Abstract
The Harpy Eagle (Harpia harpyja) is threatened with extinction throughout its distribution in the neotropical forests. In the Atlantic Forest, deforestation has reduced the number of suitable habitats, with only a few remnant forest fragments hosting active nests; currently, the only known nests in this region are in the Central Atlantic Forest Ecological Corridor (CAFEC), in Brazil. Little is known about Harpy Eagle diets in this region, despite this information being essential for developing effective conservation strategies. We classified the composition, frequency, richness, ecological attributes, and conservation status of the species that make up the Harpy Eagle’s diet in its last refuges in the CAFEC. Between 2017 and 2021, we collected and analyzed 152 prey remains and 285 camera trap photographs from seven active nests. We identified at least 16 mammal species (96.7%), one parrot and other bird remains (3.3%). The Harpy Eagle’s diet consisted mainly of medium-sized arboreal, folivorous, frugivorous, and diurnal mammals. Five prey species are currently threatened with extinction at global, six at national and seven at regional levels. The majority of the diet consists of Sapajus robustus, which is threatened, and Bradypus variegatus, which is not threatened. In addition to the effects of habitat loss and hunting, the Harpy Eagle may also suffer from the decline in the populations of their prey in the Atlantic Forest.
Similar content being viewed by others
Introduction
The Harpy Eagle (Harpia harpyja) (Fig. 1) is one of the largest eagles in the world. It is found in the neotropical forests of Central and South America, mainly in the Brazilian Amazon and Atlantic forests, and depends on forests for reproduction and foraging1,2. Breeding pairs nest in emergent and canopy trees standing over 40 m in height and usually they returns to the same tree to nest throughout their reproductive life3,4,5,6. The Harpy Eagle forages across a large area around its nest7,8, and feeds mainly on arboreal prey, such as sloths and monkeys9,10,11.
The Harpy Eagle is threatened with extinction, specifically in the Vulnerable category2. In Brazil, it is also classified as Vulnerable12. Habitat loss, hunting, and persecution comprise the major threats to wild Harpy Eagle populations2,13,14. They are sensitive to habitat modifications, and the problem is confounded by their low population density and reproductive rates, producing only one eaglet every 2.5–3 years4,9,15.
In the Brazilian Atlantic Forest, the Harpy Eagle’ situation is more alarming16, it is classified as Critically Endangered in most states that constitute its range including Espírito Santo (ES)17 and Bahia (BA)18. The Atlantic Forest is a global biodiversity hotspot19, yet over 85% of the original forest has been lost20. Although records of individual Harpy Eagles are scattered throughout the region21,22,23,24,25,26,27,28, nesting records are rare, and located primarily in the Misiones region of Argentina29. The most recent nest records in protected areas are in the Central Atlantic Forest Ecological Corridor (CAFEC) in northern ES30 and southern BA26,31.
The loss of forest cover also impacts species populations that make up the diet of the Harpy Eagle, which means resource limitations for maintaining the population of this predator. This is because some of the prey species are also dependent on intact, hunting-free forests to survive. Therefore, if prey species disappear due to habitat degradation, it can lead to the disruption of food chains and dietary overlap among species that previously did not compete for the same food resources, causing an ecological imbalance32.
Studies on the Harpy Eagle’s diet have been conducted mainly in the Amazon9,10,11,15,33,34 and in the Atlantic Forest, some information on the Harpy Eagle’s diet has been gathered from studies conducted in Argentina29 and Brazil22,30,31. However, no study has focused on the Harpy Eagle’s feeding habits in this region, where both the predator and its potential prey are dramatically at risk of extinction. In this study, we investigated the Harpy Eagle’s feeding habits in protected areas in the CAFEC and assessed the composition, foraging stratum, diet, estimated body mass, and conservation status of its prey.
Materials and methods
Study area
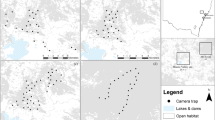
The study was conducted in four protected areas located in the CAFEC in southeastern and northeastern Brazil (Fig. 2): Vale Natural Reserve (VNR) and Sooretama Biological Reserve (SBR) are contiguous and have areas of 22,711 ha and 27,558 ha, respectively, and are located in the drainage basins of the Doce river in northern ES; Veracel Station Private Natural Heritage Reserve (VS-PNHR) covers 6069 ha in the basin of the Jequitinhonha river in southern BA; and Serra Bonita Private Natural Heritage Reserve (SB-PNHR) is a group of preserved rural properties which form an area of 2500 ha in the basin of the Pardo river in southern BA.
Location of Harpy Eagle nests monitored in Central Atlantic Forest Ecological Corridor reserves. Abbreviations: Bahia (BA), Espírito Santo (ES), Serra Bonita Private Natural Heritage Reserve (SB-PNHR), Sooretama Biological Reserve (SBR), Vale Natural Reserve (VNR), Veracel Station Private Natural Heritage Reserve (VS-PNHR). The map was made using with licensed software ArcGIS Pro version 10.8.2 (https://www.esri.com/en-us/arcgis/products/arcgis-pro/overview).
We monitored seven active Harpy Eagle nests in the CAFEC. Although the Harpy Eagle nests monitored in this study are in forested areas, it is important to highlight that each nest has its own surrounding vegetative landscape. In the VNR was monitored three nests of the species (VNR-01, VNR-02 and VNR-03). The VNR-01 nest is located approximately 600 m away from pasture areas and land intended for growing papaya and coffee. The VNR-02 nest is located approximately 1 km away from the crops, while the VNR-03 nest is further from the edge, it is approximately 5 km away from these cultivation areas. In the SBR, only one nest was monitored (SBR-01), which is located approximately 2.5 km away from BR-101 highway. Regarding VS-PNHR, two nests were monitored (VS-PNHR-01 and VS-PNHR-02), which are approximately 2 km away each from the pasture and eucalyptus plantation areas. Finally, in the SB-PNHR, only one nest was monitored (SB-PNHR-01), which is located approximately 400 m away to the cultivation areas, where cocoa was planted in the shade of the native forest, known as cabrucas.
