Abstract
The central goal of regenerative medicine is to replace damaged or diseased tissue with cells that integrate and function optimally. The capacity of pluripotent stem cells to produce unlimited numbers of differentiated cells is of considerable therapeutic interest, with several clinical trials underway. However, the host immune response represents an important barrier to clinical translation. Here we describe the role of the host innate and adaptive immune responses as triggers of allogeneic graft rejection. We discuss how the immune response is determined by the cellular therapy. Additionally, we describe the range of available in vitro and in vivo experimental approaches to examine the immunogenicity of cellular therapies, and finally we review potential strategies to ameliorate immune rejection. In conclusion, we advocate establishment of platforms that bring together the multidisciplinary expertise and infrastructure necessary to comprehensively investigate the immunogenicity of cellular therapies to ensure their clinical safety and efficacy.
Similar content being viewed by others
Introduction: why is understanding the immunogenicity of a cell therapy important?
Regenerative medicine has emerged as a promising strategy to restore damaged or diseased cells and tissues as a consequence of aging, disease, injury, or accidents. Typically, these therapies involve deriving cell types of interest from an undifferentiated source of cells with multi- or pluripotency, including human embryonic (hESC) or induced (hiPSC) pluripotent stem cell origin1. Following a thorough assessment of specific marker expression, morphology, and functionality of the derived cells in vitro, pre-clinical studies are necessary to assess the survival, integration, safety, and efficacy of the cell product. There are numerous diseases without current curative treatment such as age-related macular degeneration, Parkinson’s disease, Type 1 Diabetes Mellitus, and liver disease that could be transformed by the application of cellular therapies to restore tissue function. In fact, currently more than 20 phase I/II stem cell-based studies are registered on ClinicalTrials.gov, but only a few have published results showing survival and safety of the tested cellular therapies; efficacy outcomes are, therefore, awaited (Table 1).
Despite the fact that some cellular therapies have progressed to clinical trials, their immunogenicity remains an unresolved challenge that may impede effective long-term translation to the clinic. Although it is often assumed that autologous hiPSCs (i.e., isogenic grafts) lack immunogenicity, this may be dependent on cell type2,3,4,5. Alternatively, stem cell-derivatives generated from individuals unrelated to the recipient (i.e., allogeneic grafts) are very likely to lead to immune-mediated rejection due to their allogeneic origin. Different strategies are in place to reduce graft rejection, including the use of immunosuppressants and human leukocyte antigen (HLA) matching between donor and recipient6,7, but none are entirely successful at abolishing the immune response in a non-toxic manner. Understanding the mechanisms triggering the immune response toward a cellular product remains key to minimizing rejection and ensuring long-term graft survival and function.
What are the dominant drivers of the immune response to stem cell therapies?
The immune response is orchestrated by the innate and adaptive immune systems. The first line of defense or the innate immune response consists of fast (0–96 h) and usually non-specific physical, chemical, and cellular responses against pathogens. The second line of defense or the adaptive immune response also referred to as acquired immunity, is only found in vertebrates and is long-lasting. Although innate and adaptive immune responses are often considered as sequential and separate, there is now increasing recognition that there is an overlap between the two, both mechanistically and in timelines (Table 2).
Many of the pathways and processes in innate immunity are the same as those responsible for non-specific reactions to tissue damage, resulting in inflammation. Therefore, tissue stress and inflammation that can occur at the time of transplantation of regenerative cellular therapies is predominantly mediated by the innate immune system.
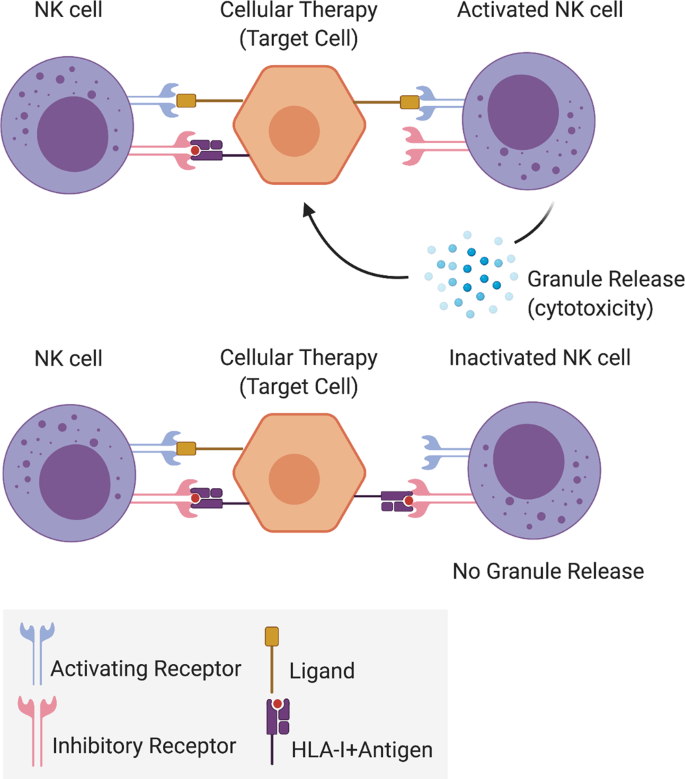
Natural killer cells (NKs), a key component of the innate immune system, are thought to play an important role in allogeneic cellular product rejection (Fig. 1 and Table 2). NKs specifically target and kill cells that express mismatched/lack self-human leukocyte antigen class I (HLA-I, also known as major histocompatibility complex [MHC]) molecules (“missing-self” hypothesis)15,16, a response that will also trigger additional adaptive immune responses via specific cytokines leading to clearance of donor graft cells. NK-mediated killing may be aggravated by specific culture conditions used during the generation of stem cell therapies, which might give rise to differentiated cellular products improperly expressing immune molecules or containing reminiscent molecules of the cell type of origin17,18,19,20,21,22 (see “What are the immunological confounders that should be taken into consideration for stem cell therapy?”). Conversely, certain tissues might, either naturally or due to the inflammatory setting generated at the time of transplantation, express inhibitory ligands or cytokines at high levels which might compensate for the degree of HLA-mismatch. This has been shown for HLA-C, HLA-E up-regulation or IL-10 secretion in hPSC-retinal pigment epithelial (RPE) cells23,24,25. In fact, recent works have demonstrated that the incorporation of specific NK inhibitory ligands (e.g., HLA-E, CD47) into the cellular therapies can ameliorate NK cell graft response26,27,28 (see “How can immunogenicity be overcome or ameliorated for the long-term success of cellular therapies?”).
NK cell activation or inactivation following activating or inhibiting receptor-target cell ligand signaling. A mismatched/lack of HLA-I-Antigen complex is a strong NK cell-activating signal. Once activated, a cytotoxic response will follow mainly through granule release. HLA: human leukocyte antigen, NK: natural killer.
The complement system, composed of more than 30 soluble and cell-bound proteins29, is another major component of the innate immune system. Its relevance is illustrated by the finding that the success of islet and hepatocyte cell transplants is limited by activation of the complement and coagulation cascades30,31. Also, hiPSC-RPE cells have shown to produce several complement components which, under inflammatory or stress conditions, had a significant impact on RPE cell survival and function in a therapeutic setting32,33.
In the context of transplantation, adaptive immunity is triggered by the recognition of foreign/donor antigens by recipient T cells. This is mostly due to the expression of highly polymorphic MHC molecules leading to T cell-mediated immune response and rejection of the transplanted allogeneic cells34,35 (Table 2). In general, HLA-I-peptide complexes (expressed on a majority of nucleated cells) are recognized by CD8+ cytotoxic T cells and following activation they mediate the direct killing of the target cells; whereas HLA-II molecules expressed mainly on antigen presenting cells (APC) present peptide derived from exogenous antigens to CD4+ helper T cells, leading to the activation of other immune cells including cytotoxic T cells and B cells36. Transplanted cells can activate the adaptive immune system (alloreactive T cells) through three pathways of allorecognition34,37 (Fig. 2). In the direct pathway of allorecognition, recipient CD8+ and CD4+ T cells recognize HLA-I or II-allo-peptide complexes, respectively, on donor APCs/cellular therapy which are consequently activated. In the indirect pathway of allorecognition, donor allo-peptide is processed by recipient APCs and presented to mainly CD4+ T cells. In the semi-direct pathway, intact donor HLA-I and -II-allo-peptide complexes are internalized, transferred to the membrane of recipient APCs, and presented to CD8+ or CD4+ T cells for their activation38. Activated recipient CD4+ T cells (with direct or indirect specificity) can provide help to cytotoxic CD8+ T cells with direct allospecificity to kill donor cells by recognizing allo-HLA-I molecules on their surface (typically leading to acute rejection); in addition to activating other immune cells e.g., NK cells and B cells which in turn will produce allograft-specific antibodies (typically leading to chronic rejection)39,40. In fact, different degrees of mismatch would be anticipated to result in different rejection strengths, as has been shown by Sugita and colleagues when allogeneic hiPSC-RPE cells with homozygous HLA-A, -B, -DRB1 alleles greatly reduced both in vitro and in vivo immune responses41,42.
Illustration representing the three different pathways of allorecognition. The direct pathway of allorecognition typically involves recognition of intact HLA-I or -II-Antigen complexes expressed by donor DC (i.e., APC)/cellular therapies by recipient CD4+ or CD8+ cells, respectively, usually leading to acute graft rejection. T cells with direct allospecificity are present in all individuals at very high frequency and this pathway is thought to play a major role immediately following transplantation. The indirect pathway of allorecognition involves processing and presentation of donor HLA molecules by recipient DC (i.e., APC) to recipient CD4+ T cells, which then provide help for CD8+ T cell-mediated cytotoxic killing and antibody production by B cells. The frequency of T cells with indirect allospecificity is undetectable but increases with time from the transplant. In line with this, this pathway was thought to be the most relevant for graft rejection late post-transplant. The semi-direct pathway involves the transfer of intact donor-derived HLA molecules to recipient APC leading to CD8+ or CD4+ T cell activation. This latter pathway implies that the direct pathway of allorecognition lasts for longer than what was initially thought and indicates that the same recipient DC can present directly and indirectly donor HLA molecules to host T cells. In all pathways, the activated recipient CD4+ T cells provide help for activation of cytotoxic CD8+ T cells which kill donor cells by binding to allo-HLA-I on their surface then leading to cellular-mediated rejection of cellular therapy (typically acute reaction). In addition, activated CD4+ T cells will trigger the innate immune system, inflammation, and B cell maturation into plasma cells that will produce allo-antigen specific antibodies which will lead to an antibody-mediated rejection of the cellular therapy (typically chronic rejection). Cellular therapy: refers to an HLA-II expressing target cell, DC: dendritic cell, TCR: T cell receptor, HLA: human leukocyte antigen, NK: natural killer.
