results were presented as mean ± SEM.
Additional Information
How to cite this article: Sun, J. et al. Induction of triglyceride accumulation and mitochondrial maintenance in muscle cells by lactate. Sci. Rep. 6, 33732; doi: 10.1038/srep33732 (2016).
References
Kamijo, Y. et al. Plasma lactate concentration and muscle blood flow during dynamic exercise with negative-pressure breathing. Journal of applied physiology 89, 2196–2205 (2000).
Hughson, R. L., Weisiger, K. H. & Swanson, G. D. Blood lactate concentration increases as a continuous function in progressive exercise. Journal of applied physiology 62, 1975–1981 (1987).
Walenta, S., Schroeder, T. & Mueller-Klieser, W. Lactate in solid malignant tumors: potential basis of a metabolic classification in clinical oncology. Current medicinal chemistry 11, 2195–2204 (2004).
Brooks, G. A. Lactate shuttles in nature. Biochemical Society transactions 30, 258–264, doi: 10.1042/ (2002).
Doherty, J. R. & Cleveland, J. L. Targeting lactate metabolism for cancer therapeutics. The Journal of clinical investigation 123, 3685–3692, doi: 10.1172/JCI69741 (2013).
Brooks, G. A. Cell-cell and intracellular lactate shuttles. The Journal of physiology 587, 5591–5600, doi: 10.1113/jphysiol.2009.178350 (2009).
Liu, C. et al. Lactate inhibits lipolysis in fat cells through activation of an orphan G-protein-coupled receptor, GPR81. The Journal of biological chemistry 284, 2811–2822, doi: 10.1074/jbc.M806409200 (2009).
Cai, T. Q. et al. Role of GPR81 in lactate-mediated reduction of adipose lipolysis. Biochemical and biophysical research communications 377, 987–991, doi: 10.1016/j.bbrc.2008.10.088 (2008).
Ahmed, K. et al. An autocrine lactate loop mediates insulin-dependent inhibition of lipolysis through GPR81. Cell metabolism 11, 311–319, doi: 10.1016/j.cmet.2010.02.012 (2010).
Langin, D. Adipose tissue lipolysis as a metabolic pathway to define pharmacological strategies against obesity and the metabolic syndrome. Pharmacological research: the official journal of the Italian Pharmacological Society 53, 482–491, doi: 10.1016/j.phrs.2006.03.009 (2006).
Hurley, B. F. et al. Muscle triglyceride utilization during exercise: effect of training. Journal of applied physiology 60, 562–567 (1986).
Hoppeler, H. et al. Endurance training in humans: aerobic capacity and structure of skeletal muscle. Journal of applied physiology 59, 320–327 (1985).
Hiltunen, J. K. et al. Mitochondrial fatty acid synthesis and respiration. Biochimica et biophysica acta 1797, 1195–1202, doi: 10.1016/j.bbabio.2010.03.006 (2010).
Zhang, L., Joshi, A. K. & Smith, S. Cloning, expression, characterization, and interaction of two components of a human mitochondrial fatty acid synthase. Malonyltransferase and acyl carrier protein. The Journal of biological chemistry 278, 40067–40074, doi: 10.1074/jbc.M306121200 (2003).
Szafranska, A. E., Hitchman, T. S., Cox, R. J., Crosby, J. & Simpson, T. J. Kinetic and mechanistic analysis of the malonyl CoA:ACP transacylase from Streptomyces coelicolor indicates a single catalytically competent serine nucleophile at the active site. Biochemistry 41, 1421–1427 (2002).
Langin, D. Adipose tissue lipolysis revisited (again!): lactate involvement in insulin antilipolytic action. Cell metabolism 11, 242–243, doi: 10.1016/j.cmet.2010.03.003 (2010).
Shen, Z. et al. Inhibition of G protein-coupled receptor 81 (GPR81) protects against ischemic brain injury. CNS neuroscience & therapeutics 21, 271–279, doi: 10.1111/cns.12362 (2015).
Smith, S. et al. Compromised mitochondrial fatty acid synthesis in transgenic mice results in defective protein lipoylation and energy disequilibrium. PloS one 7, e47196, doi: 10.1371/journal.pone.0047196 (2012).
Bonen, A. The expression of lactate transporters (MCT1 and MCT4) in heart and muscle. European journal of applied physiology 86, 6–11 (2001).
Goodpaster, B. H., He, J., Watkins, S. & Kelley, D. E. Skeletal muscle lipid content and insulin resistance: evidence for a paradox in endurance-trained athletes. The Journal of clinical endocrinology and metabolism 86, 5755–5761, doi: 10.1210/jcem.86.12.8075 (2001).
Amati, F. et al. Skeletal muscle triglycerides, diacylglycerols, and ceramides in insulin resistance: another paradox in endurance-trained athletes? Diabetes 60, 2588–2597, doi: 10.2337/db10-1221 (2011).
Sakurai, T. et al. Identification of a novel GPR81-selective agonist that suppresses lipolysis in mice without cutaneous flushing. European journal of pharmacology 727, 1–7, doi: 10.1016/j.ejphar.2014.01.029 (2014).
