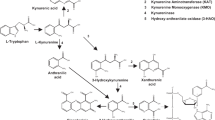
Abstract
The content of the endogenous NMDA and α7 nicotinic acetylcholine receptor antagonist kynurenate (KYNA) is increased in the cerebral cortex and cerebrospinal fluid of patients with schizophrenia. In view of the very high incidence of smoking in schizophrenic individuals, a study was designed to examine the effect of acute and prolonged nicotine administration on brain KYNA levels in experimental animals. Adult male rats received subcutaneous nicotine injections twice daily for up to 10 days, and animals were routinely killed 1 h after the last injection. Neither acute treatment nor a 2-day regimen with 1 mg/kg nicotine (=0.35 mg/kg pure base) caused changes in cerebral KYNA levels. Four- or 6 day-treatment with this dose resulted in 20–40% decreases in cerebral KYNA content. Animals treated with 1 or 10 mg/kg nicotine for 10 days showed dose-dependent, significant increases in KYNA in hippocampus, striatum, and cortex, but not in the serum. Discontinuation of nicotine treatment for 7 days restored brain KYNA to control levels. Separate animals, implanted with osmotic minipumps delivering 2 mg/kg of nicotine/day for 10 days also showed significant elevations in brain KYNA. Hippocampal microdialysis, performed in animals receiving nicotine (1 mg/kg) for 10 days, revealed a significant increase in basal extracellular KYNA levels compared to controls, whereas acute treatment with this dose produced no such change. Measurements of KYNA's bioprecursor kynurenine in brain or blood did not reveal any nicotine-induced changes. These results indicate that nicotine has a brain-specific, biphasic effect on the transamination of kynurenine to KYNA. Such nicotine-induced fluctuations in brain KYNA may cause functional changes in processes that regulate glutamatergic and cholinergic neurotransmission in the normal and diseased brain.
Similar content being viewed by others
INTRODUCTION
Several lines of evidence suggest a role of nicotinic acetylcholine receptors (nAChRs) in the pathophysiology of schizophrenia (Adler et al, 1998; Guan et al, 1999; Breese et al, 2000; Leonard et al, 2000). This connection was initially proposed because of the high incidence of heavy smoking in schizophrenic individuals (Hughes et al, 1986; Lohr and Flynn, 1992). It was found later that nicotine can normalize auditory gating and visual attention deficits, which are prominent pathological features of schizophrenia (Adler et al, 1993; Sherr et al, 2002). This could conceivably be mediated by the α7 subtype of the nAChR, which is known to be critically involved in cognitive functions (Levin and Simon, 1998). An intuitively attractive inference of these studies is, therefore, that patients smoke heavily in an attempt to self-medicate, that is to correct various sensory abnormalities that are associated with the disease (Sandyk and Kay, 1991; Adler et al, 1993; Olincy et al, 1998). In line with this reasoning, auditory gating in animals can be specifically disrupted by selective antagonists of the α7 nAChR (Luntz-Leybman et al, 1992; Stevens et al, 1996, 1998). Conversely, α7 nAChR stimulation normalizes the auditory gating deficit that is observed in rats that have been reared in social isolation (O'Neill et al, 2003). These considerations have stimulated the development of α7 nAChR agonists such as ARR-17779 (Mullen et al, 2000), which interact directly with the binding site for acetylcholine, or of drugs such as galantamine (Reminyl®), which increase nAChR activity by interacting with a site close to, but distinct from, the acetylcholine-binding site (Pereira et al, 1994, 2002; Samochocki et al, 2003). It is also noteworthy that one measure of sensory gating abnormalities, diminished inhibition of the P50 evoked response to repeated auditory stimuli, has been linked to the chromosome 15q14 locus of the α7 nAChR gene (Freedman et al, 1997; Riley et al, 2000).
Compared to control subjects with similar smoking habits, α7 nAChR density is reduced in the cerebral cortex and hippocampus of individuals with schizophrenia (Freedman et al, 1995; Adler et al, 1998; Leonard et al, 2000; Guan et al, 1999). Therefore, since cerebral α7 nAChRs are normally upregulated in heavy smokers (Benwell et al, 1988; Breese et al, 2000) and after chronic nicotine administration in animals (Olale et al, 1997; Sparks and Pauly, 1999), α7 nAChRs in patients appear to have an abnormally blunted reaction to excessive smoking. This could be due to a dysfunction in any of several distinct endogenous mechanisms, which normally control α7 nAChR expression and activity in the brain (Albuquerque et al, 1997; Pereira et al, 2002).
In a first attempt to investigate the possible role of one of these mechanisms, we examined the effect of nicotine on kynurenic acid (KYNA), a tryptophan metabolite that is present in the mammalian brain in nanomolar concentrations (Moroni et al, 1988; Turski et al, 1988). KYNA, long known as an antagonist of the glycine coagonist site of the NMDA receptor (Kessler et al, 1989), blocks α7 nAChR activity at even lower, endogenous brain concentrations (Hilmas et al, 2001), and reductions in brain KYNA levels were recently found to increase α7 nAChR function (Alkondon et al, 2004). It is therefore conceivable that nicotine-induced fluctuations in brain KYNA levels influence the activity of α7 nAChRs (Hilmas et al, 2001). This concept, and the recent demonstration that KYNA levels are elevated in cortical brain regions and cerebrospinal fluid of schizophrenic patients (Erhardt et al, 2001a; Schwarcz et al, 2001) and that elevations in brain KYNA disrupt auditory sensory gating (Shepard et al, 2003), prompted us to examine the consequences of acute and prolonged nicotine administration on the disposition of KYNA and its bioprecursor kynurenine in rats. Our data, some of which have been communicated in a preliminary fashion (Hilmas et al, 2001), revealed that nicotine causes biphasic, brain-specific changes in KYNA levels without affecting the brain concentrations of kynurenine.
