Abstract
Study design:
Cross-sectional.
Objective:
To assess cough using air stacking (AS) to assist inspiratory volume with abdominal compression (AC) during expiration in patients with American Spinal Injury Association Impairment Scale (AIS) A.
Setting:
Large tertiary hospital in Chile.
Methods:
Peak cough flow (PCF) was measured during four different interventions: spontaneous maximal expiratory effort (MEE); MEE while receiving AC (MEE-AC); MEE after AS with a manual resuscitation bag (AS-MEE); and MEE with AS and AC (AS-MEE-AC).
Results:
Fifteen in-patients with complete tetraplegia (C4–C6) were included. Median age was 33 years (16–56). PCF during the different interventions was PCF for MEE was 183±90 l min−1; PCF for MEE-AC was 273±119 l min−1; PCF for AS-MEE was 278±106 l min−1 and PCF for AS-MEE-AC was 368±129 l min−1. We observed significant differences in PCF while applying MEE-AC and AS-MEE compared with MEE (P=0.0001). However, the difference in PCF value was greater using the AS-MEE-AC technique (P=0.00001).
Conclusion:
Patients with spinal cord injury (SCI) presented an ineffective cough that constitutes a risk factor for developing respiratory complications. The application of combined techniques (AS-MEE-AC) can reach near normal PCF values. This is a low-cost, simple and easily applied intervention that could be introduced to all patients with tetraplegia.
Similar content being viewed by others
Introduction
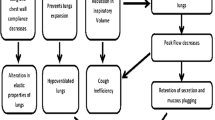
Patients with tetraplegia typically present with a restrictive respiratory pattern, reflected in reduced lung volumes and capacities and therefore commonly develop respiratory problems.1 Not only do they present with an inability to breathe deeply, but they also have an ineffective cough.2 This inability to clear secretions and lack of lung expansion has been reported to cause a higher incidence of respiratory complications.
Patients die mainly from respiratory complications, being pneumonia the most frequent cause of death during the first year post injury.3 According to the literature, two-thirds of patients with spinal cord injury (SCI) experience respiratory complications during the first year.4 The complexity of these complications varies depending on age, level and degree of injury.4 Injury level and degree have a direct effect on the impairment of the respiratory muscles. The higher the level, the more respiratory muscles will be affected and hence weakened.
Depending on the level of injury, a reduced vital capacity (VC) can be found if there is an inspiratory muscle paralysis and consequent decreased lung compliance.5 This may lead to the use of mechanical ventilation if the lesion is above C5. If the lesion involves the levels that innervate the abdominal muscles (T5–T12) then the cough is affected.6
Peak cough flow (PCF) is the maximum expiratory flow generated during a normal cough.2 Its intensity is directly related to the ability to inspire deeply, the capacity to generate an increase in intrapulmonary pressure with glottic closure and a forceful air expulsion created by contraction of the abdominal musculature.7 PCF is used to assess cough in patients with respiratory muscle weakness, particularly in patients with neuromuscular disease.8, 9 It has been successfully used as an indication of readiness for extubation and is a criterion that must be considered for tracheostomy decannulation.10 The assessment of cough effectiveness can be performed through PCF using a pneumotachometer or a portable flowmeter (generally used for measurements of peak expiratory flow by using, commonly, a mouthpiece).11
Expiratory flow values equal or below 160 l min−1 are considered as ineffective for removing airway secretions.10 Meanwhile, values equal or below 270 l min−1 generate an increased risk of developing respiratory complications, which has been demonstrated in patients with Duchenne Muscular Dystrophy (DMD).10
There are many techniques to improve cough and achieve higher PCF. Two of them, air stacking (AS) and abdominal compression (AC), are commonly used in cases of neuromuscular diseases, and have been proven to be efficient to improve expectoration in those specific populations.12 The AS technique consists of multiple inspiratory efforts delivered via a manual resuscitation bag, aiming to reach the individuals’ maximum insufflation capacity (MIC).9 This technique increases the inspired volume and replaces deep breaths, also contributing to improve thoracic mobility and possible prevention of atelectasis. Its greatest efficacy has been reported in patients with neuromuscular diseases, particularly in DMD and SCI.13 AC has been described as an assisted standard cough whereby the caregiver provides compression posteriorly and in cephalic direction through the abdomen which mimics the normal direction the diaphragm moves during a cough with functioning abdominals. This is preceded by several deep breaths with the assistance of the caregiver as the patient attempts to cough forcibly.14
We hypothesized that the PCF increases more with the use of combined techniques.
