Abstract
It has been shown previously that the selective cannabinoid CB1 receptor antagonist, rimonabant (SR141716), reduced the intake of palatable food as well as the self-administration of several drugs of abuse, suggesting that endocannabinoid systems play a role in brain reward function. The present study investigated whether a cannabinoid step was involved in food-seeking behavior induced by explicit stimuli, using an operant reinstatement procedure in rats. Experimental sessions consisted of a 15-min food rewarded period, followed by a 45-min extinction period. Rimonabant did not affect the response reinstatement induced by noncontingent delivery of food pellets, but prevented (0.03–0.3 mg/kg) the potentiation by quinelorane, a dopamine D3 receptor-preferring agonist, of food-seeking behavior. A possible link between cannabinoid processes and D3- and/or D2-mediated dopaminergic transmission was further investigated by studying Fos protein expression in cortico-limbic structures in D3 (D3−/−) and D2 (D2−/−) knockout mice. Rimonabant (10 mg/kg) increased Fos immunoreactivity in the prefrontal cortex (pFCortex) and in the shell but not the core of the nucleus accumbens (NAcc). Fos induction by this dose of rimonabant was not seen in mice lacking CB1 receptors, providing clear evidence for the involvement of CB1 receptors. In the NAcc shell, the effect of rimonabant was suppressed in D3−/−, but remained unchanged in D2−/− mice. In contrast, Fos expression by rimonabant in the pFCortex was impervious to D2 or D3 receptor deletion. In conclusion, these data indicate first that rimonabant prevented the enhancement by quinelorane of the appetitive value of food pellets unexpectedly delivered during extinction and second that rimonabant effects might involve D3 receptor-mediated processes. Overall, these results are consistent with the notion that endocannabinoid functions control brain reward processes and in particular the capacity of explicit stimuli to precipitate food-seeking behavior.
Similar content being viewed by others
INTRODUCTION
Converging electrophysiological, biochemical, and behavioral evidence supports the notion of a cannabinoid (CB) link in the neurobiological events allowing the perception of the rewarding value of various kinds of reinforcers. The selective CB1 receptor antagonist, rimonabant (Rinaldi-Carmona et al, 1994; Chaperon and Thiébot, 1999), has been shown to impair the perception of the motivational value of not only a CB receptor agonist, CP 55,940 (Braida et al, 2001) but also noncannabinoid compounds such as cocaine, morphine, or food (Chaperon et al, 1998), using place conditioning procedures in rats. Rimonabant has also been found to decrease the sensitivity to the reinforcing effects of intracranial stimulation (Deroche-Gamonet et al, 2001; but see Arnold et al, 2001), and to reduce self-administration of nicotine, ethanol, morphine, and methamphetamine (Arnone et al, 1997; Navarro et al, 2001; Cohen et al, 2002; Vinklerová et al, 2002), as well as the intake of palatable food (Arnone et al, 1997).
The rewarding effects of primary reinforcers such as palatable food and drugs of abuse, or the appetitive effects of environmental stimuli associated with food or drug intake have been related to enhanced dopamine (DA) release in cortico-limbic structures, including the nucleus accumbens (NAcc) and the preferontal cortex (pFCortex) (Phillips et al, 1991; Spanagel and Weiss, 1999; Ito et al, 2000; Beaufour et al, 2001; Bassareo et al, 2002). It has been hypothesized that rimonabant might modulate the motivational effects of drugs or natural reinforcers through its capacity to block disinhibitory effects indirectly exerted by endocannabinoids on dopaminergic neurons in meso-limbic structures (Schlicker and Kathmann, 2001). Thus, using rat brain microdialysis, rimonabant has been shown to block DA release induced by CB agonists, nicotine, and ethanol in limbic structures (Tanda et al, 1997; Cohen et al, 2002). In addition, the DA-releasing effects of morphine and ethanol were reduced in CB1 knockout mice (Mascia et al, 1999; Hungund et al, 2003). Consonant with a possible role for cannabinoid transmission in appetitive motivational processes, rimonabant enhanced Fos expression within rat cortico-limbic structures (NAcc shell, ventrolateral septum, pFCortex), while motor-related structures (NAcc core, dorsolateral caudate–putamen) were unaffected (Alonso et al, 1999).
Evidence suggests that explicit, contextual, or emotional factors such as drug priming injection, drug-associated environmental stimuli, or stress, can precipitate relapse to drug self-administration in animals. Rimonabant has been shown to prevent the reinstatement of extinguished cocaine or heroin self-administration behavior by presentation of drug-associated stimuli or by a priming dose of cocaine or heroin (De Vries et al, 2001; Fattore et al, 2003). Interestingly, this occurred at low doses that did not impair cocaine or heroin self-administration (Fattore et al, 1999; De Vries et al, 2001; Navarro et al, 2001). The present study investigated whether a cannabinoid step was involved in the processes subserving food-seeking behavior. Recently, Duarte et al (2003a, 2003b) demonstrated that extinguished food reinforced lever pressing could be reinstated by experimenter-delivered food pellets. Response reinstatement induced by food priming was clearly potentiated by very low doses of quinelorane, a DA D3 receptor preferring full agonist (Levant, 1997), which did not promoted food-seeking behavior on its own, but not by BP 897, a more selective, but partial, D3 receptor agonist (Pilla et al, 1999). The effects of rimonabant on response reinstatement induced by the noncontingent delivery of two food pellets and its potentiation by quinelorane were assessed in rats subjected to a within-session extinction procedure. A possible link between cannabinergic processes and D3- vs D2-mediated dopaminergic transmission was further investigated by studying the capacity of rimonabant to increase Fos protein expression in cortico-limbic structures in D3 vs D2 knockout mice.
MATERIALS AND METHODS
Behavioral experiments were conducted at the INSERM Research Unit U. 288 (Paris, France), and immunohistochemistry was performed at Sanofi-Synthélabo (Montpellier, France). In each case, all the experiments were conducted in accordance with the institutional guidelines for use of animals and their care, in compliance with national and international laws and policies (Council directive no. 87–848, October 19, 1987, Ministère de l'Agriculture et de la Forêt, Service Vétérinaire de la Santé et de la Protection Animale).
