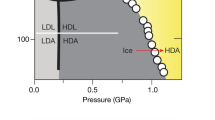
Abstract
Regelation, i.e., ice melts under compression and freezes again when the pressure is relieved, remains puzzling since its discovery in 1850’s by Faraday. Here we show that hydrogen bond (O:H-O) cooperativity and its extraordinary recoverability resolve this anomaly. The H-O bond and the O:H nonbond possesses each a specific heat ηx(T/ΘDx) whose Debye temperature ΘDx is proportional to its characteristic phonon frequency ωx according to Einstein’s relationship. A superposition of the ηx(T/ΘDx) curves for the H-O bond (x = H, ωH ~ 3200 cm−1) and the O:H nonbond (x = L, ωL ~ 200 cm−1, ΘDL = 198 K) yields two intersecting temperatures that define the liquid/quasisolid/solid phase boundaries. Compression shortens the O:H nonbond and stiffens its phonon but does the opposite to the H-O bond through O-O Coulomb repulsion, which closes up the intersection temperatures and hence depress the melting temperature of quasisolid ice. Reproduction of the Tm(P) profile clarifies that the H-O bond energy EH determines the Tm with derivative of EH = 3.97 eV for bulk water and ice. Oxygen atom always finds bonding partners to retain its sp3-orbital hybridization once the O:H breaks, which ensures O:H-O bond recoverability to its original state once the pressure is relieved.
Similar content being viewed by others
Introduction
Discovered by Faraday, James Thomson and his brother William Thomson (Later Lord Kevin) in 1850’s1,2, regelation is the phenomenon of ice melting under pressure and freezing again when the pressure is relieved at temperatures around −10 °C. In his paper Faraday1 noted that ‘two pieces of thawing ice, if put together, adhere and become one; at a place where liquefaction was proceeding, congelation suddenly occurs. The effect will take place in air, in water, or in vacuo. It will occur at every point where the two pieces of ice touch; but not with ice below the freezing-point, i.e., with dry ice, or ice so cold as to be everywhere in the solid state’. Faraday suggested that there may be a thin liquid-like layer of nascent ice on the surface, ready to be converted to solid on contact with another layer. James Thomson2 explained this observation in terms of pressure melting based on equilibrium thermodynamics available in his day and it was his brother, William, who verified the result experimentally3. This led to a dispute with Faraday, who observed that blocks of ice would stick together by freezing under mild pressure merged in 0 oC water. (which one observes with ice cubes in a basket in modern refrigerators). There is a body of modern literature suggesting that Faraday’s surmise of an anomalous ice layer may be correct but it is not actually true.
The Regelation can easily be demonstrated by looping a wire around a block of ice with a heavy weight attached to it. This loaded wire melts the local ice gradually until the wire passing through the entire block. The wire’s track will refill as soon as it passes, so the ice block will remain solid even after wire passes completely through. Another example is that a glacier can exert a sufficient amount of pressure on its lower surface to lower the melting point of its ice, allowing liquid water flow from the base of a glacier to lower elevations when the temperature of the air is above the freezing point of water (258K). The regelation is exceedingly interesting, because of its relation to glacial action under nature circumstances4, in its bearing upon molecular action5 and self-repairing of damaged living cells.
It is usual in ‘normal’ materials that compression raises the critical temperature (TC) at all phase transitions6,7,8; however, according to the phase diagram of water and ice, the freezing temperature of liquid water is lowered to −22 °C by applying 210 MPa pressure; stretching ice (i.e. tensile, or negative, pressure) has the opposite effect - ice melts at +6.5 °C when subjected to −95 MPa pressure9. Conversely, the TC for ice drops from 280 to 150 K at the transition from ordered ice-VIII to proton-disordered ice-VII phase when P is increased from 1 to 50 GPa10,11,12. A molecular-dynamics (MD) study of a nanowire cutting through ice suggests that the transition mode and the cutting rate depend on the wetting properties of the wire - hydrophobic and thicker wires cut ice faster13.