MATERIALS AND METHODS
Materials
KYNA, L-kynurenine, and nicotine (bitartrate salt) were purchased from Sigma Chemical Co. (St Louis, MO). All other chemicals were of the highest commercially available purity.
Animals
Adult male Sprague–Dawley rats (200–220 g) were purchased from Charles River Laboratories (Kingston, NY). The animals were housed in an AAALAC-approved animal facility under standard laboratory conditions, tht is, a 12/12 h light/dark cycle with free access to food and water. The experimental protocol was approved by the Institutional Animal Care and Use Committee of the University of Maryland, Baltimore.
Drug Administration
Nicotine was dissolved in phosphate-buffered saline (PBS; pH 7.4) and administered either subcutaneously (s.c.) twice daily (every 12 h) or via osmotic minipumps (Alzet, Alza Corp., Palo Alto, CA; delivering 2 mg/kg of nicotine/day). Control animals received appropriate vehicle treatments. Animals treated s.c. were killed 1 h after the final injection, and animals treated with osmotic minipumps were killed on the morning of the final day of treatment.
Microdialysis
Microdialysis was performed as reported previously (Wu et al, 1992). Briefly, the animals were anesthetized with chloral hydrate (360 mg/kg, i.p.) and mounted in a David Kopf stereotaxic frame. A guide cannula (outer diameter: 0.65 mm) was positioned on top of the hippocampus (AP: 3.4 mm posterior to bregma, L: 2.3 mm from the midline, V: 1.5 mm below the dura) and secured to the skull with anchor screws and acrylic dental cement. At least 20 h after surgery, a microdialysis probe (CMA/10, membrane length: 2 mm, Carnegie Medicin, Stockholm, Sweden) was inserted through the guide cannula, extending throughout the hippocampus. The probe was connected to a microperfusion pump (CMA/100, Carnegie Medicin) set to a speed of 1 μl/min and perfused with Ringer solution containing (in mM): NaCl, 144; KCl, 4.8; MgSO4, 1.2; CaCl2, 1.7; pH 6.7. In acute experiments, dialysate was collected every 30 min for a total of 7 h. In experiments where nicotine was administered for 10 days, animals received an additional injection of nicotine (1 mg/kg) 2 h after the collection of baseline samples, and microdialysis continued for an additional 7 h.
KYNA and Kynurenine Determination
Animals were killed by decapitation, and trunk blood was collected when indicated. The brain was removed, and hippocampus, striatum, and frontal cortex were rapidly dissected out, placed on dry ice and stored at −80°C. On the day of the assay, the tissue was thawed and homogenized (1 : 10, w/v) in ultrapure water. A 300 μl aliquot of the homogenate was acidified with 75 μl of 6% perchloric acid. After centrifugation (10 min, 12 000g), an aliquot of the supernatant was diluted (1 : 1, v/v) with HPLC mobile phase (200 mM zinc acetate containing 3.5% acetonitrile, pH 6.2). A 200 μl aliquot of the sample was applied to a C18 reverse-phase HPLC column (150 × 4.6 mm; Alltech Associates, Deerfield, IL, USA; flow rate: 1.0 ml/min), and KYNA was eluted isocratically with a retention time of approximately 5 min. KYNA was detected fluorimetrically using a Perkin–Elmer LC 240 Fluorescence Detector (Beaconsfield, UK; excitation wavelength: 344 nm; emission wavelength: 398 nm).
To determine extracellular hippocampal KYNA levels, 30 μl microdialysate fractions were directly applied to the HPLC column, and KYNA was measured as described above.
For the measurement of kynurenine, 200 μl of the diluted acidic supernatant used for KYNA determination were applied to the same HPLC system described above. Kynurenine was eluted with a retention time of approximately 4 min and detected by UV spectroscopy at 365 nm (Beckman 160 Absorbance detector, Fullerton, CA, USA).
To determine serum levels of kynurenine and KYNA, trunk blood was immediately centrifuged (10 min, 12 000g). The supernatant plasma was diluted (1 : 5, v/v) with ultrapure water and acidified with 75 μl of 6% perchloric acid. After centrifugation (5 min, 12 000g), an aliquot of the serum was further diluted (1 : 1, v/v) with HPLC mobile phase, and 200 μl were subjected to HPLC analysis as described above.
All chromatographic data were recorded using a Hewlett-Packard 3390 A integrator.
Protein Determination
Protein was determined in aliquots of the original tissue homogenate according to the method of Lowry et al (1951).
Data Analysis
Microdialysis data were not corrected for recovery from the probe (∼20%; Wu et al, 1992). For all experiments, a repeated-measures analysis of variance (ANOVA) with appropriate post hoc analysis was used. A p-value of <0.05 was considered significant in all analyses.
RESULTS
Acute Nicotine Administration
To determine the acute effects of nicotine on cerebral KYNA levels, animals received a single injection of nicotine (1 mg/kg (=0.35 mg/kg free base)). Examined after 1 or 2 h, this treatment did not cause significant changes in forebrain tissue KYNA (in fmol/mg protein: control, 163.5±17.3; 1 h, 164.1±28.6; 2 h, 166.2±33.0; n=5 per group). Measurement of extracellular KYNA concentrations in hippocampal dialysates, too, revealed no acute effect of nicotine (1 mg/kg) for up to 5 h (n=5).
Repeated Nicotine Administration: Dose Dependency
A 10-day treatment with nicotine caused dose-dependent increases in tissue KYNA levels in hippocampus, striatum, and frontal cortex. Whereas 0.1 mg/kg of nicotine was ineffective, 1 mg/kg of nicotine resulted in a 34–45% increase, and 10 mg/kg of nicotine caused an 82–107% increase in KYNA content in the three brain areas (Figure 1). These differences were found to be statistically significant in all brain regions sampled.