Because most regenerative cellular therapies are not expected to contain donor HLA-expressing APCs, it is anticipated that indirect and semi-direct allorecognition will dominate the adaptive immune response to regenerative cellular therapies. However, remnant embryonic antigens such as TRA-1, SSEA3 expressed by cellular therapies (even in an isograft setting) with direct T cell activating capability may be able to elicit an immune response. Therefore, although certain cellular products would be expected to activate the adaptive immunity directly, the majority would be expected to activate the indirect and semi-direct mechanisms of allorecognition through recipient dendritic cells (DC) or macrophages that would recognize either alive or dead donor grafted cells and present their respective allo-antigens to T cells (via processing and presentation of donor antigens or following the transfer of intact donor HLA-allo-peptide complexes)38. Nonetheless the up-regulation of certain inhibitory T cell ligands (e.g., PD-L1, CD95), the secretion of specific molecules such as pigment epithelium-derived factor (PEDF), IL-10, TGFβ, or the induction of alloantigen-specific regulatory T cells (Tregs), that may be inherent to the immunosuppressive phenotype of a specific cell type or occur under specific inflammatory conditions and cytokines (e.g., IFN-γ), could favor the inactivation of T cells even in the presence of a degree of HLA-mismatch23,25,41,42,43,44,45,46. Taking advantage of this, Yoshihara et al. elegantly over-expressed PD-L1 and showed long-term survival of the injected edited human islet-like xenografts. These xenografts restored glucose homeostasis in immune-competent diabetic mice for 50 days, and upon ex vivo IFN-γ stimulation, they showed restricted T cell activation and graft rejection compared to non-engineered cells47.
Conversely, the presence of inflammatory molecules (particularly IFN-γ) may induce the up-regulation of HLA-II molecules in the cellular therapies, which would mainly trigger the direct pathway of allograft rejection via CD4+ cells leading to B cell activation, as shown by multiple studies in hPSC-RPE23,24,25,41,42,45. Despite the intense historical interest in studying the impact of knocking out HLA genes on immunogenicity, recent advances in genetic engineering tools have finally provided a robust platform for several groups to very attractively generate cells lacking HLA genes specifically aimed at developing universal cellular therapies for regenerative medicine23,48. However, this strategy particularly intended at abolishing T-cell responses may eventually lead to NK cell activation and graft rejection, hence opening a novel window for future therapeutic strategies aiming to keep both adaptive and innate immune systems under control (see “How can immunogenicity be overcome or ameliorated for the long-term success of cellular therapies?”).
What are the available experimental platforms to study the immunogenicity of cellular therapies in vitro and in vivo?
An increasing broad range of in vitro and in vivo experimental approaches are available to investigate the immune response to cellular therapies. Due to the complexity of the immune system and the inherent deficiencies of all methodologies, it is almost always necessary to combine data from several in vitro techniques and in vivo models in order to reach definitive conclusions on the immunogenicity of cellular therapy (Table 3). However, it is not usually feasible for a research group to incorporate the complete range of in vitro and in vivo methodologies into every study. Thus, the assessment of the response of a particular immune compartment is often the focus of individual studies. Here we describe a summary of the most utilized methods to comprehensively evaluate the immunogenicity of cellular therapies.
In vitro methodologies to assess the immunogenicity of cellular therapies
-
Assessment of innate immunity
NK responses to a cellular therapy can be assessed by measurement of NK activation or degranulation (i.e., lytic granule trafficking), cytokine release, and NK cytotoxic killing. NK cell degranulation assays are usually performed by co-culturing human pre-activated NK cells and target cells (cellular therapy) in an assay-dependent ratio. Degranulation is typically evaluated by the expression of CD107 (lysosomal-associated membrane protein 1/LAMP-1) molecule and intracellular cytokines (e.g., IFN-γ) in different subsets of NK cells, which can be co-labeled with specific KIR-ligands. Cytotoxic activity of NK cells towards a target cell can be measured in vitro by quantifying the release of naturally occurring substances (e.g., lactate dehydrogenase), or through the use of target cells labeled with radioactive compounds, such as 51Chromium- or 111Indium, which are released upon NK cell killing49,50. Alternative methods to measure NK cell cytotoxicity include flow cytometry (e.g., intracellular levels of perforin, granzymes, and granulysin), enzyme-linked immunosorbent assay (ELISA)-based granzyme measurement, image cytometry, and morphometric analysis by microscopy51,52. Additionally, NK cell-mediated cytolysis and antibody-dependent cellular toxicity (ADCC) can also be studied in a novel label-free, non-invasive manner using image cytometry or a microelectronic sensor measuring impedance in adherent cells, thus avoiding the use of radioisotopes53,54. Despite these novel methods, most studies assessing NK activation by PSC aimed as genetically engineered-hypoimmunogenic cellular therapies used 51Chromium release assays, which illustrated the decrease in NK cell cytotoxic levels of the respective gene editing approaches26,27,28,55 (see section “How can immunogenicity be overcome or ameliorated for the long-term success of cellular therapies?”).
To complement the evaluation of the innate immune system, some studies have assessed by flow cytometry or immunofluorescence (IF) staining the expression of activating or inhibitory ligand molecules that might be expressed by the hPSC-derived cells. These, for instance, include HLA-A, B, C, CD112, CD155, PCNA, and NKG2A shown by Petrus-Reurer et al. in hESC-RPE cells23; or NKG2D, NKp80, NKp16, NKp44, and NKp30 shown by Deuse et al. in hiPSCs, hi-endothelial-like cells and hi-cardiomyocyte-like cells26. Although no conclusive ligand expression differences were found between edited and respective wild-type counterparts in these studies, their unique expression and combined downstream signal interaction might explain some of the mechanisms driving NK cell activity in specific cell lineages57,58.
Assessment of other components of the innate immune response includes analysis of pattern recognition receptors (PRR) by qPCR before and after stimulation with pro-inflammatory cytokines as shown by Fischer et al. with human iPSC-Hepatocytes59. However, the majority of the studies regarding PRRs have so far been aimed to define a better infectious disease model rather than defining the contribution of PRRs in cell rejection. Key components of the complement cascade (Collectin-11, MASP2, C3d, C5B-9(mac), CFB) have been analyzed before and after cell stress or pre-conditioning with TNF-α or IFN-γ32,33. A pioneering study from Sugita et al. showed by using different assays (flow cytometry, RT-PCR, and ELISA) that iPSC-RPEs expressed key components of the complement cascade that are up-regulated by IFN-γ and T Helper-1 mediated responses32. A few years later, Fanelli et al. showed by immunohistochemistry (IHC) and western blotting that in vitro cultured hiPSC-RPE under hypoxic stress upregulated their surface expression and the release of collectin-11, a molecule triggering complement activation33.
-
Assessment of cell-mediated T cell activation
The most commonly used in vitro methodology to study the response of T cells with direct allospecificity to a specific cellular therapy is a co-culture system with the cells of interest and allogeneic peripheral blood mononuclear cells (PBMCs). To try and mimic the indirect alloresponse, monocyte-derived DCs can be co-cultured with autologous T cells in the presence of the cellular product61. However, the indirect (and semi-direct) allorecognition is best modeled in vivo as it takes time for this pathway to emerge after antigen exposure. Experimental end-points for these co-culture assays comprise immune cell proliferation, expression of activation markers, target cell killing, and cytokine production. These assays are usually complemented with the evaluation of the expression of HLA and co-stimulatory and co-inhibitory molecules on the cellular product (Table 3). T cell proliferation measured in these assays can be coupled to the evaluation of naïve, activated, central and effector memory T cell sub-populations using specific markers62, thus providing a more comprehensive evaluation of the target T cells activated by the cellular product25,40,42,63,64,65,66,67,68. To understand how the inflammatory microenvironment influences a cellular therapy and its capacity to activate T cells, co-cultures can be executed following pre-conditioning of the hPSC-derived product with pro-inflammatory cytokines such as IFN-γ, TNF-α, and IL-1β (Table 3). Generally, co-cultures of the cellular therapies with PBMCs or isolated CD4+ or CD8+ tracker-labeled (e.g., CFSE) to assess T cell proliferation or IFN-γ measurements in the supernatants to assess T cell activation has been the most utilized approach to evaluate the immunogenicity of cellular therapies from matched, mismatched or genetically modified donor cells23,40,41,42,45,48,55. And in fact, most cellular products tested in this manner have shown little or no immunogenicity, thus confirming their inability to directly activate the adaptive immune system. Specifically, by co-culturing PBMCs with hESC-RPEs pre-stimulated with IFN-γ, Petrus-Reurer et al. have recently shown their lack of immunogenicity unless cultured with very specific conditions (e.g., particular target:immune cell ratio, presence of cytokines and activating molecules). In a similar manner, this lack of immunogenicity in vitro has also been shown for other cell types like hiPSC-derived cartilages69. Recently, Mehler et al. showed that hiPSC-derived neural crest stem (NCS) cells induced negligible CD3+ T cell proliferation although, conversely, hiPSC-derived smooth muscle cells (SMC) favored a high level of cell proliferation65.