However, a consistent understanding with numerical reproduction of regelation has yet been achieved despite intensive investigations. It might be true that regelation can occur for substances with the property of expanding upon freezing, but mechanisms for neither freezing expansion nor regelation is clear14. These issues are beyond the scope of classical thermodynamics in terms of equation of states, which inspires alternative ways of thinking and approaching to unlocking these puzzles.
Recent progress14,15,16,17,18,19 enables us to tackle this mystery from the perspective of hydrogen bond (O:H-O) cooperative relaxation under compression. We show in this presentation that the O:H-O bond has extreme recoverability of distortion and dissociation. Numerical reproduction of the pressure dependent melting temperature (Tm) of ice revealed that O:H-O bond relaxation disperses the critical temperatures for solid/quasisolid (gel-like form existas between 258 and 273 K for bulk; traditionally known as liquid-solid transition) phase transition.
Principle: Hydrogen bond cooperative relaxation
General bond potential
Figure 1a shows a pairing potential u(r) for the interatomic bonding. The coordinates (d, Eb) at equilibrium are the bond length and bond energy. We are concerned how the d and Eb respond to external stimulus regardless of the shape of the particular u(r). A Taylor series approximates the pairing potential u(r) as follows:
(a) The long-range, mono-well potential for paring atoms in a ‘normal’ substance and (b) the asymmetrical, short-range, double-well potentials for the O:H-O bond and their relaxation dynamics40,41. Compression stores energy by shortening and stiffening the bond whereas tension does the opposite, along an f(P) path in (a). O:H-O potentials include the O:H nonbond van der Waals like (vdW-like) interaction (EL ~ 0.1 eV, left-handed side), the H-O exchange interaction (EH ~ 4.0 eV, right-handed side) and the Coulomb repulsion (C-repulsion) between electron pairs (paring green dots) on oxygen ions. A combination of these interactions with external stimulus dislocates O atoms in the same direction by different amounts. The relaxation proceeds along the potential paths with respect to the H atom (in grey) coordination origin under compression (linked blue spheres-equivalent of cold) or tension (linked red spheres further moves left-equivalent of hot). Springs are analogous the respective interactions. The dH0 and dL0 in (b) are the respective segmental length references at 4 °C.

The zeroth differential is the bond energy at equilibrium Eb, which can be determined from photoelectron spectrometrics15. Higher-order differentials corresponding to the harmonic and nonlinear vibrations determine the shape of the u(r). The vibration amplitude x is 3% or less than atomic distance d of the substance below melting.
Generally, external stimuli, such as stressing and heating modulate the length d(T, P) and energy E(T, P) of the representative bond along a path denoted f(T, P)6. For instance, compression stores energy into a substance by shortening and stiffening all bonds with possible plastic deformation while tension does the opposite, as illustrated in Fig. 1a and formulated as follows15:

where T0 and P0 are the ambient referential conditions. The α(t) is the thermal expansion coefficient.  is the compressibility (p < 0, compressive stress) or extensibility (p > 0 tensile stress). The v is the volume of a bond (cross sectional area times length). The η(t) is the specific heat of the representative bond in Debye approximation. The integration of the η(t) from 0 K to the melting point (Tm) approximates the bond energy by omitting experimental conditions as the η(t) for constant volume deviates only 3% from that of constant pressure15.
is the compressibility (p < 0, compressive stress) or extensibility (p > 0 tensile stress). The v is the volume of a bond (cross sectional area times length). The η(t) is the specific heat of the representative bond in Debye approximation. The integration of the η(t) from 0 K to the melting point (Tm) approximates the bond energy by omitting experimental conditions as the η(t) for constant volume deviates only 3% from that of constant pressure15.
O:H-O bond asymmetric and short-range potentials
An extended tetrahedron containing two water molecules and four identical O:H-O bonds has unified the length scale and mass density of molecular packed tetrahedrally in water ice on statistical average20. This extension has also turned out the O:H-O bond with asymmetric, short-range O:H, H-O and O---O interactions, see Fig. 1b21. The O:H-O bond is segmented into a shorter H-O polar-covalent bond with a stronger exchange interaction uH(r) and a longer O:H nonbond with a weaker nonbond interaction uL(r). The two segments are coupled by Coulomb repulsion between electron pairs on adjacent oxygen atoms uC(r)18,22. All interactions are limited to the specific segment without any decay acrossing the respective region. The O:H-O bond links the O---O in both the solid and liquid H2O phase, regardless of phase structures or topologic configurations20.