Dose-dependent effects of repeated nicotine administration (s.c., b.i.d. for 10 days) on KYNA levels in various brain regions. Experiments were conducted as described in the text. Data are expressed as the mean+SEM (n=5 per group). *p<0.05 vs PBS-injected animals, #p<0.05 vs 1 mg/kg nicotine (repeated measures ANOVA followed by Bonferroni's post hoc test for multiple comparisons).
The tissue content of KYNA's bioprecursor kynurenine, too, was determined in the brains of animals receiving PBS or 1 mg/kg nicotine for 10 days (n=5 per group). The levels of kynurenine (in pmol/mg protein) in nicotine-treated rats (hippocampus, 24±8; striatum, 23±3; frontal cortex, 20±6) were not significantly different from those in PBS-treated rats (hippocampus, 20±3; striatum, 22±5; frontal cortex, 24±6).
Repeated Nicotine Administration: Time Dependency
The time course of nicotine-induced changes in tissue KYNA content was examined in separate animals (Figure 2). After repeated administration of nicotine (1 mg/kg) for 2, 4, 6, 8, or 10 days, KYNA levels in hippocampus, striatum, and frontal cortex underwent biphasic changes over time. Repeated injections for 4 or 6 days resulted in 14–35% reductions in KYNA levels, which reached statistical significance in hippocampus and striatum. In contrast, a 10-day treatment with nicotine caused an approximately 40% increases in KYNA levels in all three brain areas (cf Figure 1). Treatment for 2 or 8 days had no significant effect on endogenous KYNA.
Time-dependent effects of nicotine (1 mg/kg, s.c., b.i.d. for 2, 4, 6, 8, or 10 days) on KYNA levels in the hippocampus (a), striatum (b), and frontal cortex (c). PBS-injected control animals contained 146.4±18.4 (hippocampus), 131.1±17.4 (striatum), and 135.2±16.2 (frontal cortex) fmol KYNA/mg protein. Experiments were conducted as described in the text. Data are expressed as the mean+SEM (n=5 per time point). *p<0.05 vs PBS-injected animals (repeated measures ANOVA followed by Bonferroni's post hoc test for multiple comparisons).
Repeated Nicotine Administration: Effect on Extracellular KYNA Levels
To examine if changes in tissue levels are paralleled by changes in extracellular KYNA, hippocampal microdialysis was performed in rats treated with nicotine (1 mg/kg) for 10 days (Figure 3). The basal levels of KYNA in the nicotine-treated animals (3.6±0.4 nM) were significantly higher than in PBS-treated controls (2.4±0.5 nM). An additional acute injection of 1 mg/kg nicotine after the fourth sample collection, that is after 2 h of baseline determination, did not cause a significant change in extracellular KYNA levels in either nicotine- or PBS-treated rats.
Effect of repeated nicotine (1 mg/kg, s.c., b.i.d. for 10 days) or PBS administration on extracellular KYNA levels in the hippocampus. Experiments were conducted as described in the text. Data are the mean±SEM of five animals per group. Nicotine treatment significantly elevated baseline extracellular KYNA concentrations (p<0.05 vs PBS-injected animals; repeated measures ANOVA followed by Bonferroni's post hoc test for multiple comparisons). No changes in extracellular KYNA were seen after an additional acute injection of nicotine (1 mg/kg, s.c.) in these animals (arrow; p>0.05 vs the respective baseline; repeated measures ANOVA followed by Bonferroni's post hoc test for multiple comparisons).
Prolonged Nicotine Infusion Via Minipumps
Administration of nicotine (2 mg/kg (=0.7 mg/kg free base) per day) via osmotic minipumps for 10 days resulted in a significant, approximately 70%, increase in KYNA content in hippocampus, striatum, and frontal cortex, compared to PBS-treated controls (Figure 4).
Effects of prolonged nicotine infusion (2 mg/kg/day for 10 days) on KYNA levels in various brain regions. Experiments with osmotic minipumps were conducted as described in the text. Data are expressed as the mean+SEM (n=5 per group). *p<0.05 vs PBS-treated animals (repeated measures ANOVA followed by Bonferroni's post hoc test for multiple comparisons).
Repeated Nicotine Administration: Effect of Drug Discontinuation
To determine the reversibility of the nicotine effect, repeated treatment with nicotine (1 mg/kg) was stopped after 10 days, and KYNA levels were determined in hippocampus, striatum, and frontal cortex 7 days later. Compared to animals tested immediately after 10 days of repeated nicotine treatment, brain KYNA in these rats was significantly reduced and had in fact returned to control levels (Figure 5). In separate animals, an additional single challenge with nicotine (1 mg/kg) 7 days after nicotine discontinuation failed to affect brain KYNA levels (Figure 5).
Effect of discontinuation of prolonged nicotine treatment. Two groups of animals received either PBS or nicotine (1 mg/kg, s.c., b.i.d.) for 10 days (n=5 per group). Two other groups received nicotine (1 mg/kg, s.c., b.i.d.) for 10 days. Nicotine administration was then discontinued. After 7 days, the animals received a single s.c. injection of either PBS (‘Discont.+PBS’; n=5) or nicotine (1 mg/kg; ‘Discont.+nicotine’; n=5) and were killed 1 h later. *p<0.05 vs PBS; #p<0.05 vs nicotine (repeated measures ANOVA followed by Bonferroni's post hoc test for multiple comparisons). No changes were seen between ‘Discont.+PBS’ and ‘Discont.+nicotine’ animals (p>0.05; repeated measures ANOVA followed by Bonferroni's post hoc test for multiple comparisons).