Data collection
We collected our data between March 2017 and February 2021 (Table 1). During the study, the seven pairs of Harpy Eagles were at different stages of the reproductive cycle. In VNR-01 nest, we recorded two unsuccessful breeding attempts and one successful. In the latter, we monitored the development of the eaglet from birth (February 2019) until the two years old. In VNR-02 nest, the couple frequented the nest and hatched an egg, but the eaglet was not born. In VNR-03 nest, although the couple visited the nest and began the courtship period, no eggs were laid during the monitoring period. In SBR-01 nest, we monitored a five-month-old eaglet until the end of the reproductive cycle (September 2017–January 2019), when it left the nest. In a second cycle, the couple from the SBR-01 nest tried to reproduce again, but the eaglet died less than month old (October 2019–January 2020). In VS-PNHR-01 nest, we monitored the final stage of the couple’s reproductive cycle, with the eaglet outside the nest but spotted nearby. In VS-PNHR-02 nest, we monitored a four-month-old eaglet until the end of the reproductive cycle (May 2018–September 2019). In SB-PNHR-01 nest, we monitored a female incubating the egg until the end of the reproductive cycle (July 2018–January 2020).
We installed a nylon shade cloth under each nest, about 10–20 m2 and 1.30 m above the ground, to intercept prey remains that fell from nests, such as bone fragments, unconsumed prey remains, and regurgitated pellets33. The use of raised nets lowered the chance of ground scavengers feeding on remains. In addition, we inspected the soil and plant litter within a radius of approximately 10 m around each nest tree for prey remains and regurgitated pellets that may have fallen outside the nylon shade cloth.
A Bushnell camera trap (CT) with infrared motion detectors (Models: 119936C, 119537, 119837) was hoisted by ropes to the crown of each nesting tree and positioned approximately 5–10 m away from, and aimed towards, the nest. The CTs were programmed to take three pictures every 10 min or one picture and a 10 s video every 5 min when they were triggered by wind movement or when something moved in front of them. Occasionally, in some months it was not possible to obtain photographic records due to battery failures, equipment malfunctions, or changes in the CT’s positioning due to wind and rain.
The nests were visited by the team monthly e whenever we collected prey remains from soil and nylon shade, we also downloaded data from the CTs and changed their batteries and memory cards.
Identifying prey in remains
We examined the body parts of the collected prey remains to identify each item to the most specific taxonomic level possible. We identified bones, teeth, claws, and feathers by their external morphology through comparisons to zoological collections, guides, and the literature to identify the species35,36,37,38,39,40,41,42. We identified hairs through trichology, which involves analyzing the characteristics of the hair’s cuticle and medulla. We captured close-up images using the Leica LAS EZ 3.0 software and compared them with images from the Guia de Identificação de Pelos de Mamíferos Brasileiros (Hair Identification Guide for Brazilian Mammals)43 and other literature to identify the species44,45. When the morphological analyses only enabled us to identify prey at the genus level, we consulted available species lists for the nests’ region to identify the most likely candidate46,47,48,49.
We determined the minimum number of individuals per prey species consumed based on the quantity of cranial, mandibular, and pelvic bones and the maximum number of teeth and nails for an individual. In some cases of species without cranial or mandibular bones present, we used the thoracic or pelvic limbs, such as humeri and femora. In these cases, the events were individualized considering the paired bones (right and left). In the case of fragmented crania, mandibles, and pelves, we fit all the parts that appeared to be complementary to the various samples for each nest.
Identifying prey from CT photographs
The CTs were often triggered by eagles arriving to their nests holding prey in their talons. We used the resultant images to identify prey species by observing the coloration, size, and form of the captured prey. We compared the images to those of the species previously found in the region to identify the prey as precisely as possible, in accordance with Emmons and Feer50 and Reis et al.51. Records of a prey species in the same nest and within an interval of 24 h were considered a single record. We calculated the records effort based on the number of CTs multiplied by the number of sampling days, where each day corresponded to a 24 h period. Records success was expressed as a percentage determined by the number of records divided by the records effort and multiplied by 10052. Because of considerable overlap between photographic and remains data, we did not combine results for the number of individuals per species identified from remains and photographs.
Prey characterization
We categorized each identified mammal species by: its habits, based on the foraging attributes described by Wilman et al.53, such as main diet (folivorous, frugivorous, omnivorous, carnivorous, or insectivorous), predominant foraging stratum (arboreal, scansorial, or terrestrial), activity period (diurnal, nocturnal or cathemeral); mean adult body weight in kilograms (kg), according to Wilman et al.53; and to its conservation status at the global54, national12, and regional levels and in BA18 and ES55. Based on Eisenberg and Redford56, we classified mammals by size: small (< 1 kg), medium (1–10 kg), and large (> 10 kg). The percent contribution of each prey species to the total biomass of the Harpy Eagle diet was calculated as the percentage of potentially consumed biomass (PB = ((N * Pm)/∑ (N * Pm)) * 100), where N represents the number of consumed prey and Pm signifies the average weight of each species.
To compare species richness between the nests in BA and ES and assess whether our sampling efforts were sufficient to represent the assemblage of prey consumed by the Harpy Eagle's in these areas, we used species accumulation curves57,58 and the Jackknife-1 estimator57 with the EstimateS 9.1 software59 for the two methods used in the study. We generated the species accumulation and species richness projection plots using the R programming platform60. It is worth noting that ES contains more sampled nests when compared to BA and that, as mentioned previously, the nests had different stages of reproduction.