The O:H-O bond performs as an asymmetrical oscillator pair. Under the O---O Coulomb coupling, external excitation such as cooling14, compressing18, salting23 and clustering22 always relaxes the O:H and H-O in the same direction but by different amounts. Because of the strength disparity between the two segments, compression shortens and stiffens the O:H nonbond (left hand side of the O:H-O bond) and simultaneously lengthens and softens the H-O bond (right hand side). The COlomb repulsion makes the O:H-O bond recover completely its initial states once the compression is relieved. Conversely, once the O:H nonbond breaks, oxygen atom finds immediately bonding partner to retain its sp3-orbital hybridization that occurs at 5 K24 temperature and above even in gaseous phase25.
With the aid of quantum calculations, Lagrangian oscillating mechanics and Fourier fluid thermo dynamics and phonon spectrometrics, we have been able to consistently and quantitatively resolve quantitatively a few issues such as: 1) Mpemba effect – hot water freezes quicker than its cold16, 2) supersolid skins for the slipperiness of ice and the hydrophobic and tough skin of water liquid26, 3) ice expansion and mass density oscillation over full temperatures range14, 4) anomalies of water molecules with fewer than four nearest neighbors in clusters and droplets22, 5) Hofmeister effect – NaCl mediation of O-O repulsion 23, 6) density-geometry-dimension correlation of molecules packed in water and ice20, 7) low compressibility and proton centralization of ice18, and, 7) mapping the local potential paths for the O:H-O bond relaxing with stimulus21, etc. Progress made insofar has formed the subject of a recent treatise17.
Results and Discussion
O:H-O bond extraordinary recoverability
Figure 2a shows that a molecular dynamics (MD) decomposition of the measured V-P profile of Ice-VIII at 80 K27 truns out that the dx asymmetric relaxation proceeds until proton symmetrization occuring at 0.22 nm and 60 GPa. The subscript x = H and L reresnet for the H-O and the O:H, respectively. The dL shortens monotonically by 4.3% from 0.1768 to 0.1692 nm and the dH lengthens by 2.8% from 0.0975 to 0.1003 nm when the pressure is increased from 0 to 20 GPa18. The dL equals the dH at 0.11 nm and 60 GPa, towards proton centrolization in the O:H-O bond28,29,30. Figure 2b shows the ωx cooperative shift of ice under compression at 80 K. Phonon frequencies relax monotonically up to 60 GPa even though the pressure is increased30,31. In accordance to the length relxation, compression shifts the ωH toward higher frequencies and the ωL to lower. The length and stiffness trend of O:H-O bond relxation hold for all phases of water and ice with negligible slope variation17.
Pressure induced O:H-O bond relaxation in the (a) segmental length dx, (b) phonon frequencies ωx30,31 and (c) potential paths ux(r) for the O:H-O bond relaxing with pressure (l. to r.: P = 0, 5, 10, 15, 20, 30, 40, 50, 60 GPa)21; blue dots correspond to OH-O bond without Coulomb repulsion being involved.
The dx curves in (a) meet at the point of proton centralization occurring in phase X at 59 GPa and 0.22 nm29,30. The O:H nonbond and H-O bond responses to compression oppositely (see inset a).
A Lagrangian-Laplace transformation of the measured dx and ωx turns out the force constant kx and segmental energy Ex, which maps the potential paths of the O:H-O bond under compression21. As shown in Table 1, compression increase the EL from 0.046 to 0.250 eV up to 40 GPa and then decrease to 0.16 eV at 60 GPa; the EH decreases monotonically from 3.97 eV to 1.16 eV at 60 GPa. Different from situation of ‘normal’ substance, compression lowers the total energy of the O:H-O bond rather than raise it. The O:H-O bond will fully recover its initial states once the compression is relieved without any plastic deformation.