The aim of our study was to analyze the effects of AC and AS techniques on PCF.
Materials and methods
We recruited 15 patients with cervical SCI Association Impairment Scale (AIS) A from the Respiratory Rehabilitation Unit of Clínica Los Coihues, Santiago de Chile. The selection of patients was made using a convenience sample.
All subjects participated voluntarily and signed an informed consent form. The inclusion criteria were SCI AIS A within 1 year of initial SCI, ability to understand instructions, first admission to hospital and normal glottis function. We excluded subjects with ventilator support, tracheostomy, respiratory disease exacerbation during the previous month, smoking habits of >1 pack/year, comorbid medical disorders or inability to provide a signed consent form. The research was approved by the ethics committee.
We evaluated PCF using a MiniWright flow meter (Clement Clarke International, Essex, England) calibrated in liters per minute (l min−1), in four different interventions: (1) spontaneous maximal expiratory effort (MEE); (2) spontaneous MEE while receiving AC (MEE-AC); (3) spontaneous MEE after AS with a manual resuscitation bag (AS-MEE) and (4) spontaneous MEE with AS and AC (AS-MEE-AC). Each evaluation was performed with the subject in a semirecumbent position at 45°.15 The physiotherapist supervised and ensured an optimal lip occlusion around the peak flow meter mouthpiece to avoid any leaks that could affect the measurement. In addition, we used a nose clip. For the assessment, the order of interventions was randomized previously by an open source program to decrease the bias of a learning effect. The evaluation of PCF was performed until three maneuvers with less than a 10% difference between them were obtained. The time between each maneuver was 5 min to minimize fatigue.
We used a Wright spirometer (model Mark 14; Ferraris Development and Engineering Co, Ltd, London, UK) for VC and MIC. The MIC by AS was achieved by the patient taking a deep breath, holding it, and then using AS to consecutively deliver volumes of air until the maximum volume that could be held with a closed glottis was obtained.9
The VC and MIC assessment was performed until three maneuvers with less than a 10% difference between them were obtained.
To measure MIP and maximal expiratory pressure (MEP), an analog vacuum manometer (DHD Healthcare, New York, NY, USA) was used, calibrated from −120 to 0 cmH2O and from 0 to 120 cmH2O. The interface used was a rigid plastic flanged mouthpiece. To prevent air leakage, a nose clip was used. Pressure was registered with the system occluded at total pulmonary capacity for MEP and at residual volume for MIP. The record of the peak pressure obtained after the first second following initiation of the forced maneuver and sustained for at least 1 s was used for analysis.16
The MEE-AC consisted of the application of external pressure on the abdomen during the forced cough expiration phase, placing the therapist’s hands in the epigastric area, with spread fingers. The patient was asked to take a deep breath followed by glottis closure, and subsequently, an AC was applied by the physiotherapist from low to high, pushing the diaphragm up when the cough was produced.
For the AS-MEE maneuver, a manual resuscitation bag (model 5345; Manual Resuscitation Bags LIFESAVER, Hudson, Temecula, CA, USA) was used connected to a mouthpiece. The maximal capacity of this bag is 1600 ml. To inflate the patient during inspiration phase, the patient was asked to take a deep breath while the physiotherapist aided him by compressing the resuscitation bag manually. After that, and between each insufflation, the patient was instructed to hold the inhaled air, waiting for the next inspiration maneuver to obtain the largest possible inspiratory volume. After two or three insufflations, the subject was asked to cough as forcefully as possible to measure PCF.
For the AS-MEE-AC, PCF was measured after the application of AS-MEE followed by AC.
Statistical analysis
For the analysis of the sample data, the Stata software (version 10.1, Stata Corp, College Station, TX, USA) was used. For checking normal distribution, the Shapiro–Wilk normality test was applied. The results concluded that parametric statistics could be used, although the sample size was small.
Since the sample was observed at various times, the results are not independent samples, that is, the results correspond to the same subject during the different techniques. To compare the results, we applied ANOVA with repeated measures, with a confidence level of 95%. We estimate that using a level of 0.05 and a power of 90% will require a sample size of nine patients.
Results
A group of 15 patients with a median age of 33 years (range 16–56) with different levels of SCI (see Table 1) were included. The distribution by level of injury was C4 in 47%, C5 in 33% and C6 in 20%. Patients presented an alteration in the main pulmonary function values such as VC 2151±572 ml, MIC 3269±918 ml and peak expiratory flow 186.7±70.3 ml. The sample had a weakness of expiratory muscles with an MEP of 36.8±22.2 cmH2O and an MIP of 81.7±24.2 cmH2O (Table 1).