Based on the list of known mammals found in the studied reserves, we indicated which species could be potential prey for Harpy Eagles. We verified the list by Srbek-Araujo and Kierulff48 for VNR and SBR, the list by Falcão et al.46 for VS-PNHR, and the list by Sánchez-Lalinde et al.49 for SB-PNHR. We considered potential prey to be those belonging to the species or genera recorded in the Harpy Eagle’s diet and within the weight distribution of the prey consumed by the Harpy Eagle in this study (Supplementary Table 1).
Ethical approval
The material analyzed in this work was collected under the licenses SISBIO N° 31457-2 e N° 73444-1 by ICMBio.
Consent to participate
All authors consent to their participation in this study.
Results
We monitored the seven Harpy Eagle nests monthly over a period that varied from 15 to 41 months, totaling 275 visits. During these visits, we collected prey remains below the nests on 152 occasions, while on 123 visits we did not find any prey remains (Table 1). We collected a total of 94 regurgitated pellets below the monitored nests. The cameras recorded 2170 events in total. Among these events, 285 photographic records revealed the presence of prey brought to the seven nests, ranging from 1 to 147 records per nest (Table 1). The variation in the number of events recorded between nests occurred mainly due to battery failures, equipment malfunctions or changes in the CT position due to wind and rain.
Prey species richness and composition
We identified at least 17 species in the Harpies’ diet with the two methods we used. Sixteen of them are mammals and one is bird that were not identified at the species level, only at the family level (Psittacidae) (Table 2). In both methods, we identified 14 mammal species. Two were identified only in the photographic records (the golden-bellied capuchin (Sapajus xanthosternos) and the nine-banded armadillo, (Dasypus novemcinctus)), and two others were identified only in the trace analysis (the red-rumped agouti (Dasyprocta leporina) and the common tapeti (Sylvilagus brasiliensis)) (Table 2). With regard to remains belonging to the Psittacidae family, we recorded two events (Table 2), which may represent one or even two parrot species, although we were unable to identify them at the species level.
Among the mammals preyed upon by the Harpy Eagles, we recorded at least 13 species in the nests in ES and 14 in BA (Table 2). Ten of the species are commonly recorded in the Harpy Eagle’s diet in both regions (Table 2). The other six were exclusive to the Harpy Eagle’s diet in one of the regions. Specifically, the tayra (Eira barbara), the coastal black-handed titi (Callicebus melanochir), and Sapajus xanthosternos were found only in the diet of the Harpy Eagles in BA. Meanwhile, Dasyprocta leporina and the Atlantic titi (Callicebus personatus) were found only in ES (Table 2). We identified Dasypus novemcinctus only in ES; however, there was one identified trace of the Order Cingulata in BA (Table 2). We found bird remains in three nests in both states (Table 2), but it was not possible to identify them at the species level. In two bird findings inside pellets, one from nest VNR-01 and the other from nest VNR-03 in ES, contour feathers allowed us to identify them down to the family level, Psittacidae (Table 2). We had no photographic record of the Harpy Eagles arriving at the nest with birds as prey.
The accumulation curves of prey species did not stabilize in either region (Fig. 3A–D). Using the remains data, Jackknife-1 estimated 17 (± 1.8) species for ES (Fig. 3A) and 21 (± 2.8) for BA (Fig. 3B). When we compared the observed values with the values estimated by Jackknife-1, collection efficiency was 82% for ES and 71% for BA. Using the photographic records, Jackknife-1 estimated a total of 12 (± 0) species for ES (Fig. 3C) and 15 (± 2.1) for BA (Fig. 3D). When we compared the observed values with the values estimated by Jackknife-1, collection efficiency was 100% for ES and 73% for BA.
Richness accumulation curve observed (dashed black line) and estimated by Jackknife1 (continuous red line) of the species preyed on in the four Harpy Eagle nests located in the Espírito Santo region (A and C) and in the three nests in the Bahia region (B and D). The curves in a and b represent the results found through the methodology of analysis of prey remains found below the nests and in c and d through the camera trap records.
There are 36 known species of medium-sized and large mammals in VNR and SBR48, 33 in VS-PNHR46, and 22 in SB-PNHR49. In all, the assemblage of medium-sized and large mammals recorded in the study area consists of 45 species (Supplementary Table 1). Out of these species, 25 fall within the range of weights that made up the Harpy Eagle’s diet in this study, 28 are members of the genera or species recorded as prey in other regions, and 22 species fall in both parameters (Supplementary Table 1). In total, there are 31 potential prey species for the Harpy Eagle in the CAFEC, and this study was able to confirm 16 (52%) (Supplementary Table 1).
Among the 16 mammal species consumed by Harpy Eagles, seven (43.7%) are threatened with extinction: five at the global (37.5%), six at the national (37.5%), and seven at the regional (43.7%) levels (Table 2).
Prey taxa frequency
We identified 298 individuals from the remains analysis and 287 individuals from the photographic records of the nests (Table 2). From the remains, 288 were mammals and 10 were birds, while all the photographic records were of mammals.
We identified the Bahia porcupine (Coendou insidiosus) and bristle-spined porcupine (Chaetomys subspinosus) from the morphological differences between their spines in the remains and from photographs. However, when we found bone remains of porcupines, it was not possible to differentiate them at the species level. Thus, we quantified the records from the cranial and non-cranial bones of these species as belonging to the Family Erethizontidae, and we did not quantify the number of individuals of the species. The same occurred for the remains belonging to two armadillo individuals. We could not identify them at a more specific taxonomic level, so we only quantified them as belonging to the Order Cingulata (Table 2). However, we were able to quantify the porcupine and armadillo individuals at the species level based on the photographic records (Table 2).
