Abstract
This retrospective study used a population-based national registry to determine the impact of local treatment modalities on survival in patients with metastatic esophageal cancer (EC). The Surveillance Epidemiology and End Results (SEER) database was used to identify patients with metastatic EC from 1988 to 2012. A total of 9,125 patients were identified. There were 426 patients underwent primary surgery, 4,786 patients were administered radiotherapy (RT) alone, 847 patients underwent surgery plus RT and 3,066 patients without any local treatment. Multivariate analysis results indicated that year of diagnosis, age, race, histologic subtype, grade and local treatment modalities were independent prognostic factors for overall survival (OS). The 5-year OS were 8.4%, 4.5%, 17.5% and 3.4% in primary surgery, RT only, surgery plus RT and no local treatment, respectively (P < 0.001). Subgroup analyses showed that the impact of RT was mainly reflected by preoperative radiotherapy, as patients received preoperative radiotherapy had significantly better OS than patients who underwent primary surgery alone and postoperative RT, the 5-year OS rates were 24.7%, 6.5% and 7.8%, respectively, respectively (P < 0.001). Surgery plus RT, especially preoperative RT, may improve long-term survival of patients with metastatic EC.
Similar content being viewed by others
Introduction
Esophageal cancer (EC) is a highly lethal malignancy and the incidence is increasing1. In 2015, it has been estimated that there would be 16,980 new EC cases and 15,590 deaths in the United States2. Approximately 50% of patients had metastases to distant lymph nodes or organs at the initial diagnosis3,4. The prognosis of metastatic EC is poor and the 5-year survival rate is lower than 5%5. The palliative treatment in metastatic EC depends mainly on patients’ clinical situation with the goal of reducing cancer-related symptoms and extending survival without compromising quality of life. Systemic treatment consists of chemotherapy, targeted therapy and best supportive care. Local treatment mainly includes feeding tubes, beam radiation, brachytherapy and endoscopic management techniques such as dilation and stenting6,7.
Preoperative chemoradiotherapy may significantly increase the radical resection rate and improve survival for advanced esophageal carcinoma8, but the value of surgery plus radiotherapy (RT) for metastatic EC has not yet been clarified. RT is not a first-line treatment for metastatic EC, but RT may improve the patients’ symptoms of obstruction9. Studies with small sample sizes have shown that local treatments including surgery could prolong survival in metastatic EC10,11,12,13. Studies have shown that surgery and/or radiotherapy can improve survival in patients with stage IV malignant tumors14,15. In this study, we analyzed the metastatic EC using a population-based national registry (Surveillance Epidemiology and End Results, SEER) to determine the impact of local treatment strategies on survival in metastatic EC.
Results
Patient characteristics and treatment
The SEER database included a total of 63,759 patients with EC in 1988–2012 and 31.6% (20,168 patients) had a distant stage; 9,125 patients met the inclusion criteria of this study (Fig. 1). The patient characteristics are shown in Table 1. The median age of initial diagnosis was 64 years (range, 21–96); 83.5% (7,621/9,125) were white; 82.0% (7,486/9,125) were male; 59.2% (5,406/9,125) had adenocarcinoma; and 76.7% (6,995/9,125) had a lower thoracic esophageal cancer. Local treatment modalities were as follows: 426 (4.7%) patients underwent primary cancer-directed surgery (CDS); 4,786 (52.4%) were primary RT alone; 847 (9.3%) underwent CDS plus RT; and 3,066 (33.6%) were not administered any local treatment. Among patients who underwent CDS plus RT, 57.3% (485/847) were administered preoperative radiotherapy, while 38.3% (324/847) were received postoperative radiotherapy and 4.5% (38/847) were underwent both preoperative and postoperative RT.
Survival
The median follow-up time for all patients was 9 months (range, 4–261 months) with a median survival time was 10 months. The 1 year, 2 years, 3 years, 5 years and 10 years OS rates were 40.5%, 14.6%, 8.4%, 5.4% and 3.5%, respectively (Fig. 2).
