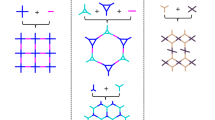
Abstract
Although the synthetic chemistry leading to interlocking molecular [n]catenanes of organic polyhedra (n = 2–3) and rings (n = 2–130) is established, the analogous chemistry that pertains to infinite three-dimensional systems ([∞]catenane) remains undeveloped. We report a series of [∞]catenane covalent organic frameworks (termed catena-COFs). These were synthesized by linking 4,4′-(1,10-phenanthroline-2,9-diyl)dibenzaldehyde to either of tris-(4-aminophenyl)-amine, -methane or -methanol through imine condensation. These combinations give discrete adamantane-like polyhedra, catenated by virtue of the copper(I) ions templating a mutually embracing arrangement of PDBs (points-of-catenation), which ultimately results in infinite catena-COF-805, 806 and 807. The crystal structures of these COFs obtained from electron microscopy and X-ray diffraction were determined to be isoreticular and to adopt the bor-y structure type.

This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
Crystallographic data for the structures reported in this Article have been deposited at the Cambridge Crystallographic Data Centre, under deposition numbers CCDC 2216102 (catena-COF-805), 2216103 (catena-COF-806) and 2216104 (catena-COF-807). Copies of the data can be obtained free of charge via https://www.ccdc.cam.ac.uk/structures/. All data are available in the main text or the Supplementary Information.
References
Wasserman, E. The preparation of interlocking rings: a catenane. J. Am. Chem. Soc. 82, 4433–4434 (1960).
Schill, G. & Lüttringhaus, A. The preparation of catena compounds by directed synthesis. Angew. Chem. Int. Ed. Engl. 3, 546–547 (1964).
Dietrich-Buchecker, C. O., Sauvage, J.-P. & Kintzinger, J. P. Une nouvelle famille de molecules: les metallo-catenanes. Tetrahedron Lett. 24, 5095–5098 (1983).
Ashton, P. R. et al. A [2] catenane made to order. Angew. Chem. Int. Ed. Engl. 28, 1396–1399 (1989).
Forgan, R. S., Sauvage, J.-P. & Stoddart, J. F. Chemical topology: complex molecular knots, links, and entanglements. Chem. Rev. 111, 5434–5464 (2011).
Gil-Ramírez, G., Leigh, D. A. & Stephens, A. J. Catenanes: fifty years of molecular links. Angew. Chem. Int. Ed. 54, 6110–6150 (2015).
Sauvage, J.-P. et al. Molecular Machines and Motors (Springer, 2001).
Browne, W. R. & Feringa, B. L. Making molecular machines work. Nat. Nanotechnol. 1, 25–35 (2006).
Kay, E. R., Leigh, D. A. & Zerbetto, F. Synthetic molecular motors and mechanical machines. Angew. Chem. Int. Ed. 46, 72–191 (2007).
Stoddart, J. F. Mechanically interlocked molecules (MIMs)—molecular shuttles, switches, and machines. Angew. Chem. Int. Ed. 56, 11094–11125 (2017).
Iino, R., Kinbara, K. & Bryant, Z. Introduction: molecular motors. Chem. Rev. 120, 1–4 (2020).
Wikoff, W. R. et al. Topologically linked protein rings in the bacteriophage HK97 capsid. Science 289, 2129–2133 (2000).
Fujita, M., Fujita, N., Ogura, K. & Yamaguchi, K. Spontaneous assembly of ten components into two interlocked, identical coordination cages. Nature 400, 52–55 (1999).
Hasell, T. et al. Triply interlocked covalent organic cages. Nat. Chem. 2, 750–755 (2010).
Zhang, G., Presly, O., White, F., Oppel, I. M. & Mastalerz, M. A shape-persistent quadruply interlocked giant cage catenane with two distinct pores in the solid state. Angew. Chem. Int. Ed. 53, 5126–5130 (2014).