The remains analyses indicated that Bradypus variegatus was the most frequent species among the Harpy Eagle’s prey (37.6%), followed by Sapajus robustus (18.1%) and species of the Family Erethizontidae (14.8%) (Table 2). The photographic records showed that Sapajus robustus was the most frequent prey (23.4%), followed by Bradypus variegatus (12.5%) and the South American coati (Nasua nasua) (6.6%) (Table 2). Eight taxa identified from the remains and six identified from the photographs presented a relative frequency of ≤ 1% in the Harpy Eagle’s diet (Table 2).
Bradypus variegatus was consumed most frequently in BA, constituting 56% of the remains and 20% of the images, while in ES it constituted 19% of the remains and 11.3% of the images (Table 2). In ES, Sapajus robustus was the most frequent species found in the remains (30%) and in the images (31.2%), while the frequency of this species in BA was 7% in the remains and 15% in the images (Table 2).
Attributes of the prey species
The majority of the species recorded in the remains were medium-sized, arboreal, frugivorous, and nocturnal mammals (Supplementary Fig. 1). However, when taking into account the number of individuals of each species, the majority were medium-sized, arboreal, folivorous, and diurnal or cathemeral mammals (Fig. 4). In the photographic records, the majority of the species were arboreal, frugivorous, and diurnal mammals (Supplementary Fig. 1). However, when taking into account the number of individuals of each species, medium-sized, arboreal, omnivorous, and diurnal mammals predominated (Fig. 4).
The mean bodyweight of the prey species we identified ranged from 0.949 to 5.515 kg for Sylvilagus brasiliensis and Tamandua tetradactyla, respectively (Table 2). Considering the average bodyweight and frequency of each adult prey species, the mean prey bodyweight in the entire study area was 3.637 kg (± 1.1) and 2.834 kg (± 1.2) (Fig. 5), based on remains and camera data, respectively. Equivalent values for ES were 3.309 kg (± 1.2) and 2.815 kg (± 1.1), and for BA were 3.943 kg (± 0.9) and 2.929 kg (± 1.3). Among the 16 mammal species found in the Harpy Eagle’s diet, 15 (93.8%) were categorized as medium-sized (1–10 kg) and 1 (6.2%) (Sylvilagus brasiliensis) was small (< 1 kg). Bradypus variegatus and Sapajus robustus presented the highest percentages of potentially consumed biomass (PB), totaling 55.6 and 15.4% in the trace analysis and 28.5 and 37.9% in the photographic records, respectively.
Discussion
This study constitutes the first systematic survey of the prey consumed by Harpy Eagles in their only known active nests in the Atlantic Forest. The high habitat quality of protected areas of the CAFEC may explain the presence of Harpy Eagle nests, which requires the availability of area, nesting trees, and prey. The northern ES and southern BA forests are within the phytogeographic region called Tabuleiro Atlantic Forest (TAF)61. It was one of the last territories in the Atlantic Forest to be exploited by the current cultural and socioeconomic model, which has generated a great loss in forest cover62. Although some nests are close to degraded areas such as pastures, highways and papaya, coffee or cocoa cultivation, the region still hosts a relevant portion of biological richness and has a rich evolutionary history63. It contains the largest fragments for mammal conservation in the Atlantic Forest64.
Prey diversity
The protected areas located in BA (VS-PNHR and SB-PNHR) are smaller in size than those in ES (VNR and SBR). Moreover, the reserves of ES altogether form the largest existing contiguous remnant forest fragment of the TAF in the same drainage basin, approximately 50,000 hectares65. Given the positive relationship between species richness and area size66, we expected the number of prey species for the Harpy Eagle to be higher in ES. Additionally, the number of nests and our efforts to collect remains and photographic records were more considerable in ES than in BA (Table 2). However, our results demonstrated that the species richness of the Harpy Eagle’s diet was similar between both states. This may be explained by the fact that the reserves in BA are surrounded by extensive forest cover that stretch beyond their boundaries, and they are about 197 km apart in two drainage basins. This may also explain the variability in the species richness of the regions we sampled, despite the reserves in BA being smaller than those in ES. Nevertheless, we detected the highest number of prey species in the three VNR nests in ES (13), followed by the two VS-PNHR nests in BA (10).
Most of the species in the Harpy Eagle’s diet in BA and ES were similar. Even though we observed only six different species between both regions, three (Eira barbara, Dasypus novemcinctus, and Dasyprocta leporina) are found in all four protected areas in which the study was conducted46,48,49, suggesting that these species rate high as potential prey for Harpy Eagles in all reserves.
Out of the potential prey belonging to species or genera that have previously been recorded in the Harpy Eagle’s diet and are found in the region, Cabassous tatouay, Cabassous unicinctus, Dasypus septemcinctus, Cerdocyon thous, Procyon cancrivorus, Leopardus pardalis, Leopardus guttulus, Leopardus wiedii, Tayassu pecari, Mazama americana, Mazama gouazoubira, and Cuniculus paca were not recorded in this study (Supplementary Table 1). However, it is important to emphasize that Harpy Eagles tend to prey on young individuals of species that reach large body sizes as adults, such as species in the genera Mazama and Tayassu67,68,69. Furthermore, of the species whose genera have not been confirmed in the Harpy Eagle’s diet but could be considered potential prey due to their size (1–10 kg), we did not observe the following three species: Euphractus sexcinctus, Galictis cuja, and Conepatus semistriatus.