-
Assessment of the immunomodulatory function of the cellular therapy
Indeed, in contrast to immune activation, suppression of T-cell responses in vitro has been described for several cellular products (Table 3). Typically, this ‘immunomodulatory’ potential is assessed by quantifying the impact of the cellular therapy on T cell proliferation in the presence of molecules that would induce it (e.g., soluble antibodies anti-CD3/OKT-3, coated beads, IL-2)23. Assessment of expression of co-stimulatory molecules (inhibitory: e.g., PD-L1, PD-L2; activating: e.g., CD80, CD86) by the cellular therapy as shown by several studies is also relevant, as this may confer T cell inhibitory properties and explain some of the inhibition of T cell proliferation and cytokine production observed in the co-culture in vitro assays63,70,71,72,73. Petrus-Reurer et al. have shown that pre-IFN-γ stimulated hESC-RPEs significantly suppressed both CD8+ and CD4+ T cell proliferation in the presence of OKT-3, a mechanism that might be mediated by the up-regulation of specific co-inhibitory molecules in the absence of co-stimulatory molecules. Similarly, hPSC-derived retinal ganglion cells had the ability to inhibit T cell proliferation in a transforming growth factor (TGF)-β2 dependent way74. TGF-β production by hiPSC-NCS is crucial for mediating their T cell proliferation immunosuppressive capacity as shown by Fujii et al67. However, by using different experimental conditions (e.g., source of hiPSC, NCS differentiation protocol, length and readout of the MLR), Mehler et al. showed the lack of this capacity for hiPSC-NCS cells. The opposite results obtained in these two studies highlight the importance of the immunological confounders present at the time of performing the different in vitro and in vivo assays (see “What are the immunological confounders that should be taken into consideration for stem cell therapy?”).
In vivo methodologies to assess the immunogenicity of hPSC-derivatives
Availability of optimized and representative in vivo animal models are a limiting factor for the definitive study of the immunogenicity of regenerative cellular therapies. Human cellular products have been previously transplanted into different anatomical locations using xeno- and immunodeficient animal models (e.g., rodents, lagomorphs, pigs, non-human primates)23,41,75,76. Despite species differences, these studies have been very useful in understanding the basic mechanisms of the host response. However, as these models do not replicate the human immune response to human cellular therapies, their ultimate utility for clinical translation is somewhat limited and they will not be further discussed in this review.
There are a number of effective strategies to model a competent human immune compartment in vivo in order to assess the immunogenicity of cellular therapies77,78,79,80. In essence, these models involve the reconstitution of severely immunodeficient rodents with human immune cells. The human immune cells used for reconstitution of the mice can either be in the form of adoptively transferred mature lymphocytes (PBMCs) or generated de novo by transfer of CD34+ hematopoietic cells. Mature lymphocytes are usually obtained from the peripheral blood of human donors, although the spleen of deceased transplant organ donors has recently been identified as an alternative source81. Alternatively, CD34+ HSCs can be obtained from umbilical cord blood, bone marrow, or fetal liver. The most common strain of mouse used for the generation of these “humanized” or human immune system (HIS) mice is NOD scid gamma (NSG) mice which lack mature B, T, and NK cells. It is important to note that the immune compartment generated in HIS mouse models is “suboptimal”, and a number of different strategies have emerged to augment and improve the immune response mounted by the animals. These include co-transplantation of fetal human thymic or other tissue and the use of genetically modified mice expressing human cytokines, HLA, or other important signaling molecules. The range of available humanized mouse models and their relative advantages and disadvantages has been reviewed in multiple studies77,78,79,80, and are also summarized in Fig. 3.
Illustration representing the methodologies behind current humanized mice models highlighting the advantages (check) and disadvantages (cross) of each model. The models include: the human peripheral blood lymphocytes (Hu-PBL-SCID) in which most of the engrafting cells are human T cells that express an activated phenotype while few B cells or myeloid cells engraft. One caveat is that these mice will develop a xenogeneic graft-versus-host disease (xeno-GVHD) that results in death, but xeno-GVHD can be delayed using immunodeficient mice lacking mouse MHC class I or class II; the human stem cell repopulating cell (Hu-SRC-SCID), which is established by engraftment of human hematopoietic stem cells (HSC) derived from bone marrow, umbilical cord blood, fetal liver, or mobilized peripheral blood HSC. Engrafting mature adult immunodeficient IL2rγ null mice with HSC permits the generation of multiple hematopoietic cell lineages but few T cells while human T cells are readily generated following engraftment of newborn or 3–4 week-old NSG and NOG mice with HSC; the SCID-HU, which is established by implantation of human fetal liver and thymus fragments under the renal capsule of immunodeficient mice and a major limitation is the paucity of human hematopoietic and immune cells in the peripheral tissues; and the bone marrow, liver, thymus (BLT), which is established by implantation of human fetal liver and thymus fragments under the renal capsule of sublethally irradiated immunodeficient mice accompanied by intravenous injection of autologous fetal liver HSC. The use of immunodeficient NOD-scid mice to establish the BLT model led to human immune system engrafted mice, which is further enhanced by the engraftment of NSG mice. A complete hematopoietic and immune system develops, and the human T cells are educated on a human thymus and are HLA-restricted. IP: intraperitoneal, IV: intravenous, IS: intrasplenic, IF: intrafemoral, IC: intracardiac, IH: intrahepatic, GvHd: graft versus host disease.
A further challenge is the occurrence of graft-versus-host (GvH) reaction which limits the experimental time-window82. This window is dependent on the HIS model and mouse strain used and is most limited in the PBMC model. Critical in interpreting the observed immune response is the use of positive and negative control tissues/cells. Positive control cells should ideally be “adult” cells and tissues that may represent an alternative treatment to cellular therapy. Where possible, autologous cells/tissues are the ideal negative controls although practically this is rarely possible. Lastly, the HIS models are subject to significant biological variation and large group sizes may be necessary to draw definitive conclusions. However, the generation of HIS mice from the spleen of deceased transplant organ donors, together with the use of control cells and generation of cellular therapies from the same donor, has the potential to address some of these challenges81.
The in vivo immunogenicity of hPSC-cell grafts can be quantified using a range of experimental endpoints, which have been summarized below from a variety of studies evaluating stem cell therapies (Table 3):
-
Assessment of survival/immune destruction of the transplanted cells by luciferase, specific human- or tissue-specific markers on IHC/immunofluorescence (IF)23,26,27,28,39,40,55,66.
-
Immune characterization of the transplanted cells (e.g., upregulation of immune markers such as HLA-I and -II, or the above-mentioned T cell and NK cell activating and inhibitory ligands) by flow cytometry or IHC/IF23,28,41.
-
Phenotypic characterization of the immune infiltration (type and amount), e.g., CD3+/CD4+/CD8+ T cells4,5,64,66,68,83; CD56+ NK cells; RAM11+ macrophages; HLA-II+ APCs by flow cytometry or IHC/IF to evaluate the immune responses elicited by the graft23,28,39,66.
-
Systemic cellular immune response (e.g., increase in the number of lymphocytes in spleen) or change in phenotype assessed by flow cytometry and IHC/IF for specific T cell and NK cell populations, but also assessment of splenic germinal center formation for B cells or deposition of complement molecules14, DAMP secretome by IHC/IF66.
-
Systemic anti-human antibody-mediated response assessed by flow cytometry or Luminex assays of the transplanted mice sera23,32,40.
-
Evaluation of the main complement molecules (e.g., C3, C5, CFH/B/I, CL-11, C3d, and C5b-9) present in the host serum or deposited at the site of the graft, measured by ELISA and/or IHC32,33.
The combination of all the described assays would be the ideal approach to get the full picture of the immune response induced in vivo, but often it is not possible and only some of the mentioned endpoints are analyzed. As an exemplar study, Petrus-Reurer et al. evaluated the immunogenicity of unedited wild-type and edited hESC-RPE lacking HLA-I and -II molecules injected in the subretinal space of albino rabbits. They assessed the survival of engrafted human cells, immune marker expression (HLA-I, HLA-II), and immune response (CD3, CD56, RAM11) with IF. Furthermore, they evaluated specific anti-human antibody production by incubation of rabbit sera that received the cellular therapies with hESC-RPE ex vivo further mixed with anti-rabbit fluorescent antibodies analyzed by flow cytometry. Overall, this study demonstrated decreased early and delayed late immune responses of HLA-I and -II knockout hESC-RPE compared to wild-type cells in a xenograft model without immunesuppression23.
These assessments can also be complemented by the use of real-time imaging techniques (e.g., using luciferase constructs, spectral domain-optical coherence tomography [SD-OCT] scans in the retina) or behavioral assays for dopaminergic cells as alternative methods to assess immune infiltration, graft cell survival, and/or function of the cellular therapy in the site of injection. This has been elegantly shown by several groups exploring survival and function of e.g., hypoimmunogenic hiPSC/hESC-RPE cells to be used as an alternative treatment modality for age-related macular degeneration23,26,28.
What are the immunological confounders that should be taken into consideration for stem cell therapy?
There are several confounders, both cell-related and assay-related, that should be taken into consideration when evaluating the immune response to any cellular product (Fig. 4).
Illustration representing the immunological confounders involved in cellular therapies including factors concerning various aspects of the cell therapy (e.g., cell source, differentiation protocol, cell type/function, specific maturation state of the cell product), the recipient’s transplantation site, and the limitations inherent to in vitro and in vivo experimental platforms. Overall these factors might trigger different degrees of rejection in the patient that will receive the cellular therapy, therefore limiting its efficacy.
The original source of the cell product has a potentially important impact. There are two common sources of pluripotent cells: hESCs and hiPSCs84,85,86,87. As hESCs and hiPSCs require different methodologies to generate the differentiated final cell product, it is, therefore, possible that they may express different antigens leading to different immunogenicity. Specifically, several factors present in the differentiation protocols and in vitro culture conditions may be sources of xeno or aberrant immunogenic antigens. Such expression can result from prolonged cultures in animal product-containing media, such as the uptake of the non-human sialic acid N-glycolyneuraminic acid (Neu5Gc) by hESC when cultured in standard conditions with serum88,89,90,91. Non-physiological media constituents, such as high concentrations of growth hormones or antibiotics have also been shown to induce the ectopic expression of CD3092,93. Finally, chromosomal abnormalities have been shown to be produced through in vitro propagation of hESC lines in multiple studies94,95,96,97.