As expected, compression shortens the dL, increases the ωL and EL of the O:H nonbond; the H-O bond responses oppositely to compression, resulting in dH elongation, ωH and bond energy EH reduction, which can be formulated in the reduced forms as follows (Ex valids at P < 30 GPa; dx/dx0 = 1 + βx1P + βx2P2 for instance):

EH dictating the Tm
The following proves that EH dictates the Tm for melting, Tm ∝ EH. According to eq (2), The Tm changes in the following relationship but x = L or H is yet to be known6,

Eq (3) defines the slope of dx(P):

Generally, pressure raises the Tm but ice responses to pressure in the opposite – Tm drops when the pressure is increased. Reproduction of the measured P-dependent Tm for melting (Fig. 3a)32 requires that the integral in eq (4) must be positive. Only the dH in Eq. (3) meets this criterion (βx1 > 0 and βx2 > 0). Therefore, the H-O bond EH dominates the Tm.
(a) Theoretical reproduction of the measured Tm(P) (−22 °C at 210 MPa; + 6.5 °C at −95 MPa)32 profiles confirms that the EH dictates the Tm for ice melting with derivative of EH = 3.97 eV for bulk water18. (b) The superposition of the ηx(T) curves yields two crossing temperatures that defines the solid/quasisolid/liquid phase boundaries. The high temperature boundary corresponds to quasisolid melting and the lower to freezing. Compression/tension (ΔP > 0)/(ΔP < 0) disperses the boundaries simultaneously and reversely by modulating the ΘDx ∝ ωDx and Ex ∝  , depressing/elevating the Tm.
, depressing/elevating the Tm.
Furthermore, matching the Tm(P) profile using Eq. (5) yields an EH value of 3.97 eV at 0.1 MPa(1 atm pressure) by taking the H atomic diameter of 0.106 nm as the diameter of the H-O bond33. This EH value agrees with the energy of 4.66 eV for dissociating the H-O bond of water molecules deposited on a TiO2 substrate with less than a monolayer coverage and 5.10 eV for dissociating water monomers in the gaseous phase34. Molecular undercoordination shortens the H-O bond and raises its cohesive energy from the bulk value of 3.97 to 4.66 and to 5.10 eV when the O:H-O bond is subject to molecular undercoordination23.
Clearly, the relaxation of the H-O bond mediates the Tm, while EL is largely irrelevant. It is not surprising, therefore, that compression softens the H-O bond and hence lowers the Tm, while negative (tensile) pressure does the opposite by shortening and stiffening the H-O bond32 and hence negative pressure elevates the Tm.
Tm(EH) and TV(EL) paradox: phase-boundary dispersivity
It is known that evaporating one H2O molecule from bulk water requires energy of 4EL = 0.38 eV35 to break four O:H nonbonds surrounding the molecule. This happens at the ambient pressure and TV = 373 K temperature. Question may arise why the EH instead of the EL dominates the Tm though the TV is higher than the Tm?
In order to clarify this paradox, let us look at the specific heat of water14. Generally, the specific heat of a ‘normal’ substance is regarded as a macroscopic quantity integrated over all bonds of the specimen, which is also the amount of energy required to raise the temperature of the substance by 1 K degree. However, in dealing with the representative for all bonds of the entire specimen, it is necessary to consider the specific heat per bond that is obtained by dividing the bulk specific heat by the total number of bonds6. For a specimen of other usual materials, one bond represents all on average; therefore the thermal response is the same for all the bonds, without any discrimination among all bonds in cooling contraction and thermal expansion36.
For water ice, however, the representative O:H-O bond is composed of two segments with strong disparity in the specific heat of the Debye approximation, ηx(T, ΘDx)14. These two segments response to a thermal excitation differently. Two parameters characterize the specific heat curves each. One is the Debye temperature ΘDx and the other is the thermal integral of the ηx(T, ΘDx) from 0 K to the Tmx. The ΘDx determines the rate at which the specific-heat curve reaches its saturation. The ηx(T, ΘDx) curve of a segment with a relatively lower ΘDx value reaches saturation more rapidly than the other segment, since the ΘDx, which is lower than Tmx, is proportional to the characteristic vibration frequency ωx of the respective segment, kΘDx = ћωx, according to Einstein’s relation37, where k and ћ are constants.