Yi, X. & Maeda, N. Endogenous production of lipoic acid is essential for mouse development. Molecular and cellular biology 25, 8387–8392, doi: 10.1128/MCB.25.18.8387-8392.2005 (2005).
Bricker, D. K. et al. A mitochondrial pyruvate carrier required for pyruvate uptake in yeast, Drosophila, and humans. Science 337, 96–100, doi: 10.1126/science.1218099 (2012).
Herzig, S. et al. Identification and functional expression of the mitochondrial pyruvate carrier. Science 337, 93–96, doi: 10.1126/science.1218530 (2012).
Bender, T., Pena, G. & Martinou, J. C. Regulation of mitochondrial pyruvate uptake by alternative pyruvate carrier complexes. The EMBO journal 34, 911–924, doi: 10.15252/embj.201490197 (2015).
Halestrap, A. P. The SLC16 gene family–structure, role and regulation in health and disease. Molecular aspects of medicine 34, 337–349, doi: 10.1016/j.mam.2012.05.003 (2013).
Hajduch, E., Heyes, R. R., Watt, P. W. & Hundal, H. S. Lactate transport in rat adipocytes: identification of monocarboxylate transporter 1 (MCT1) and its modulation during streptozotocin-induced diabetes. FEBS letters 479, 89–92 (2000).
Iwanaga, T., Kuchiiwa, T. & Saito, M. Histochemical demonstration of monocarboxylate transporters in mouse brown adipose tissue. Biomedical research 30, 217–225 (2009).
Perez de Heredia, F., Wood, I. S. & Trayhurn, P. Hypoxia stimulates lactate release and modulates monocarboxylate transporter (MCT1, MCT2, and MCT4) expression in human adipocytes. Pflugers Archiv: European journal of physiology 459, 509–518, doi: 10.1007/s00424-009-0750-3 (2010).
De Matteis, R. et al. Exercise as a new physiological stimulus for brown adipose tissue activity. Nutrition, metabolism, and cardiovascular diseases: NMCD 23, 582–590, doi: 10.1016/j.numecd.2012.01.013 (2013).
Halestrap, A. P., Wilson, M. C. et al. Lactic acid efflux from white skeletal muscle is catalyzed by the monocarboxylate transporter isoform MCT3. The Journal of biological chemistry 273, 15920–15926 (1998).
Coles, L., Litt, J., Hatta, H. & Bonen, A. Exercise rapidly increases expression of the monocarboxylate transporters MCT1 and MCT4 in rat muscle. The Journal of physiology 561, 253–261, doi: 10.1113/jphysiol.2004.073478 (2004).
Ullah, M. S., Davies, A. J. & Halestrap, A. P. The plasma membrane lactate transporter MCT4, but not MCT1, is up-regulated by hypoxia through a HIF-1alpha-dependent mechanism. The Journal of biological chemistry 281, 9030–9037, doi: 10.1074/jbc.M511397200 (2006).
Carriere, A. et al. Browning of white adipose cells by intermediate metabolites: an adaptive mechanism to alleviate redox pressure. Diabetes 63, 3253–3265, doi: 10.2337/db13-1885 (2014).
Hwang, K. A., Hwang, Y. J., Kim, G. R. & Choe, J. S. Extracts from Aralia elata (Miq) Seem alleviate hepatosteatosis via improving hepatic insulin sensitivity. BMC complementary and alternative medicine 15, 347, doi: 10.1186/s12906-015-0871-5 (2015).
Ke, B. et al. Inactivation of NF-kappaB p65 (RelA) in Liver Improves Insulin Sensitivity and Inhibits cAMP/PKA Pathway. Diabetes 64, 3355–3362, doi: 10.2337/db15-0242 (2015).
Acknowledgements
Jianping Ye is the guarantor(s) in this manuscript. This study was supported by NIH grant (DK068036 and R56 DK068036-10A1) to YJ. The qRT-PCR test was conducted in the genetic core facility supported by the NIH Grants (P30DK072476 and P20-RR021945)
Author information
Authors and Affiliations
Contributions
J.S. and X.Y. conducted the experiments and analyzed the data. M.X. and J.Y. designed the study and prepared the manuscript. All authors read and approved the final version of manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Sun, J., Ye, X., Xie, M. et al. Induction of triglyceride accumulation and mitochondrial maintenance in muscle cells by lactate. Sci Rep 6, 33732 (2016). https://doi.org/10.1038/srep33732
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep33732
This article is cited by
-
The different effects of intramuscularly-injected lactate on white and brown adipose tissue in vivo
Molecular Biology Reports (2022)
-
Lactate regulates autophagy through ROS-mediated activation of ERK1/2/m-TOR/p-70S6K pathway in skeletal muscle
Journal of Cell Communication and Signaling (2021)
-
Utilization of lactic acid in human myotubes and interplay with glucose and fatty acid metabolism
Scientific Reports (2018)
-
Citral, a Monoterpene Inhibits Adipogenesis Through Modulation of Adipogenic Transcription Factors in 3T3-L1 Cells
Indian Journal of Clinical Biochemistry (2018)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.