Benke, B. P., Kirschbaum, T., Graf, J., Gross, J. H. & Mastalerz, M. Dimeric and trimeric catenation of giant chiral [8 + 12] imine cubes driven by weak supramolecular interactions. Nat. Chem. (2022). https://doi.org/10.1038/s41557-022-01094-w
Harrison, I. T. & Harrison, S. Synthesis of a stable complex of a macrocycle and a threaded chain. J. Am. Chem. Soc. 89, 5723–5724 (1967).
Anelli, P. L., Spencer, N. & Stoddart, J. F. A molecular shuttle. J. Am. Chem. Soc. 113, 5131–5133 (1991).
Leigh, D. A., Wong, J. K. Y., Dehez, F. & Zerbetto, F. Unidirectional rotation in a mechanically interlocked molecular rotor. Nature 424, 174–179 (2003).
Niu, Z. & Gibson, H. W. Polycatenanes. Chem. Rev. 109, 6024–6046 (2009).
Wu, Q. et al. Poly[n]catenanes: synthesis of molecular interlocked chains. Science 358, 1434–1439 (2017).
Raphael, E., Gay, C. & de Gennes, P. G. Progressive construction of a ‘Olympic’ gel. J. Stat. Phys. 89, 111–118 (1997).
Fischer, J., Lang, M. & Sommer, J. U. The formation and structure of Olympic gels. J. Chem. Phys. 143, 243114 (2015).
Jin, C.-M., Lu, H., Wu, L.-Y. & Huang, J. A new infinite inorganic [n]catenane from silver and bis(2-methylimidazoyl)methane ligand. Chem. Commun. 2006, 5039–5041 (2006).
Loots, L. & Barbour, L. J. An infinite catenane self-assembled by π⋯π interactions. Chem. Commun. 49, 671–673 (2013).
Thorp-Greenwood, F. L., Kulak, A. N. & Hardie, M. J. An infinite chainmail of M6L6 metallacycles featuring multiple Borromean links. Nat. Chem. 7, 526–531 (2015).
Kuang, X. et al. Assembly of a metal–organic framework by sextuple intercatenation of discrete adamantane-like cages. Nat. Chem. 2, 461–465 (2010).
Heine, J., Schmedt auf der Günne, J. & Dehnen, S. Formation of a strandlike polycatenane of icosahedral cages for reversible one-dimensional encapsulation of guests. J. Am. Chem. Soc. 133, 10018–10021 (2011).
Jiang, L., Ju, P., Meng, X.-R., Kuang, X.-J. & Lu, T.-B. Constructions of two polycatenanes and one polypseudo-rotaxane by discrete tetrahedral cages and stool-like building units. Sci. Rep. 2, 668 (2012).
Shen, Y., Zhu, H.-B., Hu, J. & Zhao, Y. Construction of a metal–organic framework by octuple intercatenation of discrete icosahedral coordination cages. CrystEngComm 17, 2080–2082 (2015).
Chen, L., Chen, Q., Wu, M., Jiang, F. & Hong, M. Controllable coordination-driven self-assembly: from discrete metallocages to infinite cage-based frameworks. Acc. Chem. Res. 48, 201–210 (2015).
Wu, X., Xu, Z.-X., Wang, F. & Zhang, J. Catenation of homochiral metal–organic nanocages or nanotubes. Inorg. Chem. 55, 5095–5097 (2016).
Cheng, L. et al. Three-dimensional polycatenation of a uranium-based metal–organic cage: structural complexity and radiation detection. J. Am. Chem. Soc. 142, 16218–16222 (2020).
O’Keeffe, M., Peskov, M. A., Ramsden, S. J. & Yaghi, O. M. The reticular chemistry structure resource (RCSR) database of, and symbols for, crystal nets. Acc. Chem. Res. 41, 1782–1789 (2008).
Liu, Y. et al. Weaving of organic threads into a crystalline covalent organic framework. Science 351, 365–369 (2016).
Ma, T. et al. Single-crystal X-ray diffraction structures of covalent organic frameworks. Science 361, 48–52 (2018).