Repeated Nicotine Administration: Kynurenine and Kyna Content in Serum
Serum levels of kynurenine and KYNA were measured in animals that received repeated injections of nicotine (1 mg/kg) for 2, 4, 6, 8, or 10 days. None of these treatment regimens caused changes in the levels of either metabolite (Table 1).
DISCUSSION
The present study demonstrated that prolonged, but not acute, nicotine administration causes significant changes in KYNA levels in the rat brain. In agreement with an earlier, preliminary experiment (Hilmas et al, 2001), these nicotine-induced changes were biphasic in nature, that is an initial significant reduction in KYNA was observed after 4 or 6 days of repeated injections, whereas a significant increase in KYNA was seen when nicotine was given for 10 days. These nicotine-induced fluctuations were not brain region-specific since similar effects were observed in the hippocampus, striatum, and frontal cortex. However, they were not accompanied by changes in the serum levels of KYNA. The increases in brain tissue KYNA levels observed after 10 days of nicotine treatment were reversible, dose-dependent and, as documented by hippocampal microdialysis, associated with quantitatively similar elevations in the extracellular compartment. These results also resembled the increases in brain KYNA levels that were seen when the drug was injected s.c. twice daily (mimicking smoking) or administered by chronic infusion (mimicking a nicotine patch) for 10 days. Finally, nicotine treatment was found to have no effect on the concentration of KYNA's immediate bioprecursor, kynurenine, in brain or serum.
KYNA is a product of the kynurenine pathway of tryptophan degradation. In the mammalian brain, KYNA is formed through irreversible transamination of kynurenine by kynurenine aminotransferases (Guidetti et al, 1997). These enzymatic processes take place predominantly in astrocytes (Ceresoli-Borroni et al, 1999a; Guillemin et al, 2001; Kiss et al, 2003), which then readily liberate newly synthesized KYNA into the extracellular space for possible receptor interactions (Turski et al, 1989). Notably, KYNA production is controlled by several distinct factors and mechanisms. Some, such as kynurenine, competing amino-acid substrates, and 2-oxoacids, operate both in the periphery and in the brain. Others, for example the decrease in KYNA formation effected by reduced cellular energy metabolism or by depolarizing agents such as potassium or veratridine, are brain-specific (Gramsbergen et al, 1997). It remains to be seen if and to what extent astrocytic α7 nACh (Sharma and Vijayaraghavan, 2001) or NMDA (Krebs et al, 2003) receptors can directly influence cerebral KYNA formation.
KYNA formation can also be reduced by dopaminergic compounds such as amphetamine (Rassoulpour et al, 1998), L-DOPA (Wu et al, 2002), or selective dopamine receptor agonists (Poeggeler et al, 1998). These effects, too, are brain-specific and therefore qualitatively similar to the effect of prolonged nicotine administration described here. In fact, it is possible that dopaminergic mechanisms participate in the biphasic effects of nicotine on brain KYNA reported in the present study. Thus, nAChRs show both desensitization (Marks et al, 1983; Castro and Albuquerque, 1995) and subsequent supersensitivity (Schwartz and Kellar, 1983, 1985; Wonnacott et al, 1990) in response to repeated or chronic nicotine treatment, and these adaptive changes may influence the nAChRs-mediated regulation of extracellular dopamine (Harsing et al, 1992; Marshall et al, 1997). In other words, nicotine could cause biphasic changes in brain KYNA formation indirectly by controlling dopamine release linked to nAChR activation.
Alternatively or in addition, the effects of prolonged nicotine administration on brain KYNA levels may involve glutamatergic mechanisms. Thus, activation of presynaptic α7 nACh receptors is associated with enhanced glutamatergic transmission (Alkondon et al, 1996; Gray et al, 1996). Chronic nicotine treatment results in changes in astrocytic glutamate transporters (Lim and Kim, 2001) and causes the functional upregulation of ionotropic glutamate receptors (Risso et al, 2004). These phenomena result in abnormal glutamatergic activity in response to prolonged nicotine treatment and could, in turn, compromise cerebral KYNA formation (Wu et al, 1992).
In neurobiological research, high concentrations of KYNA (⩾1 mM) are frequently used as a tool to block all ionotropic glutamate receptors (Perkins and Stone, 1982). Consequently, intracerebral application of large amounts of KYNA has long been known to exert neuroprotective and anticonvulsant effects in experimental animals (Foster et al, 1984). At much lower concentrations, KYNA inhibits the strychnine-insensitive glycine coagonist site of the NMDA receptor (IC50: 8 μM; Kessler et al, 1989), and recent evidence favors a physiological action of KYNA as an allosteric, noncompetitive antagonist of the α7 nAChR (Hilmas et al, 2001; Alkondon et al, 2004). Endogenous KYNA may therefore affect neuronal excitability and vulnerability by directly or indirectly interfering with both cholinergic and glutamatergic neurotransmission (Harris et al, 1998; Poeggeler et al, 1998; Cozzi et al, 1999; Wu et al, 2000; Pereira et al, 2002; Schwarcz and Pellicciari, 2002; Sapko et al, 2003). Further, the changes in cerebral KYNA levels observed after prolonged nicotine administration may account for the ability of nicotine to influence neuronal viability in vivo (Akaike et al, 1994; Marin et al, 1994; O'Neill et al, 1998).