Conversely, the integral of ηx(T, ΘDx) from 0 K to the Tmx determines the cohesive energy per bond Ex6. The Tmx is the temperature at which the vibration amplitude of an atom or a molecule expands abruptly to more than 3% of its diameter irrespective of the environment or the size of a molecular cluster37,38. Thus we have:

Analysis of the temperature-dependence of water surface tension35 yielded ΘDL = 198 K < 273 K (Tm) and EL = 0.095 eV compared with EH = 3.97 eV for bulk water ice23. Hence,  . The O:H specific heat nL ends at 273 K and the H-O specific heat nH ends at T ≥ 3200 K (TmH). The area covered by the ηH curve is 40 times greater that covered by the ηL curve.
. The O:H specific heat nL ends at 273 K and the H-O specific heat nH ends at T ≥ 3200 K (TmH). The area covered by the ηH curve is 40 times greater that covered by the ηL curve.
The superposition of these two ηx(T, ΘDx) curves implies that the heat capacity of water ice differs from that of other, ‘normal’, materials. Such a ηx(T, ΘDx) disparity yields temperature regions with different ηL/ηH ratios over the full temperature range; see Fig. 3b. These regions correspond to phases of liquid and solid (ηL/ηH < 1) and quasisolid (ηL/ηH > 1). The intersecting temperatures (ηL/ηH = 1) correspond to extreme densities at boundaries of the quasisolid phase (viscose and jelly like). The high-temperature boundary corresponds to the maximal density at 4 °C and the lower to the crystallization of bulk water.
Numerical and experimental observations14,17,20 confirmed that cooling shortens the O:H nonbond in the liquid phase at temperature above 4 °C and in the solid phase below 258 K for bulk at different rates because ηL/ηH < 1 in both regime. However, Cooling shortens the H-O bond in the quasisolid phase (277-258 K). The other counterpart in the O:H-O bond responses to cooling in the opposite direction. This observation clarifies that the segment with lower ηx value follows the general rule of thermal expansion and drives the thermal relaxation of the O:H-O bond, which evidences the essentiality of considering the disparity of the specific heat of water ice14.
One can imagine what will happen to the crossing temperatures if one depresses the ΘDH(ωH) and EH and meanwhile, elevates the ΘDL(ωL) and EL by compression or the inverse. Compression (ΔP > 0) raises the ΘDL and EL by stiffening ωL and meanwhile, lowers the ΘDH and EH by stiffening ωL; however, tension (ΔP < 0) does the opposite. Figure 3b illustrates how the positive P squeezes the quasisolid phase boundaries. The EH determines approximately the Tm through dispersing the upper phase boundary. The ΘDx(ωx) always relax simultaneously in opposite direction under a given stimulus, which will disperse the quasisolid phase boundaries resulting in the observed ‘superheating/supercooling’, as one often refers. In fact, external stimulus can raise/depress the melting/freezing point by phonon relaxation, which is different from the effect of superheating/supercooling39.
Once the O:H bond breaks, oxygen atoms will find new partners to retain the sp3-orbital hybridization, which is the same to diamond oxidation and metal corrosion – oxygen atoms penetrate into the bulk when corrosion occurs15,25. Therefore, O:H-O bond has the strong recoverability for O:H-O bond relaxation and dissociation without any plastic deformation.