Regarding the PCF values obtained during the different maneuvers (Table 2), we observed 183±69 l min−1 during the MEE technique, 273±119 l min−1 with the MEE-AC technique, 278±106 l min−1 with the AS-MEE and 368±129 l min−1 using the AS-MEE-AC combined technique. Significant differences existed in PCF between MEE and the three maneuvers: MEE-AC, AS-MEE and AS-MEE-AC (P<0.0001 in all cases). When comparing MEE-AC vs AS-MEE, there were no statistically significant differences (P=0.86) (Figure 1).
Discussion
During our study we observed a low PCF in tetraplegic patients. When the physiotherapist applied the techniques during the inspiratory, the expiratory or both phases, a significant increase in PCF was obtained, which can transform the cough maneuver into an effective technique to expectorate secretions.
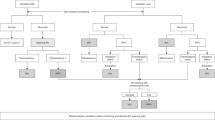
We found previous studies that investigated AC in patients with SCI (Figure 2). Kang et al.17 evaluated the relationship between the ability to cough and inspiratory muscle strength. A secondary objective was obtaining PCF difference between assisted and unassisted cough maneuvers. They observed similar results when the combined techniques, inspiratory insufflation plus manually assisted maneuvers, were applied. However, the study assessed 40 patients with SCI, including about 30% with SCI AIS B injury. Our study, although consisting of a smaller sample, only included subjects with SCI AIS A injury. Also, patients in the Kang study used sitting and supine positions. Our study adopted the semirecumbent position instead. The instability of the sitting position can lead to patients not being able to generate a maximum effort to cough. We therefore believe that the semirecumbent position is the more stable alternative for patients with complete cervical SCI.
Comparative PCF values obtained in the four studies of patients with complete tetraplegia. The figure represents the values obtained during different maneuvers applied to assist cough in the four studies done in complete tetraplegia patients.2, 17, 18 Unassisted: PCF obtained in spontaneous cough without assistance; Ins Vol Assist: PCF obtained after giving an extra inspiratory volume by resuscitation bag or positive-pressure pump, previous cough maneuver; Esp Manu Assist: PCF obtained with abdominal manual compression during cough; Combined: PCF obtained with the combination of inspiratory volume assisted maneuver plus expiratory abdominal manual compression. In all studies, except in Torres-Castro, all measurements were done in supine or sitting positions. The semirecumbent position increases patient stability and ensures efficient expiratory efforts during cough.
On the other hand, the Kirby study evaluated 12 tetraplegic patients using corsets, manual thoracic-AC and a mechanical insufflation device delivering a positive pressure of 50 cmH2O.18 Kirby concluded that an optimal cough resulted from combining positive insufflation pressure with manual assistance. The difference with our investigation lies in the use of a pump device that provides to the patients the maximum amount of air they can inhale. This is relevant given that cough depends mainly on the insufflation capacity, although a high pressure does not necessarily guarantee the amount of MIC can be reached. The physiologic mechanism involved is why the AS increases MIC thereby, raising the capacity for airflow generation. Also, AC increases intrathoracic pressure during expiration, which in turn increases expired airflow over and above what the patients are capable of given their respiratory muscle weakness.
The data obtained showed that our patients with a high level SCI had an average baseline PCF of 183 l min−1. According to Bach and Saporito10 this is considered a near to insufficient flow for generating an effective cough, increases mucus retention and risk for lung infection. However, the combination of AS and AC during the cough maneuver improves the average PCF up to the level of 368 l min−1, which indicates cough effectiveness compared with MEE.10
In patients with DMD, Kang and Bach9 and Ishikawa et al.8 concluded that it is necessary to produce high intrathoracic pressures to obtain an efficient cough when the expiratory muscles are weak by using manual methods that increase the ability to cough. Their results have supported those observed by Bach and Alba,19 who demonstrated the effectiveness of manually assisted cough in patients with high SCI. The results obtained in our study are consistent with the authors mentioned above, who described that during the assisted cough, the PCF increases significantly compared with the MEE. Bach and Alba’s research was performed in subjects with non-invasive ventilation dependence, who traditionally receive advanced respiratory care and have a restrictive respiratory pattern.19 We propose an early intervention in patients with an evolution of <1 year, given that in the long term, these subjects will have a restrictive respiratory pattern, and the AS with AC technique appears to be an effective alternative to assist cough preventing lung complications and decrease respiratory morbidity.