The present findings are also relevant for the pathophysiology of schizophrenia since both glutamate receptor and α7 nAChR dysfunction have been implicated in the disease process (Carlsson and Carlsson, 1990; Freedman et al, 1995; Tamminga, 1998; Leonard et al, 2000; Schilstrőm et al, 2000; Coyle and Tsai, 2004). Thus, the elevated levels of KYNA measured in the brain and cerebrospinal fluid (Erhardt et al, 2001a; Schwarcz et al, 2001) may contribute to the presumed hypoglutamatergic and hypocholinergic tone in schizophrenic individuals. Since brain KYNA levels are decreased 4 and 6 days after repeated nicotine administration, excessive smoking in the schizophrenic population could constitute an attempt to self-medicate (cf Introduction). Indeed, a reduction in brain KYNA enhances nicotinic and glutamatergic transmission (Alkondon et al, 2004) and could thus normalize gating (Adler et al, 1993) and eye-tracking (Olincy et al, 1998; Avila et al, 2003), deficit, and improve cognitive function (Mori and Mishina, 2003). However, the present data suggest that more prolonged exposure to nicotine may have the opposite, detrimental effects on sensory and cognitive modalities since brain KYNA levels are elevated (cf Shepard et al, 2003). In fact, even relatively modest increases in brain KYNA are known to significantly influence the electrophysiological properties of monoaminergic neurons (Erhardt et al, 2000, 2001b, 2002; Schwieler and Erhardt, 2003).
The results described here raise several interesting issues for future research. For example, further studies will be required to elucidate the molecular and cellular mechanisms that underlie the biphasic effects of extended nicotine administration on cerebral KYNA formation and the reversal of brain KYNA to normal levels upon treatment cessation. In addition, the functional consequences of fluctuations in cerebral KYNA levels in response to prolonged exposure to nicotine, especially the effects on cholinergic and glutamatergic neurotransmission, need to be explored in detail. It will be especially important to evaluate whether concurrent treatment with antipsychotic drugs influences nicotine-induced changes in brain KYNA. Thus, neuroleptics are known to have reciprocal interactions with nicotine (Jann et al, 1986; Miller et al, 1990; Lee et al, 2001), affect smoking behavior (George et al, 1995) and, when administered chronically, reduce brain KYNA levels in rats (Ceresoli-Borroni et al, 1999b; cf also Schwieler and Erhardt, 2003). Evidence for interactions between prolonged nicotine and neuroleptic treatments in determining brain KYNA formation would further support the suggestion (Court et al, 1998) that smoking history be carefully assessed and considered in future biochemical studies in schizophrenic individuals.
In summary, the present results demonstrate that prolonged nicotine administration in rats has a brain-specific, biphasic and reversible effect on KYNA levels. Nicotine-induced fluctuations in brain KYNA may cause changes in glutamatergic and cholinergic neurotransmission and may play a role in the beneficial effects of nicotine in patients suffering from schizophrenia or other brain diseases. By inference, our study suggests possible clinical benefits from direct pharmacological manipulations of cerebral KYNA levels, which can be achieved by targeting enzymes of the kynurenine pathway (Schwarcz and Pellicciari, 2002).
References
Adler LE, Hoffer LD, Wiser A, Freedman R (1993). Normalization of auditory physiology by cigarette smoking in schizophrenic patients. Am J Psychiatry 150: 1856–1861.
Adler LE, Olincy A, Waldo M, Harris JG, Griffith J, Stevens K et al (1998). Schizophrenia, sensory gating, and nicotinic receptors. Schizophr Bull 24: 189–202.
Akaike A, Tamura Y, Yokota T, Shimohama S, Kimura J (1994). Nicotine-induced protection of cultured cortical neurons against N-methyl-D-aspartate receptor-mediated glutamate cytotoxicity. Brain Res 644: 181–187.
Albuquerque EX, Alkondon M, Pereira EF, Castro NG, Schrattenholz A, Barbosa CT et al (1997). Properties of neuronal nicotinic acetylcholine receptors: pharmacological characterization and modulation of synaptic function. J Pharmacol Exp Ther 280: 1117–1136.
Alkondon M, Pereira EFR, Yu P, Arruda EZ, Almeida LEF, Guidetti P et al (2004). Targeted deletion of the kynurenine aminotransferase II gene reveals a critical role of endogenous kynurenic acid in the regulation of synaptic transmission via α7 nicotinic receptors in the hippocampus. J Neurosci 24: 4635–4648.
Alkondon M, Rocha ES, Maelicke A, Albuquerque EX (1996). Diversity of nicotinic acetylcholine receptors in rat brain. V. Alpha-bungarotoxin-sensitive nicotinic receptors in olfactory bulb neurons and presynaptic modulation of glutamate release. J Pharmacol Exp Ther 278: 1460–1471.
Avila MT, Sherr JD, Hong E, Myers CS, Thaker GK (2003). Effects of nicotine on leading saccades during smooth pursuit eye movements in smokers and nonsmokers with schizophrenia. Neuropsychopharmacology 28: 2184–2191.
Benwell ME, Balfour DJ, Anderson JM (1988). Evidence that tobacco smoking increases the density of (−)-[3H]nicotine binding sites in human brain. J Neurochem 50: 1243–1247.
Breese CR, Lee MJ, Adams CE, Sullivan B, Logel J, Gillen KM et al (2000). Abnormal regulation of high affinity nicotinic receptors in subjects with schizophrenia. Neuropsychopharmacology 23: 351–364.
Carlsson M, Carlsson A (1990). Interactions between glutamatergic and monoaminergic systems within the basal ganglia—implications for schizophrenia and Parkinson's disease. Trends Neurosci 13: 272–276.
Castro NG, Albuquerque EX (1995). Alpha-bungarotoxin-sensitive hippocampal nicotinic receptor channel has a high calcium permeability. Biophys J 68: 516–524.
Ceresoli-Borroni G, Guidetti P, Schwarcz R (1999a). Acute and chronic changes in kynurenate formation following an intrastriatal quinolinate injection in rats. J Neural Transm 106: 229–242.
Ceresoli-Borroni G, Wu HQ, Guidetti P, Rassoulpour A, Roberts RC, Schwarcz R (1999b). Chronic haloperidol administration decreases kynurenic acid levels in rat brain. Soc Neurosci Abstr 25: 727.8.