Conclusion
Numerical reproduction of the pressure effect on Tm clarifies that O:H-O bond relaxation in length, energy and phonon frequency disperses the quasisolid phase boundaries defined by the supposition of the ηx(T) curves. Compression stiffens the O:H nonbond and softens the H-O bond, which closes up the separation between the crossing points and depresses the melting temperature of ice. Negative pressure does the opposite to raise the Tm. Numerical duplication of the Tm(P) gives rise to the H-O bond cohesive energy of 3.97 eV for the bulk water and ice. Unlike ‘normal’ substance that gains energy with potential plastic deformation under compression, O:H-O bond demonstrates extreme recoverability of relaxation and dissociation because of not only the nature of oxygen sp3-orbital hybridization but also energy loss at compressed state. The O:H-O always tends to recover from its higher-energy state to initially lower state. Coulomb repulsion between electron pairs on adjacent oxygen ions and the O:H-O bond segmental disparity form the soul dictating its adaptivity, cooperativity, sensitivity, memory and recoverability when subject to stimulus. Observations may extend to damage recovery of living cells of which O:H-O bond dominates.
Additional Information
How to cite this article: Zhang, X. et al. Ice Regelation: Hydrogen-bond extraordinary recoverability and water quasisolid-phase-boundary dispersivity. Sci. Rep. 5, 13655; doi: 10.1038/srep13655 (2015).
References
Faraday, M. Note on Regelation. Proc. R. Soc. London 10, 440–450 (1859).
Thomson, J. Note on Professor Faraday’s Recent Experiments on Regelation. Proceedings of the Royal Society of London 10, 151–160 (1859).
James, T. B. Melting and Regelation of Ice. Nature (London) 5, 185 (1872).
Goddard, J. D. The viscous drag on solids moving through solids. AlChE J. 60, 1488–1498 (2014).
Möhlmann, D. T. Are nanometric films of liquid undercooled interfacial water bio-relevant? Cryobiology 58, 256–261 (2009).
Sun, C. Q. Thermo-mechanical behavior of low-dimensional systems: The local bond average approach. Prog. Mater Sci. 54, 179–307 (2009).
Errandonea, D. et al. Systematics of transition-metal melting. Phys. Rev. B 63, 132104 (2001).
Chen, Z. W., Sun, C. Q., Zhou, Y. C. & Gang, O. Y. Size dependence of the pressure-induced phase transition in nanocrystals. J. Phys. Chem. C 112, 2423–2427 (2008).
Green, J. L., Durben, D. J., Wolf, G. H. & Angell, C. A. Water and Solutions at Negative Pressure: Raman Spectroscopic Study to -80 Megapascals. Science 249, 649–652 (1990).
Aoki, K., Yamawaki, H. & Sakashita, M. Observation of Fano interference in high-pressure ice VII. Phys. Rev. Lett. 76, 784–786 (1996).
Song, M. et al. Infrared absorption study of Fermi resonance and hydrogen-bond symmetrization of ice up to 141 GPa. Phys. Rev. B 60, 12644 (1999).
Pruzan, P. et al. Phase diagram of ice in the VII-VIII-X domain. Vibrational and structural data for strongly compressed ice VIII. Journal of Raman Spectroscopy 34, 591–610 (2003).
Hynninen, T. et al. Cutting ice: nanowire regelation. Phys. Rev. Lett. 105, 086102 (2010).
Sun, C. Q. et al. Density and phonon-stiffness anomalies of water and ice in the full temperature range. J Phys Chem Lett 4, 3238–3244 (2013).
Sun, C. Q. Relaxation of the Chemical Bond. Vol. 108 (Springer, 2014).
Zhang, X. et al. Hydrogen-bond memory and water-skin supersolidity resolving the Mpemba paradox. Phys. Chem. Chem. Phys. 16, 22995–23002 (2014).
Huang, Y. et al. Hydrogen-bond relaxation dynamics: resolving mysteries of water ice. Coord. Chem. Rev. 285, 109–165 (2015).
Sun, C. Q., Zhang, X. & Zheng, W. T. Hidden force opposing ice compression. Chem Sci 3, 1455–1460 (2012).
Zhang, X. et al. Water nanodroplet thermodynamics: quasi-solid phase-boundary dispersivity. J. Phys. Chem. B 119, 5265–5269 (2015).
Huang, Y. et al. Size, separation, structure order and mass density of molecules packing in water and ice. Scientific Reports 3, 3005 (2013).
Huang, Y. et al. Hydrogen-bond asymmetric local potentials in compressed ice. J. Phys. Chem. B 117, 13639–13645 (2013).