Animals
Behavioral experiments were carried out on male Wistar AF rats (Centre d'Élevage R Janvier, Le Genest, France) weighing 100±10 g at the beginning of the training and 250–300 g at the time of the test sessions (ca 3 months later). They were housed eight per cage with free access to water in their home cage. At 1 week prior to the beginning of the conditioning, rats were placed on a daily schedule of food restriction (105 g of standard chow per day for eight rats), which was maintained until the end of the experiments. Heterozygous D2 male and female mice (D2+/−) were supplied by Dr E Borrelli (IGBMC, Strasbourg, France) (Baik et al, 1995). Mating these mice gave birth to homozygous D2-deficient (D2−/−) and control (D2+/+) mice used for the experiments. Genotypes were determined by PCR analysis of products derived from tail genomic DNA. Homozygous D3-deficient (D3−/−) male and female C57BL/6J-Drd3tmlDac mice (Accili et al, 1996) were supplied by The Jackson Laboratory (Bar Harbor, ME, USA) and bred at Sanofi-Synthélabo Recherche. This D3 knockout strain was back-crossed five times to the C57BL/6J genetic background, which resulted in a 95% C57BL/6J genome. Controls (D3+/+) were C57BL/6JIco mice (Iffa-Credo, Lyon, France) as recommended by The Jackson Laboratory. Cannabinoid CB1 receptor knockout mice (CB1−/−) were from a C57BL/6x129/Ola F2 genetic background and generated as described previously (Ravinet-Trillou et al, 2003).
Male wild-type and mutant mice were housed separately in groups of four with food and water available ad libitum. The age of mice ranged from 2 to 3 months and their weight varied between 22 and 25 g at the time of testing. All animals were housed in a temperature- and humidity-controlled room, with a 12-h light–dark cycle.
Drugs
Rimonabant base (Sanofi-Synthélabo, France) was suspended in Tween 80 (0.01%) in saline (0.9% NaCl) or distilled water and injected intraperitoneally (i.p.). Quinelorane HCl (Eli-Lilly, Indianapolis, IN, USA) was dissolved in saline and administered subcutaneously (s.c.). Drugs, or their vehicle, were administered in a volume of 5 ml/kg body weight in rats and 20 ml/kg in mice. The doses (expressed as the base or the salt, as appropriate) were based on previous published data (Chaperon et al, 1998; Alonso et al, 1999; Duarte et al, 2003a). In particular, the dose of rimonabant used in the immunohistochemistry study was linked to the sensitivity of the method and voluntarily high in order to amplify Fos responses and differences between D3 and D2 knockout mice.
Experiment 1—Behavioral Study
Apparatus
The experiments were conducted in four standard ventilated, sound-attenuated operant chambers (Campden Instruments Ltd, UK). Each cage (24 × 22 × 20 cm) was fitted with a grid floor, white stimulus lights (24 V; 3 W), and a food tray located between two levers. The operant schedules were automatically controlled and the behavioral data were collected by an Acorn computer with software written in Arachnid version of BASIC (CeNeS Cognition, UK), situated in an adjoining room.
Reinstatement procedure
Training and test sessions were conducted as previously described by Duarte et al (2003a). Rats were trained to press the right lever according to a fixed ratio 1 (FR1) schedule of food reinforcement (45 mg pellets, Bioserv, Frenchtown, NJ, USA). The light located above the right lever provided the sole illumination of the chamber. After stabilization of FR1 responding, an FR1:TO10s schedule was initiated, that is, each lever press delivered one pellet and initiated a 10-s time-out (TO) period, signaled by the extinction of the light. During TO, responses had no scheduled consequence. Once the baseline rate of lever pressing was achieved, the sessions were lengthened to 60 min and divided into two components. During the initial 15-min period, the FR1:TO10s schedule was in effect as during the previous sessions (reward component). During the next 45-min period, right lever presses no longer resulted in food delivery (extinction component). The sound of the lever and the 10-s light off stimuli associated with responding were the same as during the reward component, but food-paired stimuli, that is, the sounds generated by the food dispenser and the pellet falling into the tray, were no longer present. No discriminative stimulus was associated with the two components of the session. The number of appropriate right lever presses in the presence of the light on signal, the number of inappropriate right lever presses during the TO periods and the number of responses on the left lever (always inactive), were recorded every minute. During the extinction component, lever pressing progressively diminished and, after about five sessions, rats emitted less than five responses during the final 40 min of the session.
Rats were habituated to the injection procedure by receiving saline (i.p.) immediately before the session, and saline (s.c.), 30 min after the beginning of the session. The operant schedule and data recording were stopped for the injection duration.
After stabilization of responding, rimonabant/quinelorane interaction studies were conducted during the course of test sessions scheduled as training sessions (15-min reward period followed by a 45-min extinction period), but during which two food pellets were noncontingently delivered, together with food-paired stimuli, at the end of the 45th minute, during the extinction component. Rimonabant (0.03–0.1–0.3 mg/kg) was injected i.p., immediately before the test session and quinelorane (15 μg/kg) was administered s.c., 30 min after the beginning of the test session.
The potentiation of the reinstating effect of two noncontingent pellets by quinelorane and the effects of rimonabant thereon were investigated during the course of three test sessions, conducted 2 weeks apart. Before each test session, rats were divided into treatment groups of six to nine animals according to their performance during the preceding training session, defined as the baseline. Rats were matched for the number of right lever presses emitted in the presence of the light on signal, during the 46–60-min time interval (extinction component), the number of food-reinforced responses and the number of TO right lever presses, recorded during the 15-min rewarded component. The same dose of rimonabant was never injected twice to the same rat on successive test sessions. Each individual test session included a vehicle+saline and a vehicle+quinelorane group of rats; between-session results of these two groups were not statistically different, and were pooled for the analysis of the entire dose-range study. This experimental schedule accounts for the between-group differences in the number of animals in each experimental group. Between successive test sessions, rats were subjected to eight additional training sessions.
Data analysis
The results are the mean (±SEM) number of right lever presses performed in the presence of the ‘light on' signal, defined as ‘appropriate' responses, during the final 15 min of the extinction component. The numbers of right lever presses in the presence of the ‘light on' signal during the initial 15-min rewarded period, and presses of the inactive left lever, are also reported where appropriate. Data were analyzed by one-way analysis of variance (ANOVA), followed by planned pairwise comparisons between drug and vehicle groups using two-tailed Dunn's t-tests. Within-group comparisons to baseline responding recorded during the previous training session were made using two-tailed paired Student's t-test.