Court JA, Lloyd S, Thomas N, Piggott MA, Marshall EF, Morris CM et al (1998). Dopamine and nicotinic receptor binding and the levels of dopamine and homovanillic acid in human brain related to tobacco use. Neuroscience 87: 63–78.
Coyle JT, Tsai G (2004). NMDA receptor function, neuroplasticity, and the pathophysiology of schizophrenia. Int Rev Neurobiol 59: 491–515.
Cozzi A, Carpenedo R, Moroni F (1999). Kynurenine hydroxylase inhibitors reduce ischemic brain damage: studies with (m-nitrobenzoyl)-alanine (mNBA) and 3,4-dimethoxy-[-N-4-(nitrophenyl)thiazol-2yl]-benzenesulfonamide (Ro 61-8048) in models of focal or global brain ischemia. J Cereb Blood Flow Metab 19: 771–777.
Erhardt S, Blennow K, Nordin C, Skogh E, Lindström LH, Engberg G (2001a). Kynurenic acid levels are elevated in the cerebrospinal fluid of patients with schizophrenia. Neurosci Lett 313: 96–98.
Erhardt S, Hajos M, Lindberg A, Engberg G (2000). Nicotine-induced excitation of locus coeruleus neurons is blocked by elevated levels of endogenous kynurenic acid. Synapse 37: 104–108.
Erhardt S, Öberg H, Engberg G (2001b). Pharmacologically elevated levels of endogenous kynurenic acid prevent nicotine-induced activation of nigral dopamine neurons. Naunyn-Schmiedeberg's Arch Pharmacol 363: 21–27.
Erhardt S, Schwieler L, Engberg G (2002). Excitatory and inhibitory responses of dopamine neurons in the ventral tegmental area to nicotine. Synapse 43: 227–237.
Foster AC, Vezzani A, French ED, Schwarcz R (1984). Kynurenic acid blocks neurotoxicity and seizures induced in rats by the related brain metabolite quinolinic acid. Neurosci Lett 48: 273–278.
Freedman R, Coon H, Myles-Worsley M, Orr-Urtreger A, Olincy A, Davis A et al (1997). Linkage of a neurophysiological deficit in schizophrenia to a chromosome 15 locus. Proc Natl Acad Sci USA 94: 587–592.
Freedman R, Hall M, Adler LE, Leonard S (1995). Evidence in postmortem brain tissue for decreased numbers of hippocampal nicotinic receptors in schizophrenia. Biol Psychiatry 38: 22–33.
George TP, Sernyak MJ, Ziedonis DM, Woods SW (1995). Effects of clozapine on smoking in chronic schizophrenic outpatients. J Clin Psychiatry 56: 344–346.
Gramsbergen JBP, Hodgkins PS, Rassoulpour A, Turski WA, Guidetti P, Schwarcz R (1997). Brain-specific modulation of kynurenic acid synthesis in the rat. J Neurochem 69: 290–298.
Gray R, Rajan AS, Radcliffe KA, Yakehiro M, Dani JA (1996). Hippocampal synaptic transmission enhanced by low concentrations of nicotine. Nature 383: 713–716.
Guan ZZ, Zhang X, Blennow K, Nordberg A (1999). Decreased protein level of nicotinic receptor alpha7 subunit in the frontal cortex from schizophrenic brain. Neuroreport 10: 1779–1782.
Guidetti P, Okuno E., Schwarcz R (1997). Characterization of rat brain kynurenine amino-transferases I and II. J Neurosci Res 50: 457–465.
Guillemin GJ, Kerr SJ, Smythe GA, Smith DG, Kapoor V, Armati PJ et al (2001). Kynurenine pathway metabolism in human astrocytes: a paradox for neuronal protection. J Neurochem 78: 842–853.
Harris CA, Miranda AF, Tanguay JJ, Boegman RJ, Beninger RJ, Jhamandas K (1998). Modulation of striatal quinolinate neurotoxicity by elevation of endogenous brain kynurenic acid. Br J Pharmacol 124: 391–399.
Harsing Jr LG, Sershen H, Lajtha A (1992). Dopamine efflux from striatum after chronic nicotine: evidence for autoreceptor desensitization. J Neurochem 59: 48–54.
Hilmas C, Pereira EF, Alkondon M, Rassoulpour A, Schwarcz R, Albuquerque EX (2001). The brain metabolite kynurenic acid inhibits α7 nicotinic receptor activity and increases non-α7 nicotinic receptor expression: physiopathological implications. J Neurosci 21: 7463–7473.
Hughes JR, Hatsukami DK, Mitchell JE, Dahlgren LA (1986). Prevalence of smoking among psychiatric outpatients. Am J Psychiatry 143: 993–997.
Jann MW, Saklad SR, Ereshefsky L, Richards AL, Harrington CA, Davis CM (1986). Effects of smoking on haloperidol and reduced haloperidol plasma concentrations and haloperidol clearance. Psychopharmacology 90: 468–470.
Kessler M, Terramani T, Lynch G, Baudry M (1989). A glycine site associated with N-methyl-D-aspartic acid receptors: characterization and identification of a new class of antagonists. J Neurochem 52: 1319–1328.
Kiss C, Ceresoli-Borroni G, Guidetti P, Zielke CL, Zielke HR, Schwarcz R (2003). Kynurenate production by cultured human astrocytes. J Neural Transm 110: 1–14.
Krebs C, Fernandes HB, Sheldon C, Raymond LA, Baimbridge KG (2003). Functional NMDA receptor subtype 2B is expressed in astrocytes after ischemia in vivo and anoxia in vitro. J Neurosci 23: 3364–3372.
Lee MJ, Breese CR, Strook ML, Leonard S (2001). The effect of nicotine and haloperidol co-treatment on nicotinic receptor levels in the rat brain. Brain Res Mol Brain Res 86: 115–124.