When analyzing each technique, the AS technique achieves an increase in inspiratory lung volume, stimulates alveolar recruitment, increases lung compliance, reduces atelectasis and improves the effectiveness of cough.13 Trebbia et al.20 conducted a study which demonstrated that MIC contributes 44%, the expiratory reserve volume 13% and the MEP 2% in a PCF increase in patients with neuromuscular diseases. The importance of this low-cost and highly effective technique, as observed in our study, where the PCF with AS-MEE technique was higher compared with the MEE technique, is that it produces a significant increase in PCF of 52%. The average PCF obtained when introducing the AS-MEE technique is 277 l min−1, which coincides with the results expressed by Bach,21 who observed that AS can increase the PCF above 160 l min−1. Comparing the PCF obtained during the MEE-AC vs AS-MEE techniques, there were no statistically significant differences in the studied population, indicating that on its own no technique is superior to the other. However, both are effective in increasing the PCF, which is consistent with the results shown by Brito et al.22 and Ishikawa et al.,8 observed in DMD.
In the case of DMD, the patient has a general weakness of all respiratory muscles. In contrast, in patients with SCI, the damage is variable. Some muscles may be unaffected, while others may be completely compromised. This means that patients with DMD will cough with weak muscles while patients with spinal injuries adopt strategies according to which muscles remain unaffected or are partially affected. This can change the outcome of assisted cough techniques.
Other techniques to assist cough strength are described in the literature. One of the best known is the use of mechanical in-exsufflator. Bach23 reported the use of assisted cough to improve PCF in individuals with neuromuscular disease among patients with SCI. Another method to normalize cough flow is electrical stimulation. Di Marco et al.24 used this technique in nine cases of cervical SCI where implants were inserted between the levels T9 and L1 and demonstrated that the pressures and the flows can reach normal values. While technology allows helping patients, there are some restrictions that must be taken into account. The strongest limitation, especially in underdeveloped countries, is the cost of technology. Second, managing technology can be complicated and restrictive. AS and AC are easy to learn and can be taught to relatives, caregivers and staff. Also, they could be applied at home. In these cases, the use of AS is a good and low-cost alternative for cough assistance.
The technique of manually assisted coughing can be performed in different ways, with rib compression, AC or the use of both depending on patient comfort and ease of implementation for the caregiver. Both techniques are effective: however, the risks of each must be considered (for example, risk of reflux by AC).
Finally, there are no studies of how the use of this technique in cases of SCI affects respiratory morbidity, hospital admissions or mortality. Further studies using these indicators to analyze the effectiveness of this intervention in long-term periods are needed.
A limitation of this study was the small sample size. However, it was above the calculated sample size necessary for statistical significance (n=9). Our results may have been affected by the level of SCI ranging between C4 and C6, where a higher level implies greater complications as discussed by Brown et al.25 This could be a limitation when trying to extrapolate our results to lower or higher level of SCI. In addition, the results could have been influenced by the patient’s motivation, which was affected after long periods of hospitalization.
In conclusion, the combination of AS with AC can achieve normal values of PCF in high SCI patients and expectorating airway secretions. This study contributes to generate stronger evidence for the use of these techniques to improve the efficacy of cough in any neurological disease that produces respiratory muscle impairment. The assisted cough and AS is cheaper and easier to teach caregivers and likely just as effective with potential to reduce chest infections, hospital admissions and death if used as a preventive method.
Improving the effectiveness of cough in patients with neurologic diseases is a goal that would achieve lower respiratory morbidity with subsequent lower utilization of health resources. In the natural evolution of neurological and neuromuscular diseases, cough assistance should be considered, not only as a therapeutic aim, but also on a preventive basis. This would ensure adequate mucociliary transport and increase the patient’s chances of survival and improve quality of life.
Data Archiving
There were no data to deposit.
References
Schilero GJ, Spungen AM, Bauman WA, Radulovic M, Lesser M . Pulmonary function and spinal cord injury. Respir Physiol Neurobiol 2009; 166: 129–141.
Braun SR, Giovannoni R, O’Connor M . Improving the cough in patients with spinal cord injury. Am J Phys Med 1984; 63: 1–10.
DeVivo MJ, Black KJ, Stover SL . Causes of death during the first 12 years after spinal cord injury. Arch Phys Med Rehabil 1993; 74: 248–254.
Jackson AB, Groomes TE . Incidence of respiratory complications following spinal cord injury. Arch Phys Med Rehabil 1994; 75: 270–275.