Experiment 2—Fos Immunohistochemistry
Immunohistochemistry
At 2 h after rimonabant (10 mg/kg, i.p.) administration, mice were deeply anesthetized with sodium pentobarbital (80 mg/kg, i.p.) and perfused transcardially with saline followed by 4% (w/v) paraformaldehyde in 0.1 M phosphate-buffered saline (PBS; pH 7.4). Brains were removed and allowed to postfix overnight in 4% paraformaldehyde. Coronal sections (50 μm) were cut from each brain using a Vibratome (Leica). Immunohistochemistry was performed on free-floating tissue sections according to a standard avidin–biotin–peroxidase procedure using an anti-Fos rabbit polyclonal antibody (Sc-52, Santa Cruz Biotechnology Inc., Santa Cruz, CA, USA). Sections were rinsed with 0.02 M PBS and then pretreated for 10 min at room temperature with the same buffer containing 0.3% hydrogen peroxide. Next, they were rinsed three times in PBS, incubated with PBS containing 5% normal goat serum (Vector Laboratories, Burlingame, CA, USA) for 1 h at room temperature, and placed for 72 h at 4°C in the Fos primary antiserum (diluted 1 : 10000) solution, in PBS containing 1% normal goat serum and 0.3% Triton X-100. Thereafter, the sections were incubated successively with a biotinylated goat anti-rabbit secondary antibody (Vector Laboratories; diluted 1 : 300) for 2 h at room temperature and with an avidin-biotinylated horseradish peroxidase complex (Vector Laboratories; diluted 1 : 200) for 3 h at room temperature, in PBS containing 0.1% Triton X-100. The reaction product was visualized with diaminobenzidine in the presence of nickel. Sections were mounted, air-dried, dehydrated, and covered with Acrytol. Omission of the primary antibody from the immunohistochemical procedure or preadsorption of this antibody with a synthetic peptide corresponding to the N-terminal antigenic sequence of the Fos protein (Sc-52P, Santa Cruz Biotechnology Inc.) eliminated Fos immunoreactivity.
Data analysis
Sections were imaged through a Leica DMRB microscope and the Fos immunoreactive signal was quantified with an image analysis system (Samba Technologies, Meylan, France) by counting the number of Fos-positive cells within square areas (0.25 mm2) positioned within the pFCortex, the shell, and the core compartments of the NAcc. Particular attention was paid to the sample treatment and image analysis procedure to ensure automated counts as close as possible to manual counts. In each experiment, sections from wild-type and knockout mice were treated in parallel, and the duration of DAB exposure was strictly controlled to minimize the influence of variations in background staining. Before each section analysis, an empty field image was captured to correct for possible variations in transmitted light intensity.
For each section, the counting areas were outlined on a reduced image of the whole section built from images of connected fields captured at low magnification ( × 4). Analysis was subsequently performed at a higher magnification ( × 10) on automatically taken images of the counting areas. Cells were localized within digital images using a top hat transform to correct for trends in background values. Artifacts were removed following the filtration of segmented objects, and computation of Fos-positive cells was performed on the basis of the area criterion (mean area=25 μm2). We have determined in pilot experiments that this procedure gave automated counts that did not vary for more than 6% from manual counts.
Three to four sections per mouse were analyzed at each anatomical level, and the data were averaged. The number of immunoreactive cells (mean±SEM of six to nine mice per group) were analyzed by two-way (genotype, drug treatment) ANOVAs, followed by planned pairwise comparisons between drug- and vehicle-treated mice, and between wild-type and knockout mice given rimonabant, using two-tailed Dunn's t-tests.
RESULTS
Experiment 1—Behavioral Study
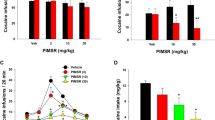
The results are shown in Figure 1. During training sessions, rats usually emitted 0–1 response on the right lever in the final 15-min interval of the extinction component. Whatever the treatment group, the animals consumed the two pellets noncontingently delivered 45 min into the test session. In control rats, food-priming reinstated nonreinforced appropriate right lever presses during 3–4 min after food collection. This effect was small (four to five presses, on average), but was stable across the test sessions and statistically significant compared to individual baseline responses recorded during the 45–60-min interval of the preceding training session (t=6.07; p<0.0001). The ANOVA indicated an overall treatment effect on the responses made in the presence of the light on signal (F5,92=5.36; p<0.0002). Planned pairwise comparisons showed that compared to control performance, quinelorane (15 μg/kg, 30 min into the test session) significantly increased the number of nonrewarded lever presses emitted after noncontingent pellet delivery (t=4.55; p<0.01). The administration of rimonabant (0.03–0.3 mg/kg) prior to the session prevented the enhancement by quinelorane of food-induced response reinstatement, and this effect was significant at 0.1 and 0.3 mg/kg (t=3.71 and 3.17, respectively; p<0.01). In the absence of quinelorane, rimonabant (0.3 mg/kg) did not modify food-induced response reinstatement (t=0.35; NS). After noncontingent pellet delivery, the number of left (inactive) lever presses, and right lever presses during the TO periods, did not differ across the treatment groups (F5,92=0.36 and 1.65, respectively; NS), although the number of TO responses per TO period was significantly higher in the vehicle–saline control group as compared to all other groups (F5,84=3.26; p<0.01; lowest t=2.55; p<0.05) (Table 1). The number of pellets obtained during the initial 15-min reinforced period (ie before quinelorane injection and noncontingent pellet delivery) was not modified by rimonabant (vehicle (n=25+28): 81.68±0.56; 0.03 mg/kg (n=6): 82.50±0.85; 0.1 mg/kg (n=16): 80.88±0.53; 0.3 mg/kg (n=8+15): 81.38±0.60; F3,92<1; NS).
Reversal by rimonabant of the potentiation by quinelorane of response reinstatement induced by noncontingent pellet delivery. Histograms represent the mean (+SEM) number of nonreinforced appropriate right lever presses performed during the 46–60-min extinction component, after noncontingent delivery of two food pellets at the 45th minute of the test session. Rats received rimonabant (0.03–0.3 mg/kg, i.p.), or its vehicle (0), immediately before the test session, and quinelorane (15 μg/kg, s.c.), or saline (0), 30 min into the test session. The black histogram indicates baseline performance without noncontingent pellet delivery, that is, the mean number of nonreinforced presses during the final 15 min of the preceding training session. ††p<0.01; vehicle+saline control group vs baseline responding (paired Student's t-test). **p<0.01; rats given quinelorane alone vs vehicle+saline control group; ‡‡p<0.01; rats given rimonabant+quinelorane vs quinelorane alone (Dunn's t-test after ANOVA).