Leonard S, Breese C, Adams C, Benhammou K, Gault J, Stevens K et al (2000). Smoking and schizophrenia: abnormal nicotinic receptor expression. Eur J Pharmacol 393: 237–242.
Levin ED, Simon BB (1998). Nicotinic acetylcholine involvement in cognitive function in animals. Psychopharmacology 138: 217–230.
Lim DK, Kim HS (2001). Changes in the glutamate release and uptake of cerebellar cells in perinatally nicotine-exposed rat pups. Neurochem Res 26: 1119–1125.
Lohr JB, Flynn K (1992). Smoking and schizophrenia. Schizophr Res 8: 93–102.
Lowry OH, Rosebrough NJ, Farr NJ, Randall RJ (1951). Protein measurement with the Folin phenol reagent. J Biol Chem 193: 265–275.
Luntz-Leybman V, Bickford PC, Freedman R (1992). Cholinergic gating of response to auditory stimuli in rat hippocampus. Brain Res 587: 130–136.
Marin P, Maus M, Desagher S, Glowinski J, Premont J (1994). Nicotine protects cultured striatal neurones against N-methyl-D-aspartate receptor-mediated neurotoxicity. NeuroReport 5: 1977–1980.
Marks MJ, Burch JB, Collins AC (1983). Effects of chronic nicotine infusion on tolerance development and nicotinic receptors. J Pharmacol Exp Ther 226: 817–825.
Marshall DL, Redfern PH, Wonnacott S (1997). Presynaptic nicotinic modulation of dopamine release in the three ascending pathways studied by in vivo microdialysis: comparison of naive and chronic nicotine-treated rats. J Neurochem 68: 1511–1519.
Miller DD, Kelly MW, Perry PJ, Coryell WH (1990). The influence of cigarette smoking on haloperidol pharmacokinetics. Biol Psychiatry 28: 529–531.
Mori H, Mishina M (2003). Roles of diverse glutamate receptors in brain functions elucidated by subunit-specific and region-specific gene targeting. Life Sci 74: 329–336.
Moroni F, Russi P, Lombardi G, Beni M, Carlà V (1988). Presence of kynurenic acid in the mammalian brain. J Neurochem 51: 177–180.
Mullen G, Napier J, Balestra M, DeCory T, Hale G, Macor J et al (2000). Spiro[1-azabicyclo[2.2.2]octane-3,5′-oxazolidin-2′-one], a conformationally restricted analogue of acetylcholine, is a highly selective full agonist at the alpha 7 nicotinic acetylcholine receptor. J Med Chem 43: 4045–4050.
Olale F, Gerzanich V, Kuryatov A, Wang F, Lindstrom J (1997). Chronic nicotine exposure differentially affects the function of human alpha3, alpha4, andalpha7 neuronal nicotinic receptor subtypes. J Pharmacol Exp Ther 283: 675–683.
Olincy A, Ross RG, Young DA, Roath M, Freedman R (1998). Improvement in smooth pursuit eye movements after cigarette smoking in schizophrenic patients. Neuropsychopharmacology 18: 175–185.
O'Neill AB, Morgan SJ, Brioni JD (1998). Histological and behavioral protection by (−)-nicotine against quinolinic acid-induced neurodegeneration in the hippocampus. Neurobiol Learn Mem 69: 46–64.
O'Neill HC, Rieger K, Kem WR, Stevens KE (2003). DMXB, an α7 nicotinic agonist, normalizes auditory gating in isolation-reared rats. Psychopharmacology 169: 332–339.
Pereira EF, Alkondon M, Reinhardt S, Maelicke A, Peng X, Lindstrom J et al (1994). Physostigmine and galanthamine: probes for a novel binding site on the alpha 4 beta 2 subtype of neuronal nicotinic acetylcholine receptors stably expressed in fibroblast cells. J Pharmacol Exp Ther 270: 768–778.
Pereira EF, Hilmas C, Santos MD, Alkondon M, Maelicke A, Albuquerque EX (2002). Unconventional ligands and modulators of nicotinic receptors. J Neurobiol 53: 479–500.
Perkins MN, Stone TW (1982). An iontophoretic investigation of the actions of convulsant kynurenines and their interaction with the endogenous excitant quinolinic acid. Brain Res 247: 184–187.
Poeggeler B, Rassoulpour A, Guidetti P, Wu HQ, Schwarcz R (1998). Dopaminergic control of kynurenate levels and N-methyl-D-aspartate toxicity in the developing rat striatum. Dev Neurosci 20: 146–153.
Rassoulpour A, Wu HQ, Poeggeler B, Schwarcz R (1998). Systemic d-amphetamine administration causes a reduction of kynurenic acid levels in rat brain. Brain Res 802: 111–118.
Riley BP, Makoff A, Mogudi-Carter M, Jenkins T, Williamson R, Collier D et al (2000). Haplotype transmission disequilibrium and evidence for linkage of the CHRNA7 gene region to schizophrenia in Southern African Bantu families. Am J Med Genet 96: 196–201.
Risso F, Parodi M, Grilli M, Molfino F, Raiteri M, Marchi M (2004). Chronic nicotine causes functional upregulation of ionotropic glutamate receptors mediating hippocampal noradrenaline and striatal dopamine release. Neurochem Int 44: 293–301.
Samochocki M, Hoffle A, Fehrenbacher A, Jostock R, Ludwig J, Christner C et al (2003). Galantamine is an allosterically potentiating ligand of neuronal nicotinic but not of muscarinic acetylcholine receptors. J Pharmacol Exp Ther 305: 1024–1036.
Sandyk R, Kay SR (1991). Tobacco addiction as a marker of age at onset of schizophrenia. Int J Neurosci 57: 259–262.