A large proportion of the potential Harpy Eagle prey species were present in protected areas are strictly terrestrial, but only 17.7% were found in the Harpy Eagle’s diet in this study (Supplementary Table 1). The inclusion of terrestrial prey in this predator's diet is often associated with the presence of areas of open vegetation or secondary forests close to the nest, such as pastures, plantations or clearings70. In this study, only nests VNR-01, VNR-02, VS-PNRH-02 and SB-PNRH-01 registered exclusively terrestrial prey, which, incidentally, are nests that are located close to pasture and plantation areas. Five armadillo species were considered potential prey: Cabassous tatouay, Cabassous unicinctus, Dasypus novemcinctus, Dasypus septemcinctus, and Euphractus sexcinctus (Supplementary Table 1). Camera trap recordings showed only Dasypus novemcinctus in ES. However, at least two other armadillo species may have been the two individuals found among the remains in the study area, but we were unable to identify the remains at the species level. Therefore, such traces may belong to species of the Family Chlamyphoridae or Dasypodidae.
The only potential arboreal prey we did not find in the remains below the nests was Sapajus xanthosternos, but we identified an event in the photographs in which an individual was brought to a nest in the SB-PNHR. Sánchez-Lalinde et al.26 had already suggested Sapajus xanthosternos as potential prey for the Harpy Eagle in the SB-PNHR. Furthermore, between the years 2013 and 2015, predator–prey interactions between the Harpy Eagle and Sapajus xanthosternos were observed in the Una Biological Reserve, which is about 50 km away from the SB-PNHR, but without predatory success71.
Sánchez-Lalinde et al.26 also suggested six other potential mammalian prey for the Harpy Eagle in the SB-PNHR. We confirmed three in this study: Nasua nasua, Bradypus variegatus, and Callicebus melanochir. Although they are arboreal, we did not consider the other species indicated by Sánchez-Lalinde et al.26, Wied’s marmoset (Callithrix kuhlli), the golden-headed lion tamarin (Leontopithecus chrysomelas), and the painted-tree rat (Callistomys pictus), to be potential prey in this study (Supplementary Table 1). They weight less than the species we found (Supplementary Table 1), and their genera, Callithrix, Leontopithecus, and Callistomys, have not been observed in the Harpy Eagle’s prey in previous studies. Moreover, the presence of Leontopithecus chrysomelas and Callistomys pictus were not confirmed in the list of mammals of the SB-PNHR provided by Sánchez-Lalinde et al.49.
In 1991, Galetti et al.22 recorded a Harpy Eagle at the Pau-Brasil Experimental Station, an area annexed to the VS-PNHR, and suggested that the white-headed marmoset (Callithrix geoffroyi), the tufted capuchin (Cebus apella syn. Sapajus robustus), and the brown-throated sloth (Bradypus variegatus) are potential prey for the Harpy Eagle. When the same authors recorded an individual of Bradypus variegatus preyed upon by a Harpy Eagle in the VNR, they indicated that Cebus apella (syn. Sapajus robustus), Alouatta guariba, Callicebus personatus, Callicebus geoffroyi, and Potos flavus are preyed upon by large raptors so are potential prey for the Harpy Eagle. Except for Callithrix, which did not fall into our parameters for potential prey, we confirmed all the other genera to be in the Harpy Eagle’s diet in the VS-PNHR and VNR in this study.
In the Atlantic Forest of Argentina, Anfuso et al.29 found two species that had not been previously recorded in the Harpy Eagle’s diet: a primate, Cebus nigritus (syn. Sapajus nigritus), and a feline, Leopardus wiedii. Species of the genus Sapajus have since been recorded in this and other studies. Although species of the genus Leopardus are known to be present in our study area, they were not recorded as prey. Because the Harpy Eagle demonstrates high acceptance for domestic rabbits as prey in captivity, Anfuso et al.29 suggested that Sylvilagus brasiliensis have high potential as prey in the wild. We confirmed this hypothesis. Though the species presented a low frequency, we recorded two individuals of Sylvilagus brasiliensis in the Harpy Eagle’s diet, one in ES and another in BA.
This study has added nine species which had not yet been recorded in other regions to the Harpy Eagle’s known diet7,9,10,11,15,33: five primate species, Alouatta guariba, Sapajus robustus, Sapajus xanthosternos, Callicebus personatus, Callicebus melanochir; two rodents, Chaetomys subspinosus and Coendou insidiosus; one marsupial, Didelphis aurita; and one lagomorph, Sylvilagus brasiliensis. Except for Sylvilagus, different species of the same genera had been previously recorded in the Harpy Eagle diet in other regions.
Although a great diversity of reptiles is present in the study area of this investigation72,73, no reptile species were recorded. Reptiles were the least common items recorded in the Harpy Eagle’s diet in other studies9,14,33,67,69,74. The study area also contains a large diversity of birds23,72,75, including species belonging to groups that are more common in the Harpy Eagle’s diet in Central and South America, such as the Galliformes and Piciformes9,10,11,14,15,33,69,76. Nevertheless, we found only one Psittacid and other unidentified remains. Even so, the frequency of birds in the Harpy Eagle’s diet in this study (3.3%) was like that of other studies conducted in different regions6,7,33.
Attributes of the prey species
In this study, mammal species, particularly arboreal mammals, made up the majority of the Harpy Eagle’s diet (Table 2). Studies conducted in other regions found similar results7,10,11,15,33. For example, Muñiz-López et al.15 monitored 11 Harpy Eagles nests in Ecuador and found that 84% of the mammal prey consumed by Harpy Eagles were arboreal species. In Parintins, Amazonas, Aguiar-Silva et al.11 found that arboreal mammal prey made up 99% of the Harpy Eagle’s diet in five nests.