Another important confounder that may impact rejection is the expression of potentially immunogenic or immunomodulatory surface molecules and the release of soluble mediators. All these factors can be dependent on the differentiation or maturation state of the cells, independently of their cellular source. Specifically, accelerated in vitro differentiation processes might be associated with immunological immaturity, since it is well established that somatic cells acquire such maturity during fetal development that continues after birth in a process mediated by intricate and gradual feedback mechanisms between the nascent immune system and developing somatic cells98,99. This process will dictate and shape the repertoire of activating and inhibitory signals that would then orchestrate the immune system. Such fine-tuning is unlikely to occur in vitro with methodologies that recapitulate long periods of normal development in just a few weeks, and also in the absence of biologically normal immune cell interactions. This may result in incompletely differentiated cells that may express ligands reminiscent of an embryonic origin that could potentially be retained and recognized as foreign antigens, such as early glycans, proteins, or other unique surface molecule modifications (e.g., TRA-1-81, TRA-1-60, SSEA3, SSEA4) that were not present during immune education of the recipient’s immune system in an already-mature antigenic microenvironment17,18,19. As mentioned in previous sections, incompletely differentiated cells may also lack or exhibit a reduced expression of ligands that are essential for immune cell inhibition, including reduced expression of HLA-I (easily altered in in vitro conditions due to its complex regulation and response to inflammatory cytokines20,21), or absence/presence of specific KIR-ligands in the recipient’s NK cells that were not properly educated to recognize certain HLA-I epitopes as self22,100,101.
Conversely, expression of other ligands such as HLA-E and HLA-G, which are known to confer foeto-maternal tolerance, may be maintained and may protect the product against NK cell cytotoxicity102. Some of these mechanisms have been exploited by groups attempting to reduce cellular product rejection27,28 (see “How can immunogenicity be overcome or ameliorated for the long-term success of cellular therapies?”). Other immunomodulatory mechanisms, reminiscent of those contributing to foeto-maternal tolerance could include: secretion of Arginase I resulting in decreased CD3 expression and lymphocyte proliferation103, activation of hemoxygenase I enzyme to produce anti-inflammatory molecules104, expression of high levels of cathepsin B and a serine protease inhibitor (serpin, SPI-9) that allows the destruction of granzyme-B released by T cells and NK cells105, or expression of the Fas ligand (FasL, CD95L) resulting in T cell apoptosis106,107,108,109.
In contrast to hESC-derived cells, the reprogramming procedure for the generation of hIPSCs could result in aberrant antigen presentation due to partial epigenetic memory retained from the parental induced cells. This could produce chimeric surface molecules of more than one cell type, thus eliciting an immune response even in autologous transplants66,110.
Some cellular therapies may require further differentiation or maturation in vivo through interaction with other immune or neighboring cells, as well as through exposure to blood flow or tissue mechanics. It is therefore likely that in some cases the immunogenicity captured in vitro may not fully recapitulate the immune response ultimately observed in vivo. Examples include stem cell-derived β-like cells needing in vivo cues from the islet niche to reach a fully mature and specialized state111,112,113, or dopaminergic neurons that must undergo further maturation after transplantation in the midbrain compartment114. Of note, immunosuppression treatment may have a detrimental effect on this process, either directly or by modifying the immune microenvironment, but further work is required to quantify these effects.
Cellular therapies may be less prone to immune rejection where the derived cells inherently have an immunomodulatory immuno-protective function. Examples include cells acting as anatomical barriers (e.g., certain types of endothelial cells, astrocytes, or pericytes in the blood-brain barrier) or RPEs in the blood-retinal barrier (which endogenously express ligands such as PD-L1, CD95L, or secreted molecules as PEDF, TGF-β3, IL-10)25,43,44,45. Similarly, transplantation of cellular therapies in immune-privileged anatomical niches (e.g., eye, brain, testes, or placenta), may induce different immune responses. If not compromised by the specific disease or by the process of transplantation, such sites may confer an advantage for allogeneic donor cell engraftment.
How can immunogenicity be overcome or ameliorated for the long-term success of cellular therapies?
A small number of early-stage hPSC-based clinical trials for multiple diseases are currently underway (Table 1). As classical immunosuppression is associated with a range of side effects6, a number of strategies are being explored to reduce or eliminate the immunogenicity of stem cell-derived cellular therapies (Table 4).
Immunosuppressive agents
Immunosuppression is the current standard approach to prevent the rejection of solid organ transplants. This strategy has also been adopted by recent clinical trials to prevent the rejection of cellular therapies (Table 1). In general, there are five broad classes of immunosuppressant drugs: (i) glucocorticoids/steroids (e.g., prednisolone, dexamethasone, hydrocortisone) that act by reducing inflammation; (ii) cytostatics (e.g., alkylating, antimetabolites, methotrexate, azathioprine, and mercaptopurine, cytotoxic antibiotics) that block T cell proliferation; (iii) specific antibodies (polyclonal, monoclonal, anti-T cell receptor, anti-IL-2 receptor) that deplete subsets of immune cells such as macrophages/APC, T cells and/or B cells; (iv) drugs acting on immunophilins (e.g., cyclosporine, tacrolimus, sirolimus, everolimus) that inhibit cytokine production by for instance calcineurin inhibitors; and (v) drugs with other mechanisms of actions (e.g., interferons, opioids, TNF binding proteins, mycophenolate, and small biological agents)120,121.
While classical immunosuppressive drugs are an appealing choice to reduce rejection of cellular therapies, they are known to lead to potentially serious toxic side-effects, including increased risk of infection (e.g., viral, bacterial, or fungal), cancers (e.g., skin or lymphoma), and organ damage (e.g., renal failure)6,122,123,124. Moreover, classical immunosuppressive drugs do not specifically and effectively modulate the innate immune response. Also, it is unclear what impact, if any, immunosuppression may have on cell survival, maturation, and function following transplantation of the differentiated cells aimed for cellular therapy. Similarly, it is still not clear if a different regime would need to be used depending on the hESC or hiPSC origin of the cellular source. Until now, clinical studies using stem cell therapies either opted for local suppression (e.g., corticosteroids) or more general regimes of immunosuppression (e.g., cyclosporine, MMF, tacrolimus) (Table 4). These regimes have not been fine-tuned as they were aimed to maximally avoid donor cell rejection. Therefore, the use of immunosuppressive agents in the context of cellular therapies requires further investigation to bypass toxic side-effects in the recipients, and it is generally accepted that the development of more specific strategies to reduce immune-mediated rejection of cellular therapies is desirable.
HLA-matching
Clinical outcomes after solid-organ transplantation are dependent on the degree of HLA disparity between the donor and the recipient7,125. Development of HLA-typed cell banks that allow optimal matching of donor and recipient cells represents an attractive strategy to reduce the risk of immune rejection. This approach is particularly attractive for populations in which major HLA-alleles are relatively conserved. The required degree of matching will differ depending on the cell type of interest (e.g., whether HLA-II is expressed by the cells) and the site of transplantation. It is estimated that a total of 150 or 140 cell lines would be required to provide well-matched cellular therapies for 93% or 90% of the UK and Japanese populations, respectively126,127,128. It is important to note, however, that the generation of cell banks with sufficient diversity for large and heterogeneous populations such as the US will be challenging. Moreover, the risk of rejection would still remain due to the presence of mismatched minor HLA antigens. Also, patients with less common haplotypes would remain difficult to match and alternative strategies would be needed to prevent rejection. This approach has not been tested yet with regenerative cell therapies per se but the reduction in immune response using matched donors is evidenced in solid organ transplants129.
Genetically engineered cells
The emergence of genetic engineering tools such as the CRISPR/Cas9 technology has enabled the pursuit of low- or non-immunogenic “universal” cellular therapies. This is predominantly explored through the elimination of HLA genes (individually or both HLA-I and HLA-II), most effectively by targeting essential molecules for their correct location and expression, namely β2M (required for proper surface expression of HLA-I) and CIITA or RFXANK (master transcriptional regulators for HLA-II genes)23,48,55. Since HLA is the predominant driver of the alloimmune response, HLA-knock-out (HLA-KO) cells are expected to evade CD8+ and CD4+ T cell responses. However, HLA-I deficient cells may nonetheless activate recipient NK cells (triggered by the “missing-self” signal), thus posing an obstacle for successful transplantation. To overcome this, engineered cells overexpressing non-polymorphic HLA-I molecules such as HLA-E, HLA-F, and HLA-G, acting as inhibitors of NK cell-mediated lysis via NKG2A/CD94 (HLA-E), KIR2DL4, and ILT2 (HLA-G) receptors have been generated. This approach has been shown to have at least some efficacy in enabling allogeneic cells to escape immune attack in vitro and in xenotransplantation experiments, as shown with hPSC, hESC-RPE, and hESC-neural progenitor cells using NK cytotoxic/51Chromium release assays27,28,130,131.
An alternative strategy is the generation of cell lines that express a common allele of a specific polymorphic HLA-I molecule (HLA-A, -B or -C) that should be matched and that would then be able to bind NK inhibitory receptors. For example, HLA-C retained cells that also lack HLA-II demonstrated the ability to suppress both NK and T cell responses while maintaining antigen presentation both in vitro and in vivo55. Another approach used in HLA-KO cells is the induction of overexpression of CD47 as a potent inhibitor of phagocytosis, thus avoiding macrophage and NK cell-mediated rejection in allogeneic hosts. This approach has been exemplified by demonstrating survival and proliferation of luciferase-labeled hiPSC and hiPSC-derivative allografts in immunocompetent mice26. Finally, “immune cloaking” is a more holistic approach that includes disruption of several pathways of immune activation and has been attempted by over-expression of immunomodulatory transgenes PD-L1, FASL, CD47, CD200, CCL21, MFGE8, H2-M3, and SPI6 in mouse ESCs, which allowed them to survive indefinitely as allografts132. Universal non-immunogenic cells could also be engineered so as not to express auto- or neo-antigens that normally trigger responses in autoimmune or genetic diseases.