Prognostic factors analysis
Univariate analysis showed that year of diagnosis, age, race, tumor histology, grade and local treatment modalities were risk factors for OS (Table 2).
Multivariate analysis indicated that year of diagnosis, age at diagnosis, race, tumor histology, grade and local treatment modalities were independent prognostic factors for OS. Patients who underwent primary CDS was significantly better OS than that of patients who were primary RT alone (HR, 1.440; 95% CI, 1.287–1.611; P < 0.001) and who were not received any local treatment (HR, 1.602; 95% CI, 1.427–1.799; P < 0.001). Surgery combined with RT could further improve survival (HR, 0.793; 95% CI, 0.693–0.908; P = 0.001) (Table 3).
Survival after local treatment
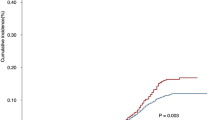
The 5-year OS rates were 8.4%, 4.5%, 17.5% and 3.4% for primary CDS, RT alone, CDS plus RT and no local treatment, respectively, with a median survival time of 11.0, 9.0, 15.0 and 9.0 months, respectively (P < 0.001) (Fig. 3). Patients who were received preoperative RT was significantly better OS than that of patients who underwent primary CDS and CDS plus postoperative RT, with 5-year OS rates of 24.7%, 6.5% and 7.8%, respectively and a median survival time of 20.0, 11.0 and 12.0 months, respectively (P < 0.001) (Fig. 4).
We determined the effect of local treatment modalities on OS by year of diagnosis. It was significantly associated with OS from 1988 to 1999 (log-rank test P < 0.001) (Figure S1A) and from 2000 to 2012 (P < 0.001) (Figure S1B). However, the survival benefit was significantly better from 2000 to 2012 for those treated with CDS plus RT.
The prognostic effect of local treatment modalities was examined based on different ethnicities. In black patients, CDS with or without RT improved OS than that of patients who underwent primary RT alone or did not have any local treatment (P < 0.001) (Figure S2A). In white patients, OS was better for patients who underwent CDS plus RT, as compared with other local treatment modalities (P < 0.001) (Figure S2B).
The effect of local treatment modalities on OS was examined based on sex. In patients who underwent CDS plus RT, OS was significantly better than that of patients who were received other local treatment modalities (P < 0.001 for male patients; P < 0.001 for female patients) (Figure S3A,S3B). The results were also significant difference in patients who were aged ≤60 years (P < 0.001) and aged >60 years (P < 0.001) (Figure S4A,S4B).
In addition, CDS plus RT provided the OS benefit in patients with squamous cell carcinoma (P < 0.001) (Figure S5A), adenocarcinoma (P < 0.001) (Figure S5B), grade I-II (P < 0.001) (Figure S6A) and grade III-IV P < 0.001) metastatic EC patients. (Figure S6B).
The OS rates were compared based on tumor location for patients who were underwent different local treatment modalities (Figure S7A–S7C). The prognostic effect of local treatment modalities was also found in patients with tumors located in the middle thoracic esophagus (P < 0.001) and lower thoracic esophagus (P < 0.001). However, in patients with tumors located in the upper thoracic esophagus, local treatment modalities were not associated with OS (P = 0.272).
Discussion
Given the limited of studies with small sample sizes investigating the effect of local treatment in metastatic EC10,11,12,13. In this study, we explored the prognostic value of local treatment modalities including CDS and RT in metastatic EC based on 9,125 metastatic EC patients in the SEER database and our results found that surgery plus RT could significantly improve survival in metastatic EC.