Sapko MT, Yu P, Guidetti P, Pellicciari R, Tagle DA, Schwarcz R (2003). Endogenous brain kynurenic acid modulates susceptibility to striatal quinolinic acid excitotoxicity. Soc Neurosci Abstr 29: 805.20.
Schilstrőm B, Fagerquist MV, Zhang X, Hertel P, Panagis G, Nomikos GG et al (2000). Putative role of presynaptic alpha7* nicotinic receptors in nicotine stimulated increases of extracellular levels of glutamate and aspartate in the ventral tegmental area. Synapse 38: 375–783.
Schwarcz R, Pellicciari R (2002). Manipulation of brain kynurenines: glial targets, neuronal effects and clinical opportunities. J Pharmacol Exp Ther 303: 1–10.
Schwarcz R, Rassoulpour A, Wu HQ, Medoff D, Tamminga CA, Roberts RC (2001). Increased cortical kynurenate content in schizophrenia. Biol Psychiatry 50: 521–530.
Schwartz RD, Kellar KJ (1983). Nicotinic cholinergic receptor binding sites in the brain: regulation in vivo. Science 220: 214–216.
Schwartz RD, Kellar KJ (1985). In vivo regulation of [3H]acetylcholine recognition sites in brain by nicotinic cholinergic drugs. J Neurochem 45: 427–433.
Schwieler L, Erhardt S (2003). Inhibitory action of clozapine on rat ventral tegmental area dopamine neurons following increased levels of endogenous kynurenic acid. Neuropsychopharmacology 28: 1770–1777.
Sharma G, Vijayaraghavan S (2001). Nicotinic cholinergic signaling in hippocampal astrocytes involves calcium-induced calcium release from intracellular stores. Proc Natl Acad Sci USA 98: 4148–4153.
Shepard PD, Joy B, Clerkin L, Schwarcz R (2003). Micromolar brain levels of kynurenic acid are associated with a disruption of auditory sensory gating in the rat. Neuropsychopharmacology 28: 1454–1462.
Sherr JD, Myers C, Avila MT, Elliott A, Blaxton TA, Thaker GK (2002). The effects of nicotine on specific eye tracking measures in schizophrenia. Biol Psychiatry 52: 721–728.
Sparks JA, Pauly JR (1999). Effects of continuous oral nicotine administration on brain nicotinic receptors and responsiveness to nicotine in C57Bl/6 mice. Psychopharmacology 141: 145–153.
Stevens KE, Freedman R, Collins AC, Hall M, Leonard S, Marks MJ et al (1996). Genetic correlation of inhibitory gating of hippocampal auditory evoked response and alpha-bungarotoxin-binding nicotinic cholinergic receptors in inbred mouse strains. Neuropsychopharmacology 15: 152–162.
Stevens KE, Kem WR, Mahnir VM, Freedman R (1998). Selective alpha7-nicotinic agonists normalize inhibition of auditory response in DBA mice. Psychopharmacology 136: 320–327.
Tamminga CA (1998). Schizophrenia and glutamatergic transmission. Crit Rev Neurobiol 12: 21–36.
Turski WA, Gramsbergen JBP, Traitler H, Schwarcz R (1989). Rat brain slices produce and liberate kynurenic acid upon exposure to L-kynurenine. J Neurochem 52: 1629–1636.
Turski WA, Nakamura M, Todd WP, Carpenter BK, Whetsell Jr WO, Schwarcz R (1988). Identification and quantification of kynurenic acid in human brain tissue. Brain Res 454: 164–169.
Wonnacott S, Drasdo A, Sanderson E, Rowell P (1990). Presynaptic nicotinic receptors and the modulation of transmitter release. Ciba Found Symp 152: 87–101.
Wu HQ, Baran H, Ungerstedt U, Schwarcz R (1992). Kynurenic acid in the quinolinate-lesioned rat hippocampus: studies in vitro and in vivo. Eur J Neurosci 4: 1264–1270.
Wu HQ, Guidetti P, Goodman JH, Varasi M, Ceresoli-Borroni G, Speciale C et al (2000). Kynurenergic manipulations influence excitatory amino acid receptor function and excitotoxic vulnerability in the rat hippocampus in vivo. Neuroscience 97: 243–251.
Wu HQ, Rassoulpour A, Schwarcz R (2002). Effect of systemic L-dopa administration on extracellular kynurenate levels in the rat striatum. J Neural Transm 109: 239–249.
Acknowledgements
We are grateful to Dr Edna FR Pereira for fruitful discussions and review of the manuscript. This work was supported in part by an NARSAD Distinguished Investigator award (to RS) and USPHS Grants NS 25296 and NS 41671 (to EXA).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Rassoulpour, A., Wu, HQ., Albuquerque, E. et al. Prolonged Nicotine Administration Results in Biphasic, Brain-Specific Changes in Kynurenate Levels in the Rat. Neuropsychopharmacol 30, 697–704 (2005). https://doi.org/10.1038/sj.npp.1300583
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.npp.1300583
Keywords
This article is cited by
-
Associations between expression of indoleamine 2, 3-dioxygenase enzyme and inflammatory cytokines in patients with first-episode drug-naive Schizophrenia
Translational Psychiatry (2021)
-
Influence of plasma cytokines on kynurenine and kynurenic acid in schizophrenia
Neuropsychopharmacology (2018)
-
Attenuating Nicotine Reinforcement and Relapse by Enhancing Endogenous Brain Levels of Kynurenic Acid in Rats and Squirrel Monkeys
Neuropsychopharmacology (2017)
-
Abnormal kynurenine pathway of tryptophan catabolism in cardiovascular diseases
Cellular and Molecular Life Sciences (2017)
-
Nicotine and nicotinic system in hypoglutamatergic models of schizophrenia
Neurotoxicity Research (2007)