The Harpy Eagles also preyed on species that forage exclusively in the terrestrial stratum, but these species occurred less frequently in the diet. The following species were classified as terrestrial: Dasyprocta leporina, Dasypus sp., Sylvilagus brasiliensis, and Eira barbara53. However, other authors do not consider Eira barbara to be an exclusively terrestrial species, as the species was seen in or near the canopy layer in one in four direct observations77. Moreover, there has been one record of the species visiting the interior of a Harpy Eagle’s nest in the Cerrado78. According to Aguiar-Silva et al.33, records ground-dwelling species such as armadillos in the Harpy Eagle’s diet may indicate that the Harpy Eagle also forages in open areas, possibly at the edge or in the matrix of forest fragments, and in the low vegetation and clearings of reserves. Nonetheless, it would likely be difficult for only terrestrial prey to maintain Harpy Eagles in the wild, considering their preference and specialization for arboreal prey.
There is a relationship between the foraging stratum and diet of the prey species. Folivorous, frugivorous, and omnivorous prey species were primarily arboreal, while insectivorous or carnivorous species were primarily terrestrial. Thus, it is likely that the low capture frequency of insectivorous and carnivorous prey is due to their foraging stratum, since obscurement by the understory likely interferes with the Harpy Eagle’s ability to find and hunt prey67. Consequently, herbivorous arboreal animals are visually and acoustically more accessible to the predator67.
The Harpy Eagles in our study preyed on cathemeral species. Since the Harpy Eagle’s activity period is diurnal, it is likely that nocturnal prey is captured when the individuals move during periods when they exhibit greater lethargy10 or sleeping, thus facilitating capture. Miranda et al.79 associated the rise in the predation rates of nocturnal mammals with darker nights, when nocturnal prey species such as anteaters, opossums, and armadillos are more active. Our camera-monitoring efforts in the seven nests did not record events in which the Harpy Eagle arrived at the nest with prey during the twilight or nighttime period. As for predation on diurnal species, the Harpy Eagle possibly takes advantage of moments when the prey is more active, such as when foraging, as this is when they are most visually and acoustically detectable67. At the same time, the Harpy Eagle has a more precise and enhanced predation strategy in comparison to other raptors. They possess a combination of extremely acute vision and a retractable facial disc which favors visual and acoustic detection79. These features make it capable of locating even relatively cryptic prey.
The species that contributed the most biomass to the Harpy Eagle’s diet in the region studied were Bradypus variegatus and Sapajus robustus; both species are medium-sized (1–10 kg). While this study adopted the body weight of adult mammalian prey species provided by Wilman et al.53, the mean weight of consumed prey was similar to that estimated by other authors who applied other methods to estimate prey weight. The mean prey weight (2.834–3.637 kg) in this study was similar to that of studies conducted in the Amazon Rainforest, which ranged from 2.6 kg11 to 4 kg4. Harpy Eagles do not capture only adult prey, juvenile and sub-adult individuals are also part of the Harpy Eagle’s diet11,67,80. Therefore, since we used adult weights, our results may be an overestimation, but we believe they still adequately demonstrate the size of the Harpy Eagle’s prey.
The Harpy Eagle’s diet is apparently opportunistic and adaptive; it allows them to sustain themselves in impacted forests by feeding on disturbance-tolerant prey76,80. Raptors species with less specialized diets are able to survive and forage in more degraded areas, such as cabrucas81. Because the conservation status of the Atlantic Forest is worse than that of the Amazon, the frequency of arboreal prey consumption in the Atlantic Forest may be lower than in the Amazon. Furthermore, forest loss is directly linked to severe reductions in prey capture rate and biomass14. However, the results found herein indicate that the Harpy Eagle in protected areas of the CAFEC still maintains its diet within the expectations of its specialized predation, even with nests very close (< 1 km) to pasture and crop areas, such as the VNR-01 and SB-PNHR-01. The fauna and flora of the TAF in the CAFEC is often compared to the Amazon Rainforest61,82. However, these characteristics are not present throughout the extent of the Atlantic Forest. The region’s environmental conditions and conservation status vary greatly throughout its distribution, as does species composition83. Therefore, it is not possible to extrapolate the results found herein to the Harpy Eagle’s entire distribution in the Atlantic Forest. Although there have been recent records of Harpy Eagles in the southern Brazilian states of Rio Grande do Sul28 or even in the mountainous region of Espírito Santo25 in the CAFEC, no other nests have been found in the Brazilian Atlantic Forest recently.
Abundance and conservation status of the prey species
Except for Bradypus variegatus and Coendou insidiosus, all of the major arboreal mammal species that make up the Harpy Eagle’s diet in this study are threatened with extinction. This is a troubling factor since Harpy Eagles depend on arboreal mammals for food. The scarcity of these prey can interrupt natural food chains and force the predator to look for food alternatives, which can lead to food overlap with other species with which it did not previously compete for the same food resources32. Bradypus variegatus was the species that contributed the most in terms of biomass to the Harpy Eagle’s diet in our study. It is also one of the species that Harpy Eagles consume the most in other regions10,11,15,33,67,69.
This can be explained by the prey’s own behavior. The species is commonly found in the upper canopy trees of the forest eating and exposing itself to the sun, and it can stay between one and three days in the same tree37,68,80. The species’ bodily movements are slower than those of the other prey species identified in the study, such as primates, so it is easier to capture. However, the Harpy Eagles preyed more upon Bradypus variegatus in BA but preyed more upon Sapajus robustus in ES. Such differences may indicate that these prey species’ abundance between the regions in ES and BA may be different, with a greater abundance of Bradypus variegatus in BA and of Sapajus robustus in ES. It is worth noting that the SB-PNHR-01 nest, the only one with the presence of eaglet in BA during the study, recorded porcupine and coatis prey more frequently. This difference can be explained by the area where the nest is located, close to the cabrucas.