Systematic therapy is still the first-line treatment for metastatic EC. The main purpose of local treatment lies in effective control of dysphagia, pain, bleeding and other symptoms. The potential value of surgery and RT in metastatic EC remains controversial. In a study by Schauer et al., 19 patients with stage IV Barrett’s adenocarcinoma received multimodality therapy including resection of the primary tumor. No significant difference was found in postoperative morbidity and mortality between metastatic EC and locally advanced EC, but the median survival was only 9 months16. Tanaka et al. also found that surgery did not improve survival in stage IVB EC with distant organ metastasis (P = 0.1291)5. Wang et al. included 96 patients with stage IV EC who were received palliative chemotherapy and concurrent chemoradiotherapy (CRT), of which 14 patients underwent surgery after neoadjuvant therapy and surgery had significantly better survival than those who did not11. Two related studies also showed that long-term survival could be achieved after resection of the primary tumor and metastases of stage IV EC12,13. In our study, 1,273 patients received surgery with or without RT and surgery combined with RT could significantly improve survival. Thus, multimodality therapy including surgery and RT has the potential to prolong survival in metastatic EC.
Multimodality therapy is the dominant research direction in metastatic EC. Our subgroup analysis showed that in 2000–2012, patients who underwent surgery plus RT obtained a significantly better survival than patients in 1988–1999. Although the SEER data could not reflect specific conditions in patients regarding chemotherapy and targeted therapy, we speculated that it was closely correlated with of the effect of systemic treatment in metastatic EC17,18,19,20. Systemic therapy is the primary treatment of metastatic EC, but local treatment including surgery or RT after effective systemic therapy could further reduce the tumor burden. Therefore, we recommend for future prospective studies to investigate the effect of local treatment in metastatic EC.
Our study showed that patients with upper thoracic esophageal cancer did not benefit from local treatment, which might be related to greater difficulties in surgical treatment in upper thoracic esophageal cancer than middle and lower thoracic esophageal cancer. We could not clarify the effect of surgical treatment in upper thoracic metastatic EC, as only 30 patients underwent surgery with or without RT in this study.
In this study, the 5-year OS for preoperative RT plus CDS could reach 24.7%, while no significant difference in survival was seen for primary CDS and CDS plus postoperative RT (5-year OS, 6.5% and 7.8%, respectively), indicating that preoperative neoadjuvant therapy has a greater value in metastatic EC. Our study found that the OS improvement for surgery plus RT was mainly reflected by preoperative RT which could provide the best chance for the complete resection of primary tumors.
There are several limitations in our study. First, inherent biases exist in any retrospective study. Second, due to the limitations of SEER data, we could not obtain related information including chemotherapy, indications for surgery and RT and range of non-regional lymph node metastases and distant metastases. In addition, patients with distant SEER stage were intended to approximate stage IV in the TNM staging system and our results also promoted that OS of distant stage in SEER was substantially similar to that of stage IV esophageal carcinoma. Several different extent of disease schemes have been used in the SEER database. Therefore, a potential difference in the two staging systems should be considered. However, the primary strength of this study was the ability to assess the epidemiology, prognostic factors and local treatment modalities in metastatic EC using a SEER registry. Although retrospective reviews are generally considered inferior to prospective studies, no prospective study design has been performed to assess the clinical value of local treatment in metastatic EC.
In conclusion, surgery plus RT, especially preoperative RT, may improve long-term survival of patients with metastatic EC. A prospective study on metastatic EC should be conducted to investigate the effect of local treatment in metastatic EC. Our findings may play an important role in local treatment considerations in metastatic EC if further confirmed in studies with larger sample sizes.
Methods and Materials
Patients
Data were obtained from the current SEER database to identify patients with EC diagnosed in 1988–2012. We obtained permission to access research data files with the reference number 11252-Nov 201421. Patients included in this study had the following criteria: 1) metastatic thoracic esophageal cancer with a known tumor location; and 2) local treatment modalities including cancer-directed surgery (CDS), beam radiotherapy, CDS plus RT, or no local treatment. Metastatic disease was defined as having a distant stage at diagnosis according to the SEER historic stage. Distant stage was defined as a neoplasm that had spread to parts of the body remote from the primary tumor through direct extension, discontinuous metastasis (e.g., implantation or seeding) to distant organs and tissues, or the lymphatic system to distant lymph nodes21. Patients were excluded from the analysis if they had an estimated survival of ≤3 months after diagnosis. SEER data did not require informed consent and this study was approved by the ethics committee of the Sun Yat-sen University Cancer Center.