Gemmi, M. & Oleynikov, P. Scanning reciprocal space for solving unknown structures: energy filtered diffraction tomography and rotation diffraction tomography methods. Z. Kristallogr. 228, 51–58 (2013).
Skowronek, P., Warżajtis, B., Rychlewska, U. & Gawroński, J. Self-assembly of a covalent organic cage with exceptionally large and symmetrical interior cavity: the role of entropy of symmetry. Chem. Commun. 49, 2524–2526 (2013).
Ivanova, S. et al. Isoreticular crystallization of highly porous cubic covalent organic cage compounds. Angew. Chem. Int. Ed. 60, 17455–17463 (2021).
Yaghi, O. M., Kalmutzki, M. J. & Diercks, C. S. Introduction to Reticular Chemistry: Metal–Organic Frameworks and Covalent Organic Frameworks (Wiley-VCH, 2019).
Acknowledgements
This research was partly supported by King Abdulaziz City for Science and Technology (Center of Excellence for Nanomaterials and Clean Energy Applications), and mechanical property measurements funded by the Defense Advanced Research Projects Agency (DARPA) under contract HR001-119-S-0048. This research used resources of the Advanced Light Source (beamline 7.3.3) at Lawrence Berkeley National Laboratory, which is a DOE Office of Science user facility under contract DE-AC02-05CH11231. Y.Z. and O.T. acknowledge the support of the Center for High-resolution Electron Microscopy (CℏEM), ShanghaiTech University (EM02161943), Shanghai Science and Technology Plan (21DZ2260400) and National Natural Science Foundation of China (21835002). We thank C. Zhu for assistance in acquiring synchrotron PXRD data on beamline 7.3.3 of the Advanced Light Source, A. Lund and H. Celik of the School of Chemical Sciences at the University of California-Berkeley NMR facility for assistance with solid-state NMR data acquisition, Z. Wang at the Cornell High Energy Synchrotron Source and T. Matsumoto and A. Yamano at the Rigaku Co., Japan, for the initial trials on acquiring single-crystal XRD and PXRD data. D.M.P. thanks V. A. Blatov at the Samara Center for Theoretical Materials Science for providing the ToposPro software.
Author information
Authors and Affiliations
Contributions
C.S.D., T.M. and O.M.Y. conceived the idea. T.M. and O.M.Y. led the project and interpreted the results. T.M. conducted the syntheses, structure analyses and characterizations for all the samples and interpreted the data. Y.Z. and O.T. collected and analysed the TEM data, and supported the comparison of TEM and PXRD results. J.K. and R.O.R. collected and analysed the nanoindentation data. F.G. and P.P.S. finalized the PXRD refinement. H.L. and Y.Z. supported the scanning electron microscopy measurements. N.H. conducted the THF sorption experiment. Y.L. and N.J.D. supported the linker synthesis. D.M.P. helped in the literature and topological analysis of the organic polyhedra and catenation. T.M., C.S.D. and O.M.Y. wrote the manuscript and all the authors reviewed it.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Synthesis thanks the anonymous reviewers for their contribution to the peer review of this work. Primary Handling Editor: Alison Stoddart, in collaboration with the Nature Synthesis team.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figs. 1–57, Tables 1–5 and Scheme 1.
Supplementary Data 1
Crystal structure of catena-COF-805; CCDC 2216102.
Supplementary Data 2
Crystal structure of catena-COF-806; CCDC 2216103.
Supplementary Data 3
Crystal structure of catena-COF-807; CCDC 2216104.
Source data
Source Data Fig. 5
Experimental PXRD data of catena-COF-805 and -806.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ma, T., Zhou, Y., Diercks, C.S. et al. Catenated covalent organic frameworks constructed from polyhedra. Nat. Synth 2, 286–295 (2023). https://doi.org/10.1038/s44160-022-00224-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s44160-022-00224-z
This article is cited by
-
Encoding ordered structural complexity to covalent organic frameworks
Nature Communications (2024)