There are important outstanding safety and efficacy considerations that must be addressed in relation to genetically engineered cellular therapies. First, it is essential to demonstrate that the cells do not undergo malignant transformation or infection by virtue of their ability to evade immune recognition. The addition of a fail-safe/suicidal gene cassette system has thus been suggested to selectively eliminate cells that are, for example, more proliferative133. Second, it is important to demonstrate that the elimination of HLA (or other) molecules does not interfere with cell survival, differentiation, and function after transplantation. Despite the current high interest to adopt such technology by several pharmaceutical companies, the clinical translation of this promising approach is still immature and requires careful study in the context of a competent HIS to better anticipate the outcome of clinical trials.
Induction of tolerance
Immune tolerance is defined as the absence of a deleterious immune response despite the continued presence of the antigen (e.g., derived from the transplanted cells) in an intact immune system setting. It is a desirable state that could potentially be achieved through a combination of specific deletions, cell-intrinsic checkpoints, and suppression by regulatory mechanisms such as in vivo induction or expansion of Tregs134,135,136,137,138,139,140,141,142. The targeted manipulation of specific T cell co-stimulatory or inhibitory pathways by for instance inhibiting the second signal via specific monoclonal antibodies (e.g., anti-CD40L, CTLA4Ig, anti-LFA-1), might represent a way to induce tolerance47,143. Another approach to achieve tolerance that has recently reached the clinic in solid organ transplantation144,145,146,147,148 is ex vivo expansion of patient-derived polyclonal or donor-specific Tregs that would be transferred back to the recipient149,150,151,152. None of these approaches have been directly tested in the context of regenerative cell therapies. Nonetheless, they are promising strategies to efficiently regulate the recipient’s immune system in the long term with minimal induction of toxic effects.
Cell shielding/encapsulation
Shielding of the cellular therapy with alginate-beads or specific encapsulation devices has been used for transplantation of β-like cells153,154,155,156. Excitingly, the company ViaCyte has developed different cell-encapsulated versions of SC-derived human pancreatic progenitor cells to treat Type 1 Diabetes, which has now entered Phase 2 clinical trials (https://viacyte.com/). However, the challenge remains on the maintenance of full oxygenation and vascularization of the cells for optimal function, while still providing sufficient immunoprotection over time. This has been largely achieved with materials of particular permeability and with a specific size and distribution of nanopores157. In addition, the use of such technology critically depends on the transplantation site and its accessibility to the recipient’s immune system, which if protected (e.g., immunoprivileged sites), might confer natural tolerance of the grafted cells. Finally, a potentially more refined approach would be to incorporate biocompatible materials in the matrix that contains molecules (e.g., anti-inflammatory cytokines, blocking antibodies) that would protect the cell product against the recipient’s immune response. This technology is still in its infancy, but in proof-of-concept studies, hydrogels functionalized with IFN-γ, TGF-β1, or IL-10 siRNA were able to effectively modulate the immune response to several immune cells types158,159,160,161,162,163,164,165,166. This represents an elegant strategy free of both in vitro and ex vivo manipulation, although the challenge might be keeping the cellular therapy free from immune invasion as the biocompatible material degrades.
In conclusion, while none of the above-mentioned approaches are perfect on their own, they may be combined synergistically to minimize immunogenicity. An example of such a combined strategy may be to use engineered cells or HLA-typed cells transplanted with low-dose immunosuppression or with strategies to induce tolerance while under a specific shielding material. Such combinations would be patient-dependent (e.g., if suffering from a precondition that prevents the use of immunosuppressants), cellular therapy-dependent (e.g., if a cell type is specially affected by the genetic manipulation or the immunosuppressive regime), and site-dependent (e.g., in an immune-privileged site, use of shielding material may not be necessary). In summary, a bespoke and multi-dimensional case-specific approach is likely to be necessary to avoid graft rejection while guaranteeing the survival and function of the specific cellular therapy.
Outlook
Concluding remarks: the need for multi-disciplinary approach and infrastructure for immune-assessment of cellular therapies
As outlined in this review, characterizing and modulating the immune response to regenerative cellular therapies is an essential step for their clinical translation. The immune response is highly complex, consisting of multiple overlapping and interconnected pathways (Table 2). A combination of in vitro and in vivo methodologies must, therefore, be used in concert for the definitive evaluation of both the innate and adaptive immune responses (Table 3). Importantly, all experimental paradigms have inherent weaknesses and advantages, and extrapolation of the findings to the clinical scenario should be made with caution. We described several confounders that can affect the conclusions of immunogenicity studies, including the original cell source, differentiation procedure, the identity of the cell type of interest, maturation status, site of transplantation, or methodological limitations (Fig. 4). This complexity is mirrored by the plethora of approaches under investigation to ameliorate or eliminate the immune response to cellular therapies (Table 4). In view of the redundancy displayed by the immune system, it is likely that a combined methodology is would be the most feasible and cost-effective treatment modality.
We conclude that the immunological assessment of cellular therapies is as necessary as functional or safety verification prior to clinical translation. Few research groups possess the complete range of resources, experimental models, and expertise to conduct the full breadth of studies required to generate definitive and validated immunogenicity data that would be relevant to multiple cellular therapies. We, therefore, suggest that a reasonable and practical approach would be the establishment of collaborative networks and platforms combining diverse expertise and infrastructure, which would warrant standardized methods for their immunogenicity assessment (in a similar way to the recent global initiative ARDAT for advanced cell and gene therapies: http://ardat.org/). If supported by the full breadth of in vitro and in vivo experimental models currently available, such a consortium approach would be well placed to plan and execute definitive immunogenicity studies that ultimately ensure the safe and efficient clinical translation of any cellular product.
References
Blau, H. M. & Daley, G. Q. Stem cells in the treatment of disease. N. Engl. J. Med. 380, 1748–1760 (2019).
Takahashi, K. et al. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell 131, 861–872 (2007).
Park, I.-H. et al. Reprogramming of human somatic cells to pluripotency with defined factors. Nature 451, 141–146 (2008).
Zhao, T., Zhang, Z.-N., Rong, Z. & Xu, Y. Immunogenicity of induced pluripotent stem cells. Nature 474, 212–215 (2011).
Guha, P., Morgan, J. W., Mostoslavsky, G., Rodrigues, N. P. & Boyd, A. S. Lack of immune response to differentiated cells derived from syngeneic induced pluripotent stem cells. Cell Stem Cell 12, 407–412 (2013).
Kennedy, L. B. & Salama, A. K. S. A review of cancer immunotherapy toxicity. CA Cancer J. Clin. 70, 86–104 (2020).
Larkins, N. G., Wong, G., Taverniti, A. & Lim, W. H. Epitope matching in kidney transplantation: recent advances and current limitations. Curr. Opin. Organ Transpl. 24, 370 (2019).
Amarante-Mendes, G. P. et al. Pattern recognition receptors and the host cell death molecular machinery. Front. Immunol. 9, 2379 (2018).
Abel, A. M., Yang, C., Thakar, M. S. & Malarkannan, S. Natural killer cells: development, maturation, and clinical utilization. Front. Immunol. 9, 1869 (2018).
Krzewski, K. & Strominger, J. L. The killer’s kiss: the many functions of NK cell immunological synapses. Curr. Opin. Cell Biol. 20, 597–605 (2008).
Karahan, G. E., Claas, F. H. J. & Heidt, S. B cell immunity in solid organ transplantation. Front. Immunol. 7, 686 (2016).
Ng, Y.-H., Oberbarnscheidt, M. H., Chandramoorthy, H. C. K., Hoffman, R. & Chalasani, G. B cells help alloreactive T cells differentiate into memory T cells. Am. J. Transpl. 10, 1970–1980 (2010).
Becker, P. D. et al. B lymphocytes contribute to indirect pathway T cell sensitization via acquisition of extracellular vesicles. Am. J. Transpl. (2020) https://doi.org/10.1111/ajt.16088.
Vazquez, M. I., Catalan-Dibene, J. & Zlotnik, A. B cells responses and cytokine production are regulated by their immune microenvironment. Cytokine 74, 318–326 (2015).
Karre, K., Ljunggren, H. G., Piontek, G. & Kiessling, R. Selective rejection of H-2-deficient lymphoma variants suggests alternative immune defence strategy. Nature 319, 675–678 (1986).
Ljunggren, H. G. & Karre, K. In search of the ‘missing self’: MHC molecules and NK cell recognition. Immunol. Today 11, 237–244 (1990).
Nagano, K., Yoshida, Y. & Isobe, T. Cell surface biomarkers of embryonic stem cells. Proteomics 8, 4025–4035 (2008).
Schopperle, W. M. & DeWolf, W. C. The TRA‐1‐60 and TRA‐1‐81 human pluripotent stem cell markers are expressed on podocalyxin in embryonal carcinoma. Stem Cells (2007).
Brimble, S. N. et al. The cell surface glycosphingolipids SSEA-3 and SSEA-4 are not essential for human ESC pluripotency. Stem Cells 25, 54–62 (2007).
Drukker, M. et al. Characterization of the expression of MHC proteins in human embryonic stem cells. Proc. Natl Acad. Sci. USA 99, 9864–9869 (2002).
Boyd, A. S. & Wood, K. J. Variation in MHC expression between undifferentiated mouse ES cells and ES cell-derived insulin-producing cell clusters. Transplantation 87, 1300–1304 (2009).
Yokoyama, W. M. & Kim, S. How do natural killer cells find self to achieve tolerance? Immunity 24, 249–257 (2006).
Petrus-Reurer, S. et al. Generation of retinal pigment epithelial cells derived from human embryonic stem cells lacking human leukocyte antigen class I and II. Stem Cell Rep. (2020).