Clinicopathological and treatment factors
The following clinicopathological and treatment factors were collected from the SEER database: year of diagnosis, age at diagnosis, race, tumor histology, tumor location, grade and local treatment modalities. Vital status including cause of death and duration of follow-up was recorded.
Statistical analysis
The χ2 and Fisher’s exact probability tests were used to analyze differences between qualitative data. Univariate and multivariate Cox regression analyses were generated to analyze risk factors for overall survival (OS). Multivariable analyses were performed for factors that were significantly associated with OS in univariate analyses. Survival rates were calculated and plotted using the Kaplan–Meier method and compared using the log-rank test. All data were analyzed using the SPSS statistical software package, version 21.0 (IBM Corporation, Armonk, NY, USA). A P value of <0.05 was considered significant.
Additional Information
How to cite this article: Wu, S.-G. et al. Surgery Combined with Radiotherapy Improved Survival in Metastatic Esophageal Cancer in a Surveillance Epidemiology and End Results Population-based Study. Sci. Rep. 6, 28280; doi: 10.1038/srep28280 (2016).
References
Rustgi, A. K. & El-Serag, H. B. Esophageal carcinoma. N Engl J Med 371, 2499–509 (2014).
Siegel, R. L., Miller, K. D. & Jemal, A. Cancer statistics, 2015. CA Cancer J Clin 65, 5–29 (2015).
Enzinger, P. C. & Mayer, R. J. Esophageal cancer. N Engl J Med 349:2241–2252 (2003).
Horner, M. J. et al. SEER Cancer Statistics Review, 1975-2006, National Cancer Institute. Bethesda, MD, http://seer.cancer.gov/csr/1975_2006/, based on November 2008 SEER data submission, posted to the SEER web site, 2009. (2009) (Date of access: 02/11/2015).
Tanaka, T. et al. Outcomes of multimodality therapy for stage IVB esophageal cancer with distant organ metastasis (M1-Org). Dis Esophagus 23, 646–651 (2010).
Berry, M. F. Esophageal cancer: staging system and guidelines for staging and treatment. J Thorac Dis 6, S289–297 (2014).
Stah, M. et al. Oesophageal cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 24, vi51–56 (2013).
Shapiro, J. et al. Neoadjuvant chemoradiotherapy plus surgery versus surgery alone for oesophageal or junctional cancer(CROSS): long-term results of a randomised controlled trial. Lancet Oncol 16, 1090–1098 (2015).
Nieman, D. R. & Peters, J. H. Treatment strategies for esophageal cancer. Gastroenterol Clin North Am 42, 187–197 (2013).
Yamashita, K. et al. Multimodality Treatment and Prognosis in Esophageal Squamous Cell Carcinoma Requiring Esophagectomy. Hepatogastroenterology 61, 1042–1048 (2014).
Wang, J. et al. Factors Predictive of Improved Outcomes With Multimodality Local Therapy After Palliative Chemotherapy forStage IV Esophageal Cancer. Am J Clin Oncol. 39, 228–235 (2016).
Chao, Y. K. et al. Distant nodal metastases from intrathoracic esophageal squamous cell carcinoma: characteristics of long-term survivors after chemoradiotherapy. J Surg Oncol 102, 158–162 (2010).
Blank, S. et al. A reliable risk score for stage IV esophagogastric cancer. Eur J Surg Oncol 39, 823–830 (2013).
Ouyang, W. W. et al. Radiation dose and survival of patients with stage IV non-small cell lung cancer undergoing concurrentchemotherapy and thoracic three-dimensional radiotherapy: reanalysis of the findings of a single-centerprospective study. BMC Cancer 14, 491 (2014).