Sapajus robustus is classified as Endangered throughout its distribution. In the VNR, there are an estimated 1725 individuals or a density of 8.025 ind/km284. In 2021, the Federal Police arrested a gang that killed mothers and captured the babies of Sapajus robustus in the VNR and SBR for trafficking. In BA, studies involving the loss of area potentially occupied by Sapajus robustus due to forest fragmentation showed a 50.7% decrease in suitable habitats for the species between 1995 and 201085. This decrease in the species’ home range may suggest a strong decline in population density in BA, and it may explain its lower occurrence in the Harpy Eagle’s diet in this region compared to ES. These primates are diurnal and travel in troops, and their howling at dusk, dawn, and when they are moving through the canopy in search of fruit67. These features likely make them easy for Harpy Eagles to locate and hunt on a regular basis. Additionally, Harpy Eagles have a habit of capturing younger individuals that are inexperienced at escaping, thus easier to catch11,78,86.
Sapajus xanthosternos is classified as Critically Endangered globally87 and as Endangered in Brazil12, and was among the 25 most endangered primates in the world88. Its total remaining population size is estimated to be 3000 to 5000 individuals throughout its distribution89. A study carried out in the Una Biological Reserve, located in southern Bahia close to the SB-PNHR, estimated a group of 25 individuals occupying a territory of 1030 ha, generating a density of 0.024 ind/ha90. The low density found for this species is a result of hunting practices and the pet trade91,92,93. The small size of its population may explain why only record of the species was found in the SB-PNHR.
Alouatta guariba is classified as Critically Endangered in Brazil12 and BA18, Endangered in ES55 and Vulnerable by the IUCN54. The species’ population is declining, and the current population size is suspected to be extremely small94. Alouatta guariba represents the genus of primates that lost the most individuals and populations during the yellow fever epidemic in Brazil in 2016, but the outbreak did not reach the protected areas of this study95.
Callicebus personatus and Callicebus melanochir, which were identified as prey in this study, are classified as Vulnerable at all scales of their distribution12,18,55,96,97. Throughout the distribution of these two species, estimates indicate that the number of mature individuals is less than 10,000 for Callicebus personatus98 and probably not much greater than 10,000 for Callicebus melanochir99. In the VNR, the population size for Callicebus personatus is estimated to be 2252 (1768–3252) individuals100. In a study on Callicebus melanochir conducted in a remnant forest in southern BA, Costa-Araújo et al.101 found that the species is more likely to be present in larger, higher-quality forest fragments. The low proportion of these species in Harpy Eagle diets probably reflects the low abundance of these species in the reserves, especially in BA, where the forest fragments are smaller. Moreover, these species are among the lightest arboreal prey (1.359 kg) found in this study.
Out of the two porcupine species consumed by Harpy Eagles, Chaetomys subspinosus is rare in the wild102, and its population is declining103. Furthermore, it is classified as Vulnerable throughout its distribution12,18,55,103. Coendou insidiosus, on the other hand, is considered Least Concern. The camera-trap data evidenced a total of 18 individuals of Coendou insidiosus and three individuals of Chaetomys subspinosus across all nests. Therefore, this result likely reflects the low abundance of Chaetomys subspinosus relative to Coendou insidiosus.
The terrestrial Sylvilagus brasiliensis is also threatened, classified as globally Endangered104. However, the species is not classified as threatened in Brazil and in the regions where the study was conducted12,18,55. Finally, Dasyprocta leporina is classified as Vulnerable only in ES, where its main threats are hunting and capture55. Both species presented a low frequency in the Harpy Eagle’s diet in the CAFEC, which is probably attributed to the Harpy Eagle's low foraging in the terrestrial stratum and its most frequent consumption of arboreal prey.
In the CAFEC, 50% of the species in the Harpy Eagle’s diet are threatened with extinction, and between 47 and 50% of the potentially consumed biomass are individuals of threatened species (Table 2). Endangered species are experiencing population declines, mainly as a result of habitat loss and fragmentation generated by anthropogenic activities such as livestock farming, agriculture, silviculture, roads, and overexploitation, among others105. The Atlantic Forest hosts many mammals that are on the brink of extinction106, which includes the identified prey of the Harpy Eagle. The population decline of prey in the CAFEC signifies declining resources for the Harpy Eagle. In addition to threats to Harpy Eagles due to declining nesting sites79, poor habitat quality in their home range14, and the hunting of individuals34,74,107,108, Harpy Eagles may be under even greater pressure as their prey is also under threat15.
The limited food resources of its specialization may put pressure on this predator to change the quality of its diet. However, this did not occur in the study area of this investigation. Although many of the Harpy Eagle’s prey are under threat, the Harpy Eagle also preys on species that are not threatened, such as Bradypus variegatus and of Coendou insidiosus, which may reduce its predatory impact on the populations of prey threatened with extinction67. Still, what likely limits the Harpy Eagle from preying on species that are within its specialization and threatened with extinction is their availability in the environment. Additionally, the pressure on threatened species by the Harpy Eagle may contribute to the maintenance of their dangerously low population sizes. We suggest that more studies look into this issue and evaluate interactions between threatened prey and predators and the supportive capacity of reserves in sustaining these ecological relationships in the long term. Such results would be useful in informing conservation strategies for all species involved.