Kamao, H. et al. Characterization of human induced pluripotent stem cell-derived retinal pigment epithelium cell sheets aiming for clinical application. Stem Cell Rep. 2, 205–218 (2014).
Idelson, M. et al. Immunological properties of human embryonic stem cell-derived retinal pigment epithelial cells. Stem Cell Rep. 11, 681–695 (2018).
Deuse, T. et al. Hypoimmunogenic derivatives of induced pluripotent stem cells evade immune rejection in fully immunocompetent allogeneic recipients. Nat. Biotechnol. 37, 252–258 (2019).
Gornalusse, G. G. et al. HLA-E-expressing pluripotent stem cells escape allogeneic responses and lysis by NK cells. Nat. Biotechnol. 35, 765–772 (2017).
Sugita, S., Makabe, K., Iwasaki, Y., Fujii, S. & Takahashi, M. Natural killer cell inhibition by HLA-E molecules on induced pluripotent stem cell-derived retinal pigment epithelial cells. Investig. Ophthalmol. Vis. Sci. 59, 1719–1731 (2018).
Sacks, S. H. & Zhou, W. The role of complement in the early immune response to transplantation. Nat. Rev. Immunol. 12, 431–442 (2012).
Tjernberg, J., Ekdahl, K. N., Lambris, J. D., Korsgren, O. & Nilsson, B. Acute antibody-mediated complement activation mediates lysis of pancreatic islets cells and may cause tissue loss in clinical islet transplantation. Transplantation 85, 1193–1199 (2008).
Thorgersen, E. B. et al. The role of complement in liver injury, regeneration, and transplantation. Hepatology 70, 725–736 (2019).
Sugita, S., Makabe, K., Fujii, S. & Takahashi, M. Detection of complement activators in immune attack eyes after iPS-derived retinal pigment epithelial cell transplantation. Investig. Ophthalmol. Vis. Sci. 59, 4198–4209 (2018).
Fanelli, G. et al. Human stem cell-derived retinal epithelial cells activate complement via collectin 11 in response to stress. Sci. Rep. 7, 14625 (2017).
Marino, J., Paster, J. & Benichou, G. Allorecognition by T lymphocytes and allograft rejection. Front. Immunol. 7, 582 (2016).
Roche, P. A. & Furuta, K. The ins and outs of MHC class II-mediated antigen processing and presentation. Nat. Rev. Immunol. 15, 203–216 (2015).
Jurewicz, M. M. & Stern, L. J. Class II MHC antigen processing in immune tolerance and inflammation. Immunogenetics 71, 171–187 (2019).
Afzali, B., Lombardi, G. & Lechler, R. I. Pathways of major histocompatibility complex allorecognition. Curr. Opin. Organ Transpl. 13, 438–444 (2008).
Smyth, L. A., Lechler, R. I. & Lombardi, G. Continuous acquisition of MHC: peptide complexes by recipient cells contributes to the generation of anti-graft CD8+ T cell immunity. Am. J. Transpl. 17, 60–68 (2017).
McGill, T. J. et al. Allogeneic iPSC-derived RPE cell graft failure following transplantation into the subretinal space in nonhuman primates. Investig. Ophthalmol. Vis. Sci. 59, 1374–1383 (2018).
Sugita, S. et al. Detection of retinal pigment epithelium-specific antibody in iPSC-derived retinal pigment epithelium transplantation models. Stem Cell Rep. 9, 1501–1515 (2017).
Sugita, S. et al. Successful transplantation of retinal pigment epithelial cells from MHC homozygote iPSCs in MHC-matched models. Stem Cell Rep. 7, 635–648 (2016).
Sugita, S. et al. Lack of T cell response to iPSC-derived retinal pigment epithelial cells from HLA homozygous donors. Stem Cell Rep. 7, 619–634 (2016).
Wenkel, H. & Streilein, J. W. Evidence that retinal pigment epithelium functions as an immune-privileged tissue. Investig. Ophthalmol. Vis. Sci. 41, 3467–3473 (2000).
Zamiri, P., Masli, S., Streilein, J. W. & Taylor, A. W. Pigment epithelial growth factor suppresses inflammation by modulating macrophage activation. Investig. Ophthalmol. Vis. Sci. 47, 3912–3918 (2006).
Sugita, S. et al. Inhibition of T-cell activation by retinal pigment epithelial cells derived from induced pluripotent stem cells. Investig. Ophthalmol. Vis. Sci. 56, 1051–1062 (2015).
Cai, S. et al. iPSC-derived regulatory dendritic cells inhibit allograft rejection by generating alloantigen-specific regulatory T cells. Stem Cell Rep. 8, 1174–1189 (2017).
Yoshihara, E. et al. Immune-evasive human islet-like organoids ameliorate diabetes. Nature https://doi.org/10.1038/s41586-020-2631-z (2020).
Mattapally, S. et al. Human leukocyte antigen class I and II knockout human induced pluripotent stem cell-derived cells: universal donor for cell therapy. J. Am. Heart Assoc. 7, e010239 (2018).
Brunner, K. T., Mauel, J., Cerottini, J. C. & Chapuis, B. Quantitative assay of the lytic action of immune lymphoid cells on 51-Cr-labelled allogeneic target cells in vitro; inhibition by isoantibody and by drugs. Immunology 14, 181–196 (1968).
Ortaldo, J. R., Wiltrout, R. H. & Reynolds, C. W. Natural killer activity: early days, advances, and seminal observations. Crit. Rev. Oncog. 19, 1–13 (2014).
De Meyer, K., De Baetselier, P., Verschueren, H. & Geldhof, A. B. Morphometric analysis of cytolysis in cultured cell monolayers: a simple and versatile method for the evaluation of the lytic activity and the fate of LAK cells. J. Immunol. Methods 277, 193–211 (2003).
Somanchi, S. S., McCulley, K. J., Somanchi, A., Chan, L. L. & Lee, D. A. A novel method for assessment of natural killer cell cytotoxicity using image cytometry. PLoS ONE 10, e0141074 (2015).
Solly, K., Wang, X., Xu, X., Strulovici, B. & Zheng, W. Application of real-time cell electronic sensing (RT-CES) technology to cell-based assays. Assay. Drug Dev. Technol. 2, 363–372 (2004).
Park, K.-H. et al. Evaluation of NK cell function by flowcytometric measurement and impedance based assay using real-time cell electronic sensing system. Biomed. Res. Int. 2013, 210726 (2013).
Xu, H. et al. Targeted disruption of HLA genes via CRISPR-Cas9 generates iPSCs with enhanced immune compatibility. Cell Stem Cell 24, 566–578 e7 (2019).
Dressel, R. et al. Pluripotent stem cells are highly susceptible targets for syngeneic, allogeneic, and xenogeneic natural killer cells. FASEB J. 24, 2164–2177 (2010).
Pegram, H. J., Andrews, D. M., Smyth, M. J., Darcy, P. K. & Kershaw, M. H. Activating and inhibitory receptors of natural killer cells. Immunol. Cell Biol. 89, 216–224 (2011).
Long, E. O., Kim, H. S., Liu, D., Peterson, M. E. & Rajagopalan, S. Controlling natural killer cell responses: integration of signals for activation and inhibition. Annu. Rev. Immunol. 31, 227–258 (2013).
Fischer, L., Lucendo-Villarin, B., Hay, D. C. & O’Farrelly, C. Human PSC-derived hepatocytes express low levels of viral pathogen recognition receptors, but are capable of mounting an effective innate immune response. Int. J. Mol. Sci. 21 (2020).
Koch, C. A., Jordan, C. E. & Platt, J. L. Complement-dependent control of teratoma formation by embryonic stem cells. J. Immunol. 177, 4803–4809 (2006).
Moser, J. M. et al. Optimization of a dendritic cell-based assay for the in vitro priming of naïve human CD4+ T cells. J. Immunol. Methods 353, 8–19 (2010).
Mousset, C. M. et al. Comprehensive phenotyping of T cells using flow cytometry. Cytometry A 95, 647–654 (2019).
Liu, J. et al. Human neural stem/progenitor cells derived from embryonic stem cells and fetal nervous system present differences in immunogenicity and immunomodulatory potentials in vitro. Stem Cell Res. 10, 325–337 (2013).
Itakura, G. et al. Low immunogenicity of mouse induced pluripotent stem cell-derived neural stem/progenitor cells. Sci. Rep. 7, 12996 (2017).
Mehler, V. J., Burns, C. J., Stauss, H., Francis, R. J. & Moore, M. L. Human iPSC-derived neural crest stem cells exhibit low immunogenicity. Mol. Ther. Methods Clin. Dev. 16, 161–171 (2020).
Zhao, T. et al. Humanized mice reveal differential immunogenicity of cells derived from autologous induced pluripotent stem cells. Cell Stem Cell 17, 353–359 (2015).
Fujii, S. et al. Immunological properties of neural crest cells derived from human induced pluripotent stem cells. Stem Cells Dev. 28, 28–43 (2019).
de Almeida, P. E. et al. Transplanted terminally differentiated induced pluripotent stem cells are accepted by immune mechanisms similar to self-tolerance. Nat. Commun. 5, 3903 (2014).
Kimura, T., Yamashita, A., Ozono, K. & Tsumaki, N. Limited immunogenicity of human induced pluripotent stem cell-derived cartilages. Tissue Eng. Part A 22, 1367–1375 (2016).
Chen, L. & Flies, D. B. Molecular mechanisms of T cell co-stimulation and co-inhibition. Nat. Rev. Immunol. 13, 227–242 (2013).
Sugita, S. et al. T-cell suppression by programmed cell death 1 ligand 1 on retinal pigment epithelium during inflammatory conditions. Investig. Ophthalmol. Vis. Sci. 50, 2862–2870 (2009).
Cichocki, F. et al. iPSC-derived NK cells maintain high cytotoxicity and enhance in vivo tumor control in concert with T cells and anti-PD-1 therapy. Sci. Transl. Med. 12 (2020).