Experiment 2—Fos Immunohistochemistry
Fos-inducing effects of rimonabant in D2+/+ vs D−/− mice
In D2+/+ mice, rimonabant increased the number of Fos immunoreactive cells in the shell compartment of the NAcc and in the pFCortex, while Fos expression was not affected in the NAcc core. A similar pattern of Fos expression was found following rimonabant administration in D2−/− mice (Figure 2). In the NAcc shell, two-way ANOVA showed a significant drug effect (F1,28=65.28; p<0.0001), no genotype effect (F1,28=3.27; p=0.08), and no drug × genotype interaction (F1,28<1; NS). Pairwise comparisons indicated that rimonabant significantly increased the number of Fos immunoreactive cells in the NAcc shell of D2+/+ (vs Veh, t=4.84; p<0.01) and D2−/− mice (vs Veh, t=6.72; p<0.01). Following rimonabant administration, the number of Fos-positive cells was not significantly different in D2−/− and D2+/+ mice (t=1.85; NS).
Effect of rimonabant on Fos expression in D2+/+ and D2−/− mice. Animals were given either vehicle (Veh) or rimonabant (SR, 10 mg/kg, i.p.), 2 h before being killed. Data are the number (mean+SEM) of Fos-positive cells counted within the indicated brain region (n=7 in D2+/+ and n=9 in D2−/− mice for each treatment group). **p<0.01 rimonabant vs vehicle, in mice of the same genotype; NS: not significant (Dunn's t-test after ANOVA).
In the NAcc core, there was no significant main effect for drug treatment (F1,28=2.02; NS), no genotype effect (F1,28=2.73; NS), and no drug × genotype interaction (F1,28<1; NS). In this brain region, rimonabant did not modify the number of Fos immunoreactive cells in D2−/− or D2+/+ mice.
In the pFCortex, the two-way ANOVA showed a significant main effect for drug treatment (F1,28=40.78; p<0.0001), no genotype effect (F1,28=3.86; p=0.06), and no drug × genotype interaction (F1,28<1; NS). Further comparisons indicated that rimonabant significantly increased Fos immunolabeling in D2+/+ (vs Veh, t=4.11; p<0.01) and D2−/− mice (vs Veh, t=4.99; p<0.01) mice, this effect being not significantly different between the two genotypes (D2−/− vs D2+/+, t=1.54; NS).
Fos-inducing effects of rimonabant in D3+/+ vs D3−/− mice
Similarly to that was obtained in D2+/+ mice, D3+/+ mice given rimonabant (10 mg/kg) exhibited significant higher Fos counts than vehicle-injected mice in the NAcc shell (but not in the core compartment) and in the pFCortex. The Fos-inducing effect of rimonabant in mice lacking D3 receptors was totally suppressed in the NAcc shell and remained unaffected in the pFCortex (Figure 3).
Effect of rimonabant on Fos expression in D3+/+ and D3−/− mice. Animals were given either vehicle (Veh) or rimonabant (SR, 10 mg/kg, i.p.), 2 h before being killed. Data are the number (mean+SEM) of Fos-positive cells counted within the indicated brain region (n=8 in D3+/+ and D3−/− mice for each treatment group). *p<0.05; **p<0.01 rimonabant vs vehicle, in mice of the same genotype; ††p<0.01 vs rimonabant-treated D3+/+ mice; NS: not significant (Dunn's t-test after ANOVA).
In the NAcc shell, two-way ANOVA indicated significant main effects for drug treatment (F1,28=40.78; p<0.0001), genotype (F1,28=20.95; p<0.0001), and drug × genotype interaction (F1,28=32.17; p<0.0001). Pairwise comparisons showed that rimonabant significantly increased the number of Fos immunoreactive cells in D3+/+ (vs Veh, t=9.25; p<0.01), but not D3−/− mice (vs Veh, t=1.23; NS), thus resulting in a significant difference in rimonabant-induced Fos expression between the two genotypes (t=7.25; p<0.01).
In the NAcc core, there was no significant main effect for drug treatment (F1,28=3.47; p=0.07), no genotype effect, and no drug × genotype interaction (both F1,28<1; NS). In this brain region, the number of Fos immunoreactive cells was not altered by rimonabant in D3+/+ or D3−/− mice.
In the pFCortex cortex, there was a significant drug effect (F1,28=28.34; p<0.0001), no genotype effect (F1,28=1.16; NS), and no interaction (F1,28=2.94; p=0.09). Pairwise comparisons indicated that rimonabant significantly increased cortical Fos immunolabeling in D3+/+ (vs Veh, t=4.98; p<0.01) and D3−/− (vs Veh, t=2.55; p<0.05) mice, this effect being not significantly different between the two genotypes (D3−/− vs D3+/+, t=1.97; NS).
Fos-inducing effects of rimonabant in CB1+/+ vs CB1−/− mice
As observed in the other wild-type groups, CB1+/+ mice given rimonabant (10 mg/kg) exhibited significant higher Fos counts than vehicle-injected mice in the NAcc shell (but not in the core compartment) and in the pFCortex. The Fos-inducing effect of rimonabant in CB1−/− mice was totally suppressed in the NAcc shell and the pFCortex (Figure 4). The number of Fos-positive cells in vehicle-treated CB1−/− mice tended to be higher than that observed in wild-type mice, but this effect did not reach statistical significance except in the NAcc shell (p<0.05).
Effect of rimonabant on Fos expression in CB1+/+ and CB1−/− mice. Animals were given either vehicle (Veh) or rimonabant (SR, 10 mg/kg, i.p.), 2 h before being killed. Data are the number (mean+SEM) of Fos-positive cells counted within the indicated brain region (n=7 in vehicle- and n=6 in rimonabant-injected mice of each genotype). **p<0.01 rimonabant vs vehicle, in mice of the same genotype. *p<0.05 vs vehicle-treated CB1+/+ mice. ††p<0.01 vs rimonabant-treated CB1+/+ mice (Dunn's t-test after ANOVA).
In the NAcc shell, two-way ANOVA indicated significant main effects for drug treatment (F1,22=23.28; p<0.0001) and drug × genotype interaction (F1,22=36.80; p<0.0001); the genotype effect failed to reach the level of statistical significance (F1,22=3.96; p=0.059). Pairwise comparisons showed that rimonabant significantly increased the number of Fos immunoreactive cells in CB1+/+ (vs Veh, t=7.70; p<0.01), but not CB1−/− mice (vs Veh, t=0.88; NS), thus resulting in a significant difference in rimonabant-induced Fos expression between the two genotypes (t=5.49; p<0.01).