Urmey W, Loring S, Mead J, Slutsky AS, Sarkarati M, Rossier A et al. Upper and lower rib cage deformation during breathing in quadriplegics. J App Physiol 1986; 60: 618–622.
Estenne M, De Troyer A . Cough in tetraplegic subjects: an active process. Ann Intern Med 1990; 112: 22–28.
Chang AB . The physiology of cough. Paediatr Respir Rev 2006; 7: 2–8.
Ishikawa Y, Bach JR, Komaroff E, Miura T, Jackson-Parekh R . Cough augmentation in Duchenne muscular dystrophy. Am J Phys Med Rehabil 2008; 87: 726–730.
Kang SW, Bach JR . Maximum insufflation capacity. Chest 2000; 118: 61–65.
Bach JR, Saporito LR . Criteria for extubation and tracheostomy tube removal for patients with ventilatory failure. A different approach to weaning. Chest 1996; 110: 1566–1571.
Kang SW, Kang YS, Moon JH, Yoo TW . Assisted cough and pulmonary compliance in patients with Duchenne muscular dystrophy. Yonsei Med J 2005; 46: 233–238.
Bach JR, Ishikawa Y, Kim H . Prevention of pulmonary morbidity for patients with Duchenne muscular dystrophy. Chest 1997; 112: 1024–1028.
Boitano LJ . Equipment options for cough augmentation, ventilation, and noninvasive interfaces in neuromuscular respiratory management. Pediatrics 2009; 123 (Suppl 4): S226–S230.
Bain J, Bishop J, Olinsky A . Evaluation of directed coughing in cystic fibrosis. Br J Dis Chest 1988; 82: 138–148.
Alvisi V, Marangoni E, Zannoli S, Uneddu M, Uggento R, Farabegoli L et al. Pulmonary function and expiratory flow limitation in acute cervical spinal cord injury. Arch Phys Med Rehabil 2012; 93: 1950–1956.
Black LF, Hyatt RE . Maximal respiratory pressures: normal values and relationship to age and sex. Am Rev Respir Dis 1969; 99: 696–702.
Kang SW, Shin JC, Park CI, Moon JH, Rha DW, Cho D . Relationship between inspiratory muscle strength and cough capacity in cervical spinal cord injured patients. Spinal Cord 2006; 44: 242–248.
Kirby N, Barnerias MJ, Siebens A . An evaluation of assisted cough in quadriparetic patients. Arch Phys Med Rehabil 1966; 47: 705–710.
Bach JR, Alba AS . Noninvasive options for ventilatory support of the traumatic high level quadriplegic patient. Chest 1990; 98: 613–619.
Trebbia G, Lacombe M, Fermanian C, Falaize L, Lejaille M, Louis A et al. Cough determinants in patients with neuromuscular disease. Respir Physiol Neurobiol 2005; 146: 291–300.
Bach J . Air stacking for cough assistance. Muscle Nerve 2004; 30: 680–681.
Brito MF, Moreira GA, Pradella-Hallinan M, Tufik S . Air stacking and chest compression increase peak cough flow in patients with Duchenne muscular dystrophy. J Bras Pneumol 2009; 35: 973–979.
Bach JR . Mechanical insufflation-exsufflation. Comparison of peak expiratory flows with manually assisted and unassisted coughing techniques. Chest 1993; 104: 1553–1562.
DiMarco AF, Kowalski KE, Geertman RT, Hromyak DR, Frost FS, Creasey GH et al. Lower thoracic spinal cord stimulation to restore cough in patients with spinal cord injury: results of a National Institutes of Health-Sponsored clinical trial. Part II: clinical outcomes. Arch Phys Med Rehabil 2009; 90: 726–732.
Brown R, DiMarco AF, Hoit JD, Garshick E . Respiratory dysfunction and management in spinal cord injury. Respir Care 2006; 51: 853–868.
Acknowledgements
We thank Erika Díaz and Cintya Yañez for their contributions to the study design and analysis, and Anne Marie Bonnefoy and Alexander Plett for their contributions to the text translation.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Torres-Castro, R., Vilaró, J., Vera-Uribe, R. et al. Use of air stacking and abdominal compression for cough assistance in people with complete tetraplegia. Spinal Cord 52, 354–357 (2014). https://doi.org/10.1038/sc.2014.19
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sc.2014.19
Keywords
This article is cited by
-
Improvement in Pulmonary Function with Short-term Rehabilitation Treatment in Spinal Cord Injury Patients
Scientific Reports (2019)
-
Assessment of gas compression and lung volume during air stacking maneuver
European Journal of Applied Physiology (2017)