Santos, R. et al. Differentiation of inflammation-responsive astrocytes from glial progenitors generated from human induced pluripotent stem cells. Stem Cell Rep. 8, 1757–1769 (2017).
Edo, A. et al. Capacity of retinal ganglion cells derived from human induced pluripotent stem cells to suppress T-cells. Int. J. Mol. Sci. 21, (2020).
Sohn, E. H. et al. Allogenic iPSC-derived RPE cell transplants induce immune response in pigs: a pilot study. Sci. Rep. 5, 11791 (2015).
Fox, A., Mountford, J., Braakhuis, A. & Harrison, L. C. Innate and adaptive immune responses to nonvascular xenografts: evidence that macrophages are direct effectors of xenograft rejection. J. Immunol. 166, 2133–2140 (2001).
Stripecke, R. et al. Innovations, challenges, and minimal information for standardization of humanized mice. EMBO Mol. Med. 12, e8662 (2020).
Hogenes, M., Huibers, M., Kroone, C. & de Weger, R. Humanized mouse models in transplantation research. Transpl. Rev. 28, 103–110 (2014).
Shultz, L. D., Brehm, M. A., Garcia-Martinez, J. V. & Greiner, D. L. Humanized mice for immune system investigation: progress, promise and challenges. Nat. Rev. Immunol. 12, 786–798 (2012).
Curran, M. et al. Recent advancements and applications of human immune system mice in preclinical immuno-oncology. Toxicol. Pathol. 48, 302–316 (2020).
Matas-Céspedes, A. et al. Use of human splenocytes in an innovative humanised mouse model for prediction of immunotherapy-induced cytokine release syndrome. Clin. Transl. Immunol. 9, e1202 (2020).
Adigbli, G. et al. Humanization of immunodeficient animals for the modeling of transplantation, graft versus host disease, and regenerative medicine. Transplantation 104, 2290 (2020).
Schweitzer, J. S. et al. Personalized iPSC-derived dopamine progenitor cells for Parkinson’s disease. N. Engl. J. Med. 382, 1926–1932 (2020).
Zakrzewski, W., Dobrzyński, M., Szymonowicz, M. & Rybak, Z. Stem cells: past, present, and future. Stem Cell Res. Ther. 10, 68 (2019).
Liu, G., David, B. T., Trawczynski, M. & Fessler, R. G. Advances in pluripotent stem cells: history, mechanisms, technologies, and applications. Stem Cell Rev. Rep. 16, 3–32 (2020).
Puri, M. C. & Nagy, A. Concise review: embryonic stem cells versus induced pluripotent stem cells: the game is on. Stem Cells 30, 10–14 (2012).
Eguizabal, C. et al. Two decades of embryonic stem cells: a historical overview. Hum. Reprod. Open 2019, hoy024 (2019).
Martin, M. J., Muotri, A., Gage, F. & Varki, A. Human embryonic stem cells express an immunogenic nonhuman sialic acid. Nat. Med. 11, 228–232 (2005).
Tangvoranuntakul, P. et al. Human uptake and incorporation of an immunogenic nonhuman dietary sialic acid. Proc. Natl Acad. Sci. USA. 100, 12045–12050 (2003).
Heiskanen, A., Satomaa, T., Tiitinen, S. & Laitinen, A. N‐glycolylneuraminic acid xenoantigen contamination of human embryonic and mesenchymal stem cells is substantially reversible. Stem 25, 197–202 (2007).
Okamura, R. M. et al. Immunological properties of human embryonic stem cell-derived oligodendrocyte progenitor cells. J. Neuroimmunol. 192, 134–144 (2007).
Chung, T.-L. et al. Ascorbate promotes epigenetic activation of CD30 in human embryonic stem cells. Stem Cells 28, 1782–1793 (2010).
Prowse, A. B. J. et al. Long term culture of human embryonic stem cells on recombinant vitronectin in ascorbate free media. Biomaterials 31, 8281–8288 (2010).
Werbowetski-Ogilvie, T. E. et al. Characterization of human embryonic stem cells with features of neoplastic progression. Nat. Biotechnol. 27, 91–97 (2009).
Draper, J. S. et al. Recurrent gain of chromosomes 17q and 12 in cultured human embryonic stem cells. Nat. Biotechnol. 22, 53–54 (2004).
Spits, C. et al. Recurrent chromosomal abnormalities in human embryonic stem cells. Nat. Biotechnol. 26, 1361–1363 (2008).
International Stem Cell Initiative et al. Screening ethnically diverse human embryonic stem cells identifies a chromosome 20 minimal amplicon conferring growth advantage. Nat. Biotechnol. 29, 1132–1144 (2011).
Fernandez, N. et al. A critical review of the role of the major histocompatibility complex in fertilization, preimplantation development and feto-maternal interactions. Hum. Reprod. Update 5, 234–248 (1999).
Gardner, J. M., Fletcher, A. L., Anderson, M. S. & Turley, S. J. AIRE in the thymus and beyond. Curr. Opin. Immunol. 21, 582–589 (2009).
Jonsson, A. H. & Yokoyama, W. M. Natural killer cell tolerance licensing and other mechanisms. Adv. Immunol. 101, 27–79 (2009).
Orr, M. T. & Lanier, L. L. Natural killer cell education and tolerance. Cell 142, 847–856 (2010).
Trowsdale, J. & Betz, A. G. Mother’s little helpers: mechanisms of maternal-fetal tolerance. Nat. Immunol. 7, 241–246 (2006).
Yachimovich-Cohen, N., Even-Ram, S., Shufaro, Y., Rachmilewitz, J. & Reubinoff, B. Human embryonic stem cells suppress T cell responses via arginase I-dependent mechanism. J. Immunol. 184 1300–1308 (2010).
Trigona, W. L., Porter, C. M., Horvath-Arcidiacono, J. A., Majumdar, A. S. & Bloom, E. T. Could heme-oxygenase-1 have a role in modulating the recipient immune response to embryonic stem cells? Antioxid. Redox Signal. 9, 751–756 (2007).
Utermöhlen, O. & Krönke, M. Survival of priceless cells: active and passive protection of embryonic stem cells against immune destruction. Arch. Biochem. Biophys. 462, 273–277 (2007).
Li, L., Baroja, M. L., Majumdar, A. & Chadwick, K. Human embryonic stem cells possess immune‐privileged properties. Stem 22 448–456 (2004).
Fabricius, D., Bonde, S. & Zavazava, N. Induction of stable mixed chimerism by embryonic stem cells requires functional Fas/FasL engagement. Transplantation 79, 1040–1044 (2005).
Bonde, S. & Zavazava, N. Immunogenicity and engraftment of mouse embryonic stem cells in allogeneic recipients. Stem Cells 24, 2192–2201 (2006).
Brunlid, G., Pruszak, J., Holmes, B., Isacson, O. & Sonntag, K.-C. Immature and neurally differentiated mouse embryonic stem cells do not express a functional Fas/Fas ligand system. Stem Cells 25, 2551–2558 (2007).
Kim, K. et al. Epigenetic memory in induced pluripotent stem cells. Nature 467, 285–290 (2010).
Cabrera, O. et al. The unique cytoarchitecture of human pancreatic islets has implications for islet cell function. Proc. Natl Acad. Sci. USA. 103, 2334–2339 (2006).
Ravier, M. A. et al. Loss of connexin36 channels alters beta-cell coupling, islet synchronization of glucose-induced Ca2+ and insulin oscillations, and basal insulin release. Diabetes 54, 1798–1807 (2005).
Wojtusciszyn, A., Armanet, M., Morel, P., Berney, T. & Bosco, D. Insulin secretion from human beta cells is heterogeneous and dependent on cell-to-cell contacts. Diabetologia 51, 1843–1852 (2008).
Fan, Y., Winanto & Ng, S.-Y. Replacing what’s lost: a new era of stem cell therapy for Parkinson’s disease. Transl. Neurodegener. 9, 2 (2020).
Schwartz, S. D. et al. Human embryonic stem cell-derived retinal pigment epithelium in patients with age-related macular degeneration and Stargardt’s macular dystrophy: follow-up of two open-label phase 1/2 studies. Lancet 385, 509–516 (2015).
Schwartz, S. D., Tan, G., Hosseini, H. & Nagiel, A. Subretinal transplantation of embryonic stem cell-derived retinal pigment epithelium for the treatment of macular degeneration: an assessment at 4 years. Investig. Ophthalmol. Vis. Sci. 57, ORSFc1–9 (2016).
Schwartz, S. D. et al. Embryonic stem cell trials for macular degeneration: a preliminary report. Lancet 379, 713–720 (2012).
Sung, Y. et al. Long-term safety and tolerability of subretinal transplantation of embryonic stem cell-derived retinal pigment epithelium in Asian Stargardt disease patients. Br. J. Ophthalmol. (2020) https://doi.org/10.1136/bjophthalmol-2020-316225.
Menasche, P. et al. Human embryonic stem cell-derived cardiac progenitors for severe heart failure treatment: first clinical case report. Eur. Heart J. 36, 2011–2017 (2015).
Wiseman, A. C. Immunosuppressive medications. Clin. J. Am. Soc. Nephrol. 11, 332–343 (2016).
Hartono, C., Muthukumar, T. & Suthanthiran, M. Immunosuppressive drug therapy. Cold Spring Harb. Perspect. Med. 3, a015487 (2013).
Piselli, P. et al. De novo malignancies after organ transplantation: focus on viral infections. Curr. Mol. Med. 13, 1217–1227 (2013).
Ventura-Aguiar, P., Campistol, J. M. & Diekmann, F. Safety of mTOR inhibitors in adult solid organ transplantation. Expert Opin. Drug Saf. 15, 303–319 (2016).
Malvezzi, P. & Rostaing, L. The safety of calcineurin inhibitors for kidney-transplant patients. Expert Opin. Drug Saf. 14, 1531–1546 (2015).
Süsal, C. & Opelz, G. Current role of human leukocyte antigen matching in kidney transplantation. Curr. Opin. Organ Transpl. 18, 438–444 (2013).