In the NAcc core, there was no significant effect for drug, genotype, and drug × genotype interaction (all F1,22⩽1.05; NS). In this brain region, the number of Fos immunoreactive cells was not altered by rimonabant in CB1+/+ or CB1−/− mice.
In the pFCortex cortex, there were significant main effects for drug treatment (F1,22=22.45; p<0.0001), genotype (F1,22=13.24; p<0.002), and drug × genotype interaction (F1,22=34.12; p<0.0001). Pairwise comparisons indicated that rimonabant significantly increased cortical Fos immunolabeling in CB1+/+ (vs Veh, t=7.48; p<0.01), but not in CB1−/− (vs Veh, t=0.78; NS) mice, thus resulting in a significant difference in rimonabant-induced Fos expression between the two genotypes (t=6.46; p<0.01).
DISCUSSION
There are two main findings in the present study that suggest an impact of the selective CB1 receptor antagonist, rimonabant, on DA D3-mediated reward-related processes. Firstly, in rats subjected to a reinstatement procedure, rimonabant prevented the potentiation of relapse to food-seeking behavior by the D3 receptor preferring agonist, quinelorane. Secondly, rimonabant increased Fos expression in cortico-limbic structures, NAcc (shell, but not core) and pFCortex. Its effect in the NAcc shell was abolished in D3, but not D2, knockout mice, whereas rimonabant-induced Fos activation was present in the pFCortex of both types of mutant mice.
In the operant procedure, the noncontingent delivery of two food pellets induced a modest, but statistically significant, reinstatement of extinguished responding, an effect considered as an estimate of food seeking. As previously reported, a very low dose of quinelorane (15 μg/kg) potentiated this effect since, following noncontingent pellet delivery, nonrewarded responses were about three-fold higher in drug- than in saline-injected rats. This effect unlikely resulted from a nonselective behavioral stimulation since responses on the left (inactive) lever and right lever presses during light off TO per TO periods initiated remained very low during the reinstatement period. On the contrary, some studies in mice reported a reduction of motor activity after i.p. injection of low doses of quinelorane (Boulay et al, 1999; Frances et al, 2001). Motor activity has not been measured but even if quinelorane induced some hypoactivity in the present experimental conditions, the enhancement of food-primed response reinstatement occurred despite this effect and in no case can be accounted for by this effect. On the other hand, such a potentiation did not result from a direct activation of reward processes by quinelorane, since up to 65-fold higher doses had no reinstating action in nonprimed rats (Duarte et al, 2003a). Thus, quinelorane did not mimic the internal affective state present during food consumption, but enhanced the motivational strength of food reward. D3 receptors could play an important role in this effect. Indeed, in a previous study, the comparison of several DA agonists on reinstatement induced by food priming has shown that the more selective the agonist for D3 receptors, the more it was able to potentiate specifically the priming effect of food (Duarte et al, 2003a). This finding is consistent with evidence that the D3 receptor plays a role in drug-induced brain reward enhancement and drug seeking (Caine and Koob, 1993; Self et al, 1996; Cohen et al, 1998; Pilla et al, 1999; Vorel et al, 2002; Ashby et al, 2003; Le Foll et al, 2003). D3 receptor activation has also been shown to affect the appetitive valence of food or intracranial brain stimulation, however, both an increase and a reduction of the motivational value have been reported (Chaperon and Thiébot, 1996; Depoortere et al, 1996; Duarte et al, 2003a). It has been suggested that, depending on brain concentrations, D3 agonists might recruit different populations of receptors with opposite functional roles (possibly auto- vs hetero-receptors) and/or affect distinct neuronal pathways. However, a critical involvement of D2 receptors in the potentiation by quinelorane of relapse to food-seeking responses cannot be ruled out as the effect of quinelorane on food priming was abolished by D2, but not D3, receptor antagonists (Duarte et al, 2003a). It could be the case that mice with deletion of D3 or D2 receptor subtypes may help to unraveling the respective role of these two receptors in the action of quinelorane on food-primed food-seeking behavior. However, the reinstatement procedure is a rather complex paradigm and it is not certain that mice would be able to achieve such a task, which necessitates high cognitive capacities.
Rimonabant (0.1 and 0.3 mg/kg) prevented the potentiation by quinelorane of food-seeking behavior induced by pellet delivery, whereas it did not modify the reinstating effect of food-priming alone. The blockade of CB1 receptors has been reported to reduce food intake as measured in various procedures (Arnone et al, 1997; Simiand et al, 1998; Rowland et al, 2001). However, as already observed in a variety of operant schedules (Freedland et al, 2000; Navarro et al, 2001; Pério et al, 2001), responding during the rewarded period of the operant session was not modified by low doses of rimonabant, indicating that the reduction of response reinstatement did not result from effects on food consumption. In addition, Freedland et al (2000) showed that lever presses in an FR15 schedule of food reinforcement, a schedule that generates a high rate of responding (Ferster and Skinner, 1957), were not reduced by rimonabant (up to 0.3 mg/kg), suggesting that a rate-dependent effect unlikely accounted for the selective reduction of the potentiation by quinelorane of the reinstating effect of food priming. On the other hand, the possibility that the effects of rimonabant may be confused with a nonspecific motor effect can be ruled out since no deleterious effect on locomotor activity has been reported, even at doses larger than those used in the present study (Costa et al, 1999; Freedland et al, 2000).
The finding that rimonabant did not reduce the food-priming effect suggests that the blockade of CB1 receptors did not impair the responsiveness of rats to a signal for food availability. However, distinct from the present result, rimonabant has been reported to attenuate drug-induced cocaine- or heroin-seeking behavior in rats (De Vries et al, 2001; Fattore et al, 2003). Although one source of discrepancy between reports may be due, at least in part, to procedural differences (ie between- vs within-session extinction), these results might indicate that different neuronal circuits mediate a similar behavior driven by either drugs of abuse or natural reward. In fact, electrophysiological studies clearly indicated that cocaine, heroine, and food activated different neuronal populations in the NAcc (Chang et al, 1998; Miyazaki et al, 1998; Carelli et al, 2000). In contrast, rimonabant appears to reduce specifically the potentiation by quinelorane of the motivational strength of food pellets. Consonant with a possible role for endocannabinoid transmission in appetitive motivational processes (González et al, 2002; Poncelet et al, 2003), rimonabant blocked the acquisition of conditioned place preference to positive reinforcers such as food, morphine, or cocaine (Chaperon et al, 1998). Likewise, in rats, CB1 receptor antagonists reduced intracranial self-stimulation (Deroche-Gamonet et al, 2001; but see Arnold et al, 2001), self-administration of nicotine, ethanol, morphine, methamphetamine, or heroin (Arnone et al, 1997; Navarro et al, 2001; Cohen et al, 2002; Vinklerová et al, 2002), and rebound alcohol intake after transient deprivation in alcohol-consuming rats (Serra et al, 2002). However, the existence of an endogenous cannabinoid tone in basal conditions remains controversial since a rewarding effect of rimonabant has been reported in some studies (Sañudo-Peña et al, 1997; Cheer et al, 2000), but not in others (Chaperon et al, 1998; Hutcheson et al, 1998).