Sun, C. Q. et al. Density, elasticity and stability anomalies of water molecules with fewer than four neighbors. J. Phys. Chem. Lett. 4, 2565–2570 (2013).
Zhang, X. et al. Mediating relaxation and polarization of hydrogen-bonds in water by NaCl salting and heating. Phys. Chem. Chem. Phys. 16, 24666–24671 (2014).
Guo, J. et al. Real-space imaging of interfacial water with submolecular resolution-Supp. Nat. Mater. 13, 184–189 (2014).
Sun, C. Q. Oxidation electronics: bond-band-barrier correlation and its applications. Prog. Mater Sci. 48, 521–685 (2003).
Zhang, X. et al. A common supersolid skin covering both water and ice. Phys. Chem. Chem. Phys. 16, 22987–22994 (2014).
Yoshimura, Y. et al. High-pressure x-ray diffraction and Raman spectroscopy of ice VIII. J Chem Phys 124, 024502 (2006).
Kang, D. D. et al. Quantum similation of thermally driven phase transition and O k-edge absorption of high-pressure ice. Scientifc reports 3, 3272 (2013).
Benoit, M., Marx, D. & Parrinello, M. Tunnelling and zero-point motion in high-pressure ice. Nature 392, 258–261 (1998).
Goncharov, A. F., Struzhkin, V. V., Mao, H.-K. & Hemley, R. J. Raman Spectroscopy of Dense H2O and the Transition to Symmetric Hydrogen Bonds. Phys. Rev. Lett. 83, 1998–2001 (1999).
Yoshimura, Y. et al. Convergent Raman Features in High Density Amorphous Ice, Ice VII and Ice VIII under Pressure. J. Phys. Chem. B 115, 3756–3760 (2011).
Malenkov, G. Liquid water and ices: understanding the structure and physical properties. J. Phys.-Condes. Matter 21, 283101 (2009).
Sun, C. Q. et al. Dimension, strength and chemical and thermal stability of a single C-C bond in carbon nanotubes. J. Phys. Chem. B 107, 7544–7546 (2003).
Harich, S. A. et al. Photodissociation of H2O at 121.6 nm: A state-to-state dynamical picture. J Chem Phys 113, 10073–10090 (2000).
Zhao, M. et al. Atomistic origin, temperature dependence and responsibilities of surface energetics: An extended broken-bond rule. Phys. Rev. B 75, 085427 (2007).
Gu, M. X., Zhou, Y. C. & Sun, C. Q. Local bond average for the thermally induced lattice expansion. J. Phys. Chem. B 112, 7992–7995 (2008).
Omar, M. A. Elementary Solid State Physics: Principles and Applications. (Addison-Wesley, 1993).
Lindemann, F. A. The calculation of molecular natural frequencies. Phys. Z. 11, 609–612 (1910).
Debenedetti, P. G. & Stanley, H. E. Supercooled and glassy water. Phys. Today 56, 40–46 (2003).
Desiraju, G. R. A Bond by Any Other Name. Ang Chem Int Ed 50, 52–59 (2011).
Falvello, L. R. The Hydrogen Bond, Front and Center. Ang Chem Int Ed 49, 10045–10047 (2010).
Acknowledgements
Financial support from National Natural Science Foundation (No. 21273191) of China is acknowledged.
Author information
Authors and Affiliations
Contributions
X.Z., Y.H. and P.S. conducted calculations, Z.M. and X.J. plot all figures, Y.Z., J.Z. and W.Z. analyzed results, C.Q. conceived the project and wrote the manuscript. All authors reviewed the manuscript.
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Electronic supplementary material
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Zhang, X., Huang, Y., Sun, P. et al. Ice Regelation: Hydrogen-bond extraordinary recoverability and water quasisolid-phase-boundary dispersivity. Sci Rep 5, 13655 (2015). https://doi.org/10.1038/srep13655
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep13655
This article is cited by
-
Hydrogen-bond potential for ice VIII-X phase transition
Scientific Reports (2016)
-
From ice superlubricity to quantum friction: Electronic repulsivity and phononic elasticity
Friction (2015)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.