Taylor, C. J., Peacock, S., Chaudhry, A. N., Bradley, J. A. & Bolton, E. M. Generating an iPSC bank for HLA-matched tissue transplantation based on known donor and recipient HLA types. Cell Stem Cell 11, 147–152 (2012).
Umekage, M., Sato, Y. & Takasu, N. Overview: an iPS cell stock at CiRA. Inflamm. Regener. 39, 17 (2019).
Nakatsuji, N., Nakajima, F. & Tokunaga, K. HLA-haplotype banking and iPS cells. Nat. Biotechnol. 26, 739–740 (2008).
Opelz, G., Wujciak, T., Döhler, B., Scherer, S. & Mytilineos, J. HLA compatibility and organ transplant survival. Collaborative transplant study. Rev. Immunogenet. 1, 334–342 (1999).
Zhao, L., Teklemariam, T. & Hantash, B. M. Heterelogous expression of mutated HLA-G decreases immunogenicity of human embryonic stem cells and their epidermal derivatives. Stem Cell Res. 13, 342–354 (2014).
Zhao, H.-X. et al. Enhanced immunological tolerance by HLA-G1 from neural progenitor cells (NPCs) derived from human embryonic stem cells (hESCs). Cell. Physiol. Biochem. 44, 1435–1444 (2017).
Harding, J., Vintersten-Nagy, K., Shutova, M. & Yang, H. Induction of long-term allogeneic cell acceptance and formation of immune privileged tissue in immunocompetent hosts. bioRxiv (2019).
Liang, Q. et al. Linking a cell-division gene and a suicide gene to define and improve cell therapy safety. Nature 563, 701–704 (2018).
Chidgey, A. P., Layton, D., Trounson, A. & Boyd, R. L. Tolerance strategies for stem-cell-based therapies. Nature 453, 330–337 (2008).
Fife, B. T. & Bluestone, J. A. Control of peripheral T‐cell tolerance and autoimmunity via the CTLA‐4 and PD‐1 pathways. Immunol. Rev. (2008).
Li, J., Tan, J., Martino, M. M. & Lui, K. O. Regulatory T-cells: potential regulator of tissue repair and regeneration. Front. Immunol. 9, 585 (2018).
Romano, M., Tung, S. L., Smyth, L. A. & Lombardi, G. Treg therapy in transplantation: a general overview. Transpl. Int. 30, 745–753 (2017).
Romano, M., Fanelli, G., Albany, C. J., Giganti, G. & Lombardi, G. Past, present, and future of regulatory T cell therapy in transplantation and autoimmunity. Front. Immunol. 10, 43 (2019).
Hirakawa, M. et al. Low-dose IL-2 selectively activates subsets of CD4+ Tregs and NK cells. JCI Insight 1, e89278 (2016).
Francisco, L. M., Sage, P. T. & Sharpe, A. H. The PD-1 pathway in tolerance and autoimmunity. Immunol. Rev. 236, 219–242 (2010).
Kharfan-Dabaja, M. A. & Bazarbachi, A. Emerging role of CD20 blockade in allogeneic hematopoietic cell transplantation. Biol. Blood Marrow Transpl. 16, 1347–1354 (2010).
Sood, P. & Hariharan, S. Anti-CD20 blocker rituximab in kidney transplantation. Transplantation 102, 44–58 (2018).
Ford, M. L. & Larsen, C. P. Translating costimulation blockade to the clinic: lessons learned from three pathways. Immunol. Rev. 229, 294–306 (2009).
Landwehr-Kenzel, S. et al. Novel GMP-compatible protocol employing an allogeneic B cell bank for clonal expansion of allospecific natural regulatory T Cells: nTreg expansion by allogeneic B cells. Am. J. Transpl. 14, 594–606 (2014).
Chandran, S. et al. Polyclonal regulatory T cell therapy for control of inflammation in kidney transplants. Am. J. Transpl. 17, 2945–2954 (2017).
Sawitzki, B. et al. Regulatory cell therapy in kidney transplantation (the ONE study): a harmonised design and analysis of seven non-randomised, single-arm, phase 1/2A trials. Lancet 395, 1627–1639 (2020).
Roemhild, A. et al. Regulatory T cells for minimising immune suppression in kidney transplantation: phase I/IIa clinical trial. BMJ 371, (2020).
Sánchez‐Fueyo, A. et al. Applicability, safety, and biological activity of regulatory T cell therapy in liver transplantation. Am. J. Transpl. 20, 1125–1136 (2020).
Tang, Q. & Bluestone, J. A. Regulatory T-cell therapy in transplantation: moving to the clinic. Cold Spring Harb. Perspect. Med. 3, (2013).
Nadig, S. N. et al. In vivo prevention of transplant arteriosclerosis by ex vivo—expanded human regulatory T cells. Nat. Med. 16, 809 (2010).
Putnam, A. L. et al. Clinical grade manufacturing of human alloantigen-reactive regulatory T cells for use in transplantation: clinical grade alloantigen-reactive Tregs. Am. J. Transpl. 13, 3010–3020 (2013).
Sagoo, P. et al. Human regulatory T cells with alloantigen specificity are more potent inhibitors of alloimmune skin graft damage than polyclonal regulatory T cells. Sci. Transl. Med. 3, 83ra42 (2011).
de Vos, P., Spasojevic, M. & Faas, M. M. Treatment of diabetes with encapsulated islets. Adv. Exp. Med. Biol. 670, 38–53 (2010).
Desai, T. & Shea, L. D. Advances in islet encapsulation technologies. Nat. Rev. Drug Discov. 16, 367 (2017).
Vaithilingam, V. & Tuch, B. E. Islet transplantation and encapsulation: an update on recent developments. Rev. Diabet. Stud. 8, 51–67 (2011).
Vegas, A. J. et al. Long-term glycemic control using polymer-encapsulated human stem cell-derived beta cells in immune-competent mice. Nat. Med. 22, 306–311 (2016).
Sneddon, J. B. et al. Stem cell therapies for treating diabetes: progress and remaining challenges. Cell Stem Cell 22, 810–823 (2018).
Zimmermann, J. A., Hettiaratchi, M. H. & McDevitt, T. C. Enhanced immunosuppression of T cells by sustained presentation of bioactive interferon-γ within three-dimensional mesenchymal stem cell constructs. Stem Cells Transl. Med. 6, 223–237 (2017).
Julier, Z., Park, A. J., Briquez, P. S. & Martino, M. M. Promoting tissue regeneration by modulating the immune system. Acta Biomater. 53, 13–28 (2017).
Mao, A. S. et al. Programmable microencapsulation for enhanced mesenchymal stem cell persistence and immunomodulation. Proc. Natl Acad. Sci. USA 116, 15392–15397 (2019).
Dellacherie, M. O., Seo, B. R. & Mooney, D. J. Macroscale biomaterials strategies for local immunomodulation. Nat. Rev. Mater. 4, 379–397 (2019).
Hotaling, N. A., Tang, L., Irvine, D. J. & Babensee, J. E. Biomaterial strategies for immunomodulation. Annu. Rev. Biomed. Eng. 17, 317–349 (2015).
Hume, P. S., He, J., Haskins, K. & Anseth, K. S. Strategies to reduce dendritic cell activation through functional biomaterial design. Biomaterials 33, 3615–3625 (2012).
Singh, A., Suri, S. & Roy, K. In-situ crosslinking hydrogels for combinatorial delivery of chemokines and siRNA-DNA carrying microparticles to dendritic cells. Biomaterials 30, 5187–5200 (2009).
Singh, A. et al. An injectable synthetic immune-priming center mediates efficient T-cell class switching and T-helper 1 response against B cell lymphoma. J. Control. Release 155, 184–192 (2011).
Singh, A. et al. Efficient modulation of T-cell response by dual-mode, single-carrier delivery of cytokine-targeted siRNA and DNA vaccine to antigen-presenting cells. Mol. Ther. 16, 2011–2021 (2008).
Boonyaratanakornkit, J. & Taylor, J. J. Techniques to study antigen-specific B cell responses. Front. Immunol. 10, 1694 (2019).
Acknowledgments
This work was supported by the UK Regenerative Medicine Platform (UKRMP, [Grant number MR/S020934/1]). This research was also funded in part, by the Wellcome Trust [Grant number RG79413]. For the purpose of open access, the author has applied a CC BY public copyright licence to any Author Accepted Manuscript version arising from this submission. Figure drawings were created with BioRender.com.
Author information
Authors and Affiliations
Contributions
S.P.-R. and M.R. wrote the manuscript. K.S.P., G.L., J.L.J., and S.H. revised and edited the text. S.P.-R. made the figures. All authors contributed to discussions and structure definitions of the respective sections.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information Communications Biology thanks the anonymous reviewers for their contribution to the peer review of this work. Primary Handling Editors: Karli Montague-Cardoso. Peer reviewer reports are available.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Petrus-Reurer, S., Romano, M., Howlett, S. et al. Immunological considerations and challenges for regenerative cellular therapies. Commun Biol 4, 798 (2021). https://doi.org/10.1038/s42003-021-02237-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s42003-021-02237-4
This article is cited by
-
Plant-derived nanovesicles: harnessing nature's power for tissue protection and repair
Journal of Nanobiotechnology (2023)
-
Stem cell sources and characterization in the development of cell-based products for treating retinal disease: An NEI Town Hall report
Stem Cell Research & Therapy (2023)
-
Immunogenicity Monitoring Cell Chip Incorporating Finger-Actuated Microfluidic and Colorimetric Paper-Based Analytical Functions
BioChip Journal (2023)
-
Bone marrow-derived mesenchymal stem cells transplantation downregulates pancreatic NF-κB and pro-inflammatory cytokine profile in rats with type I and type II-induced diabetes: a comparison study
Biologia (2023)
-
Corneal epithelial differentiation of human pluripotent stem cells generates ABCB5+ and ∆Np63α+ cells with limbal cell characteristics and high wound healing capacity
Stem Cell Research & Therapy (2021)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.