A possible link between cannabinergic processes and D3- vs D2-mediated dopaminergic transmission was investigated by studying Fos protein expression by rimonabant in cortico-limbic structures of mice lacking D3 or D2 DA receptors. In wild-type mice, rimonabant (10 mg/kg) activated Fos expression in the NAcc shell and the pFCortex, but not in the NAcc core. Similar structure-selective increases in Fos immunoreactivity by rimonabant have been found in rats (Alonso et al, 1999). These stimulatory effects were not seen in CB1 knockout mice, thus providing clear evidence of Fos induction by this dose of rimonabant to be dependent on CB1 receptors. It should be mentioned that vehicle- or rimonabant-treated mice displayed more Fos-positive counts than those determined in our previous work in rat. This can be explained firstly, by species differences—in our hands, Fos expression in basal conditions was reproducibly higher in mouse than in rat—and secondly, by the use of a different antibody that markedly improved the detection of Fos-positive cells.
Remarkably, the Fos-inducing effect of rimonabant in the NAcc shell was also suppressed in mice lacking D3 receptors, but remained unchanged in D2 mutant mice. In contrast, Fos expression by rimonabant in the pFCortex was impervious to D2 or D3 receptor deletion. The D3 knockout strain was back-crossed five times to the C57BL/6J (D3+/+) genetic background, which resulted in a 95% C57BL/6J genome. It is therefore unlikely that the lack of effect of rimonabant in D3−/− mice resulted from differences in the genetic background of D3+/+ and D3−/− mice, although this possibility cannot be totally discarded. It must also be noticed that vehicle administration resulted in higher Fos counts in D3 than D2 knockout mice in the brain regions analyzed. Whether this effect was accounted for by deletion of DA receptor subtypes and/or subtle differences in the genetic background of these animals cannot be excluded.
Although the mechanism by which rimonabant increased Fos expression and its dependency on D3 receptors cannot be addressed from the present study, several comments can be made. Our findings indicate that the stimulatory effect of rimonabant requires the presence of either CB1 or D3 receptors. While binding to CB1 receptors clearly accounts for the Fos-inducing effects of rimonabant, a direct action on D3 receptors is unlikely since rimonabant is devoid of affinity for DA receptor subtypes (Rinaldi-Carmona et al, 1994). It remains possible, however, that Fos expression by rimonabant in the NAcc may result from an action on neurons expressing D3 receptors. This latter hypothesis is consistent with two sets of data: the presence of CB1 receptors in these brain regions in the rat (Mailleux and Vanderhaeghen, 1992), and the capacity of rimonabant to increase neurotensin-like immunoreactivity in the NAcc (Alonso et al, 1999), where a majority of neurons expressing D3 receptor mRNA are neurotensin-containing neurons (Diaz et al, 1995).
Although our findings do not indicate that there is a causal relationship between Fos expression and behavior, they suggest that probably both depend on the permissive role of D3 receptors. In particular, the regulation of D3 receptor-mediated processes in the NAcc shell by endocannabinoid function could be central to the action of quinelorane on the hedonic aspect of food-primed food-seeking behavior. The NAcc shell, but not the core or the pFCortex, contains high density of D3 receptors (Diaz et al, 1995). Extensive evidence indicates that primary (eg food, cannabinoid agonists, and other drugs of abuse) and secondary (eg conditioned stimuli) appetitive reinforcers consistently increase DA transmission in the NAcc (Tanda et al, 1997; Ito et al, 2000; Bassareo et al, 2002). Moreover, DA innervation in NAcc shell has been shown to code for the motivational valence of stimuli (Bassareo et al, 2002; Everitt and Wolf, 2002) and, importantly, rimonabant was found recently to decrease DA release in the NAcc shell induced by reinforcing stimuli such as nicotine and ethanol (Cohen et al, 2002).
In conclusion, these results show first, that, rimonabant reduced the potentiation by the DA D3 receptor preferring agonist, quinelorane, of food-priming-induced response reinstatement whereas it did not modify the reinstating effect of food priming alone, suggesting that rimonabant might specifically prevent the enhancement by quinelorane of the motivational strength of food pellets. Second, rimonabant effects might involve D3 receptor-mediated processes. Overall, these results are consistent with the notion that endocannabinoid functions control brain reward processes and in particular might play a role in the effects of explicit stimuli on food-seeking behavior.
References
Accili D, Fishburn CS, Drago J, Steiner H, Lachowicz JE, Park BH et al (1996). Targeted mutation of the D3 dopamine receptor gene is associated with hyperactivity in mice. Proc Natl Acad Sci USA 93: 1945–1949.
Alonso R, Voutsinos B, Fournier M, Labie C, Steinberg R, Souilhac J et al (1999). Blockade of cannabinoid receptors by SR 141716 selectively increases Fos expression in rat mesocorticolimbic areas via reduced dopamine D2 function. Neuroscience 91: 607–620.
Arnold JC, Hunt GE, McGregor IS (2001). Effects of the cannabinoid receptor agonist CP 55,940 and the cannabinoid receptor antagonist SR 141716 on intracranial self-stimulation in Lewis rats. Life Sci 70: 97–108.
Arnone M, Maruani J, Chaperon F, Thiébot MH, Poncelet M, Soubrié P et al (1997). Selective inhibition of sucrose and ethanol intake by SR 141716, an antagonist of central cannabinoid (CB1) receptors. Psychopharmacology (Berl) 132: 104–106.
Ashby Jr CR, Paul M, Gardner EL, Heidbreder CA, Hagan JJ (2003). Acute administration of the selective D3 receptor antagonist SB-277011A blocks the acquisition and expression of the conditioned place preference response to heroin in male rats. Synapse 48: 154–156.
Baik JH, Picetti R, Saiardi A, Thiriet G, Dierich A, Depaulis A et al (1995). Parkinsonian-like locomotor impairment in mice lacking dopamine D2 receptors. Nature 377: 424–428.