Khan, H., Khan, N., Ahmad, A., Olszewski, A. J. & Somasundar, P. Surgical management of metastatic colon cancer: A population-based analysis. J Geriatr Oncol 6, 446–453 (2015).
Schauer, M. et al. Results of a multimodal therapy in patients with stage IV Barrett’s adenocarcinoma. World J Surg 32, 2655–2660 (2008).
Anderson, S. E. et al. Combined modality therapy in esophageal cancer: the Memorial experience. Semin Surg Oncol 21, 228–232 (2003).
Dutton, S. J. et al. Gefitinib for oesophageal cancer progressing after chemotherapy (COG): a phase 3, multicentre, double-blind, placebo-controlled randomised trial. Lancet Oncol 15, 894–904 (2014).
Ford, H. E. et al. Docetaxel versus active symptom control for refractory oesophagogastric adenocarcinoma (COUGAR-02): an open-label, phase 3 randomised controlled trial. Lancet Oncol 15, 78–86 (2014).
Bang, Y. J. et al. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positiveadvanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlledtrial. Lancet 376, 687–697 (2010).
Surveillance, Epidemiology and End Results (SEER) Program (www.seer.cancer.gov) SEER*Stat Database: Incidence-SEER 18 Regs Research Data+Hurricane Katrina Impacted Louisiana Cases, Nov 2014 Sub (1973-2012 varying)-Linked To County Attributes-Total U.S., 1969-2013 Counties, National Cancer Institute, DCCPS, Surveillance Research Program, Surveillance Systems Branch, released April 2015, based on the November 2014 submission. (2015) (Date of access: 30/05/2015).
Acknowledgements
This work was supported by grants from the National Natural Science Foundation of China (No. 81402527), the Sci-Tech Office of Guangdong Province (No. 2013B021800157, 2013B021800458) and the Youth Foundation of Fujian Provincial Health and Family Planning Commission (No. 2014-2-63).
Author information
Authors and Affiliations
Contributions
S.-G.W., W.-H.X., Z.-Q.Z., Y.-B. and Z.-Y.H. was involved in study concept and design, drafting of the manuscript, obtained funding and study supervision. S.-G.W. and W.-H.X. was involved in acquisition of data. Z.-Q.Z., J.-Y.S. and H.-X.L. were involved in statistical analysis. S.-G.W., W.-H.X., F.-Y.L., Z.-Q.Z. and Z.-Y.H. were involved in interpretation of data revision of manuscript. All authors were involved in the interpretation of the data; revision of the manuscript; and the decision to submit the manuscript for publication.
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Electronic supplementary material
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Wu, SG., Xie, WH., Zhang, ZQ. et al. Surgery Combined with Radiotherapy Improved Survival in Metastatic Esophageal Cancer in a Surveillance Epidemiology and End Results Population-based Study. Sci Rep 6, 28280 (2016). https://doi.org/10.1038/srep28280
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep28280
This article is cited by
-
Metastatic Esophageal Carcinoma: Prognostic Factors and Survival
Journal of Gastrointestinal Cancer (2022)
-
Cost Effectiveness of Adding Pembrolizumab to Platinum and Fluoropyrimidine-Based Chemotherapy as First-Line Treatment for Advanced Esophageal Cancer: A US Healthcare Payer’s Perspective
PharmacoEconomics (2022)
-
Short-course compared to long-course palliative radiotherapy for oesophageal cancer: a single centre observational cohort study
Radiation Oncology (2021)
-
Profile of esophageal squamous cell carcinoma mutations in Brazilian patients
Scientific Reports (2021)
-
Local definitive intensity-modulated radiation therapy recommended for patients initially diagnosed with nasopharyngeal carcinoma with distant metastasis after an effective systemic chemotherapy*
Oncology and Translational Medicine (2018)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.