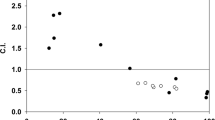
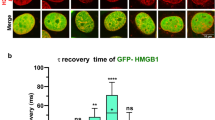
Abstract
There is a large discrepancy between the changes in drug accumulation and the changes in drug cytotoxicity that accompany development of anthracycline in multidrug-resistant cells. Moreover, although different molecular targets for anthracyclines such as DNA, cell membranes, or enzymes like topoisomerases could be involved, mechanisms by which these compounds exert their cytotoxic and differentiating effects remain unclear. Studies of correlation between the biological effects of anthracyclines and drug uptake have given conflicting conclusions. For example, a decrease in drug cytotoxicity for different incubation temperatures has been observed in spite of the same intracellular anthracycline amount, suggesting that temperature-dependent cytotoxic effects may be mediated by drug interaction with the cell membrane. What we propose in this review are results of our laboratory which are in agreement with an action mechanism targeted to the nucleus. In fact, we have shown by using microspectrofluorometry, that identical nuclear anthracycline concentration induces the same degree of cytotoxicity, independent of cellular MDR phenotype and the anthracycline structure. Thus, we could acquire information on the mechanisms of drug resistance related to drug transport. We could also give evidence that this accumulation is increased when MDR modulators, such as verapamil and S9788 and cyclosporin A or anthracyclines are used. For clinical applications, our studies have already dealt with nuclear concentration measurements of doxorubicin in leukocytes of treated patients, and in vitro measurements of drug efflux from nuclei of acute leukemic cells and its correlation with P-glycoprotein expression. However, in these studies, there was no correlation between anthracycline nuclear accumulation in vitro and P-glycoprotein expression. In addition, from preliminary results, we have shown that some modulators such as quinine do not significantly increase nuclear accumulation of anthracyclines in MDR cells but are able to restore anthracycline sensitivity. Other authors have recently shown that quinine has a relatively weak effect on cellular doxorubicin accumulation in MDR cells but is able to completely restore doxorubicin sensitivity. They concluded that quinine has essentially intracellular targets involved in drug distribution (cytoplasm → nucleus) from sequestration compartments. Our data contradict this and we believe that such modulator modifies the molecular environment of anthracyclines and/or their binding to a possible cytoplasmic target leading to different cell death. Thus, we conclude that mechanisms by which anthracyclines induce cell death, and ways by which chemotherapy fails in resistant cells remain complex and are related to more than one target.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Morjani, H., Millot, JM., Belhoussine, R. et al. Anthracycline subcellular distribution in human leukemic cells by microspectrofluorometry: factors contributing to drug-induced cell death and reversal of multidrug resistance. Leukemia 11, 1170–1179 (1997). https://doi.org/10.1038/sj.leu.2400668
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/sj.leu.2400668
Keywords
This article is cited by
-
Increased cellular accumulation and distribution of amrubicin contribute to its activity in anthracycline-resistant cancer cells
Cancer Chemotherapy and Pharmacology (2012)
-
Within the cell: analytical techniques for subcellular analysis
Analytical and Bioanalytical Chemistry (2005)
-
A Mathematical Model for Comparison of Bolus Injection, Continuous Infusion, and Liposomal Delivery of Doxorubicin to Tumor Cells
Neoplasia (2000)