Bassareo V, De Luca MA, Di Chiara G (2002). Differential expression of motivational stimulus properties by dopamine in nucleus accumbens shell versus core and prefrontal cortex. J Neurosci 22: 4709–4719.
Beaufour CC, Le Bihan C, Hamon M, Thiébot MH (2001). Extracellular dopamine in the prefrontal cortex during reward-, punishment- and novelty-associated behaviour: effects of diazepam. Pharmacol Biochem Behav 69: 133–142.
Boulay D, Depoortere R, Rostene W, Perrault G, Sanger DJ (1999). Dopamine D3 receptor agonists produce similar decreases in body temperature and locomotor activity in D3 knock-out and wild-type mice. Neuropharmacology 38: 555–565.
Braida D, Pozzi M, Cavallini R, Sala M (2001). Conditioned place preference induced by the cannabinoid agonist CP 55,940: interaction with the opioid system. Neuroscience 104: 923–926.
Caine SB, Koob GF (1993). Modulation of cocaine self-administration in the rat through D-3 dopamine receptors. Science 260: 1814–1816.
Carelli RM, Ijames SG, Crumling AJ (2000). Evidence that separate neural circuits in the nucleus accumbens encode cocaine versus ‘natural’ (water and food) reward. J Neurosci 20: 4255–4266.
Chang JY, Janak PH, Woodward DJ (1998). Comparison of mesocorticolimbic neuronal responses during cocaine and heroin self-administration in freely moving rats. J Neurosci 18: 3098–3115.
Chaperon F, Soubrié P, Puech AJ, Thiébot MH (1998). Involvement of central cannabinoid (CB1) receptors in the establishment of place conditioning in rats. Psychopharmacology (Berl) 135: 324–332.
Chaperon F, Thiébot MH (1996). Effects of dopaminergic D3-receptor-preferring ligands on the acquisition of place conditioning in rats. Behav Pharmacol 7: 105–109.
Chaperon F, Thiébot MH (1999). Behavioral effects of cannabinoid agents in animals. Crit Rev Neurobiol 13: 243–281.
Cheer JF, Kendall DA, Marsden CA (2000). Cannabinoid receptor and reward in the rat: a conditioned place preference study. Psychopharmacology (Berl) 151: 25–30.
Cohen C, Perrault G, Sanger DJ (1998). Preferential involvement of D3 versus D2 dopamine receptors in the effects of dopamine receptor ligands on oral ethanol self-administration in rats. Psychopharmacology (Berl) 140: 478–485.
Cohen C, Perrault G, Voltz C, Steinberg R, Soubrié P (2002). SR 141716, a central cannabinoid (CB1) receptor antagonist, blocks the motivational and dopamine-releasing effects of nicotine. Behav Pharmacol 13: 451–463.
Costa B, Vailati S, Colleoni M (1999). SR 141716A, a cannabinoid receptor antagonist, reverses the behavioural effects of anandamide-treated rats. Behav Pharmacol 10: 327–331.
Depoortere R, Perrault G, Sanger DJ (1996). Behavioural effects in the rat of the putative dopamine D3 receptor agonist 7-OH-DPAT: comparison with quinpirole and apomorphine. Psychopharmacology (Berl) 124: 231–240.
Deroche-Gamonet V, Le Moal M, Piazza PV, Soubrié P (2001). SR 141716, a CB1 receptor antagonist, decreases the sensitivity to the reinforcing effects of electrical brain stimulation in rats. Psychopharmacology (Berl) 157: 254–259.
De Vries TJ, Shaham Y, Homberg JR, Crombag H, Schuurman K, Dieben J et al (2001). A cannabinoid mechanism in relapse to cocaine seeking. Nat Med 7: 1151–1154.
Diaz J, Lévesque D, Lammers CH, Griffon N, Martres M-P, Schwartz J-C et al (1995). Phenotypical characterization of neurons expressing the dopamine D3 receptor in the rat brain. Neuroscience 65: 731–745.
Duarte C, Biala G, Le Bihan C, Hamon M, Thiébot MH (2003a). Respective roles of dopamine D2 and D3 receptors in food-seeking behaviour in rats. Psychopharmacology (Berl) 166: 19–32.
Duarte C, Lefebvre C, Chaperon F, Hamon M, Thiebot MH (2003b). Effects of a dopamine D(3) receptor ligand, BP 897, on acquisition and expression of food-, morphine-, and cocaine-induced conditioned place preference, and food-seeking behavior in rats. Neuropsychopharmacology 28: 1903–1915.
Everitt BJ, Wolf ME (2002). Psychomotor stimulant addiction: a neural systems perspective. J Neurosci 22: 3312–3320.
Fattore L, Martellotta MC, Cossu G, Mascia MS, Fratta W (1999). CB1 cannabinoid receptor agonist WIN-55,212-2 decreases intravenous cocaine self-administration in rats. Behav Brain Res 104: 141–146.
Fattore L, Spano MS, Cossu G, Deiana S, Fratta W (2003). Cannabinoid mechanism in reinstatement of heroin-seeking after a long period of abstinence in rats. Eur J Neurosci 17: 1723–1726.
Ferster CB, Skinner BF (1957). Schedules of reinforcement. Appleton-Century-Crofts: New York. pp 39–132.
Frances H, Smirnova M, Leyris A, Aymard N, Bourre J (2001). Influence of social isolation and 6-OHDA lesion on the effects of quinelorane. Pharmacol Biochem Behav 69: 143–149.
Freedland CS, Poston JS, Porrino LJ (2000). Effects of SR141716A, a central cannabinoid receptor antagonist, on food-maintained responding. Pharmacol Biochem Behav 67: 265–270.
González S, Cascio MG, Fernández-Ruiz J, Fezza F, Di Marzo V, Ramos JA (2002). Changes in endocannabinoid contents in the brain of rats chronically exposed to nicotine, ethanol or cocaine. Brain Res 954: 73–81.
Hungund BL, Szakall I, Adam A, Basavarajappa BS, Vadasz C (2003). Cannabinoid CB1 receptor knockout mice exhibit markedly reduced voluntary alcohol consumption and lack alcohol-induced dopamine release in the nucleus accumbens. J Neurochem 84: 698–704.
Hutcheson DM, Tzavara ET, Smadja C, Valjen E, Roques BP, Hanoune J et al (1998). Behavioural and biochemical evidence for signs of abstinence in mice chronically treated with 9-tetrahydrocannabinol. Br J Pharmacol 125: 1567–1577.
Ito R, Dalley JW, Howes SR, Robbins TW, Everitt BJ (2000). Dissociation in conditioned dopamine release in the nucleus accumbens core and shell in response to cocaine cues and during cocaine-seeking behavior in rats. J Neurosci 20: 7489–7495.
Le Foll B, Schwartz JC, Sokoloff P (2003). Disruption of nicotine conditioning by dopamine D(3) receptor ligands. Mol Psychiatry 8: 225–230.
Levant B (1997). The D3 dopamine receptor: neurobiology and potential clinical relevance. Pharmacol Rev 49: 231–252.
Mailleux P, Vanderhaeghen JJ (1992). Distribution of neural cannabinoid receptor in the adult rat brain: a comparative receptor binding radioautography and in situ hybridization histochemistry. Neuroscience 48: 655–668.
Mascia MS, Obinu MC, Ledent C, Parmentier M, Bohme GA, Imperato A et al (1999). Lack of morphine-induced dopamine release in the nucleus accumbens of cannabinoid CB(1) receptor knockout mice. Eur J Pharmacol 383: R1–R2.
Miyazaki K, Mogi E, Araki N, Matsumoto G (1998). Reward-quality dependent anticipation in rat nucleus accumbens. Neuroreport 9: 3943–3948.
Navarro M, Carrera MR, Fratta W, Valverde O, Cossu G, Fattore L et al (2001). Functional interaction between opioid and cannabinoid receptors in drug self-administration. J Neurosci 21: 5344–5350.
Pério A, Barnouin MC, Poncelet M, Soubrié P (2001). Activity of SR141716 on post-reinforcement pauses in operant responding for sucrose reward in rats. Behav Pharmacol 12: 641–645.
Phillips AG, Pfaus JG, Blaha CD (1991). Dopamine and motivated behavior: insights provided by in vivo analyses. In Willner P, Scheel-Krüger J (eds) The Mesolimbic Dopamine System: From Motivation to Action. John Wiley & Sons: Chichester. pp 199–224.
Pilla M, Perachon S, Sautel F, Garridol F, Mann A, Wermuth CG et al (1999). Selective inhibition of cocaine-seeking behaviour by a partial dopamine D3 receptor agonist. Nature 400: 371–375.
Poncelet M, Maruani J, Calassi R, Soubrié P (2003). Overeating, alcohol and sucrose consumption decrease in CB1 receptor deleted mice. Neurosci Lett 343: 216–218.
Ravinet-Trillou C, Arnone M, Delgorge C, Gonalons N, Keane P, Maffrand JP et al (2003). Anti-obesity effect of SR141716, a CB1 receptor antagonist, in diet-induced obese mice. Am J Physiol Regul Integr Comp Physiol 284: R345–R353.
Rinaldi-Carmona M, Barth F, Héaulme M, Shire D, Calandra B, Congy C et al (1994). SR 141716A, a potent and selective antagonist of the brain cannabinoid receptor. FEBS Lett 350: 240–244.
Rowland NE, Mukherjee M, Robertson K (2001). Effects of the cannabinoid receptor antagonist SR 141716, alone and in combination with dexfenfluramine or naloxone, on food intake in rats. Psychopharmacology (Berl) 159: 111–116.
Sañudo-Peña MC, Tsou K, Delay ER, Hohman AG, Force M, Walker JM (1997). Endogenous cannabinoids as an aversive or counter-rewarding system in the rat. Neurosci Lett 223: 125–128.
Schlicker E, Kathmann M (2001). Modulation of transmitter release via presynaptic cannabinoid receptors. Trends Pharmacol Sci 22: 565–572.
Self DW, Barnhart WJ, Lehman DA, Nestler EJ (1996). Opposite modulation of cocaine-seeking behavior by D1- and D2-like dopamine receptor agonists. Science 271: 1586–1589.
Serra S, Brunetti G, Pani M, Vacca G, Carai MA, Gessa GL et al (2002). Blockade by the cannabinoid CB(1) receptor antagonist, SR 141716, of alcohol deprivation effect in alcohol-preferring rats. Eur J Pharmacol 443: 95–97.
Simiand J, Keane M, Keane PE, Soubrié P (1998). SR-141716, a CB1 cannabinoid receptor antagonist, selectively reduces sweet food intake in marmoset. Behav Pharmacol 9: 179–181.
Spanagel R, Weiss F (1999). The dopamine hypothesis of reward: past and current status. Trends Neurosci 22: 521–527.
Tanda G, Pontieri FE, Di Chiara G (1997). Cannabinoid and heroin activation of mesolimbic dopamine transmission by a common μ1 opioid receptor mechanism. Science 276: 2048–2050.
Vinklerová J, Nováková J, Sulcová A (2002). Inhibition of methamphetamine self-administration in rats by cannabinoid receptor antagonist AM 251. J Psychopharmacol 16: 139–143.
Vorel SR, Ashby Jr CR, Paul M, Liu X, Hayes R, Hagan JJ et al (2002). Dopamine D3 receptor antagonism inhibits cocaine-seeking and cocaine-enhanced brain reward in rats. J Neurosci 22: 9595–9603.
Acknowledgements
These studies were supported, in part, by grants from INSERM. Christine Duarte was recipient of a grant from the European Community (REU 32, Account 64131/90, Hôpital Louis Mourier). We are grateful to Eli Lilly for the generous gift of quinelorane. We thank John Alexander for critically reading the manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Duarte, C., Alonso, R., Bichet, N. et al. Blockade by the Cannabinoid CB1 Receptor Antagonist, Rimonabant (SR141716), of the Potentiation by Quinelorane of Food-Primed Reinstatement of Food-Seeking Behavior. Neuropsychopharmacol 29, 911–920 (2004). https://doi.org/10.1038/sj.npp.1300370
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.npp.1300370
Keywords
This article is cited by
-
Moderation of antipsychotic-induced weight gain by energy balance gene variants in the RUPP autism network risperidone studies
Translational Psychiatry (2013)
-
Sensitization to cocaine is inhibited after intra-accumbal GR103691 or rimonabant, but it is enhanced after co-infusion indicating functional interaction between accumbens D3 and CB1 receptors
Psychopharmacology (2011)
-
Cannabinoids in Eating Disorders and Obesity
Molecular Neurobiology (2007)
-
Synergistic Interactions between Cannabinoids and Environmental Stress in the Activation of the Central Amygdala
Neuropsychopharmacology (2005)
-
Endocannabinoid control of food intake and energy balance
Nature Neuroscience (2005)