Conclusion
The presence of Harpy Eagle nests in the CAFEC reserves and the diet presented herein attests to the quality of these forests in maintaining the only currently known Harpy Eagle breeding sites in the Brazilian Atlantic Forest and contribute to knowledge about the Harpy Eagle’s diet. Most species captured by the Harpy Eagle in the region were medium-sized, folivorous, frugivorous, and omnivorous mammals that forage in the upper canopy, a pattern similar to that recorded in the diet of Harpy Eagles in the Amazon. New species have been added to the knowledge on the Harpy Eagle’s diet. However, important species in the Harpy Eagle’s diet are threatened with extinction, making them limited resources in the Atlantic Forest, although some of them were found to be frequent in this predator’s diet. Nevertheless, Harpy Eagles may face an even greater threat due to the population reduction of their prey. The Harpy Eagle is a top predator of the ecological chain and requires large areas with availability and quality of prey for its existence in the medium and long term. Thus, actions focused on its conservation in the reserves for the protection of its nests in the Atlantic Forest, may benefit a range of other threatened species that interact with the Harpy Eagle in the predator–prey relationship.
Data availability
All data generated or analysed during this study are included in this published article and its supplementary information files.
References
Vargas, G. J. J. et al. Estado y distribución actual del Águila Arpía (Harpia harpyja) en Centro y Sur América. Ornitol. Neotrop. 17, 39–55 (2006).
BirdLife International. Species factsheet: Harpia harpyja. https://www.birdlife.org/ (2022).
Fowler, J. M. & Cope, J. B. Notes on the Harpy Eagle in British Guiana. Auk 81, 257–273. https://doi.org/10.2307/4082683 (1964).
Rettig, N. L. Breeding behavior of the Harpy Eagle (Harpia harpyja). Auk 95, 629–643. https://doi.org/10.1093/auk/95.4.629 (1978).
Luz, B. B. Características de árvores emergentes utilizadas por gavião-real (Harpia harpyja) para nidificação no centro e leste da Amazônia Brasileira (Instituto Nacional de Pesquisas da Amazônia/Universidade Federal do Amazonas, 2005).
Giudice, R., Piana, R. & Williams, M. Tree architecture as a determinant factor in nest-tree selection by Harpy Eagles. In Neotropical Raptors (eds Bildstein, K. L. et al.) 14–22 (Hawk Mountain Sanctuary, 2007).
Muñiz-López, R. Revisión de la situación del Águila Harpía Harpia harpyja en Ecuador. Cotinga 29, 42–47 (2008).
González, J. J. V. & Vargas, F. H. Nesting density of Harpy Eagles in Darien with population size estimates for Panama. J. Raptor Res. 45, 199–210. https://doi.org/10.3356/JRR-10-57.1 (2011).
Álvarez-Cordero, E. Biology and conservation of the Harpy Eagle in Venezuela and Panamá (University of Florida, 1996).
Piana, R. Anidamiento y dieta de Harpia harpyja Linnaeus en la Comunidad Nativa de Infierno, Madre de Dios. Perú. Rev. Peru. Biol. 14, 135–138 (2007).
Aguiar-Silva, F. H., Sanaiotti, T. M. & Luz, B. B. Food habits of the Harpy Eagle, a top predator from the Amazonian Rainforest Canopy. J. Raptor Res. 48, 24–35. https://doi.org/10.3356/JRR-13-00017.1 (2014).
Brasil. Lista Nacional de Espécies Ameaçadas de Extinção (Ministério do Meio Ambiente, 2022).
Giraldo-Amaya, M. A., Aguiar-Silva, F. H., Aparicio-U, K. M. & Zuluaga, S. Human persecution of the Harpy Eagle: A widespread threat?. J. Raptor Res. 55, 281–286. https://doi.org/10.3356/0892-1016-55.2.281 (2021).
Miranda, E. B. P. et al. Tropical deforestation induces thresholds of reproductive viability and habitat suitability in Earth’s largest eagles. Sci. Rep. 11, 13048. https://doi.org/10.1038/s41598-021-92372-z (2021).
Muñiz-López, R., Criollo, O. & Mendúa, A. Results of five years of the “Harpy Eagle (Harpia harpyja) Research Program” in the Ecuadorian tropical forest. In Neotropical Raptors (eds Bildstein, K. L. et al.) 22–32 (Hawk Mountain Sanctuary, 2007).
Banhos, A. et al. Harpia harpyja (Linnaeus, 1758). In Livro Vermelho da Fauna Brasileira Ameaçada de Extinção Vol. 3 124–125 (ICMBio/MMA, 2018).
Chaves, F. G. et al. Aves Ameaçadas de extinção no estado do Espírito Santo. In Fauna e flora ameaçadas de extinção no estado do Espírito Santo (eds Fraga, C. N. et al.) 294–313 (Instituto Nacional da Mata Atlântica, 2019).
Bahia. Lista Oficial das Espécies da Fauna Ameaçadas de Extinção do Estado da Bahia (Secretaria do Meio Ambiente, 2017).
Mittermeier, R. A. et al. Hotspots Revisited: Earth’s Biologically Richest and Most Endangered Terrestrial Ecoregions (CEMEX, 2004).
Ribeiro, M. C., Metzger, J. P., Martensen, A. C., Ponzoni, F. J. & Hirota, M. M. The Brazilian Atlantic Forest: How much is left, and how is the remaining forest distributed? Implications for conservation. Biol. Conserv. 142, 1141–1153. https://doi.org/10.1016/j.biocon.2009.02.021 (2009).
Albuquerque, J. L. B. Observations of rare raptors in southern Atlantic Rainforest of Brazil. J. F. Ornithol. 66, 363–369 (1995).
Galetti, M., Martuscelli, P., Pizo, M. A. & Simão, I. Records of Harpy and Crested Eagles in the Brazilian Atlantic forest. Bull. Ornithol. Club 117, 27–31 (1997).
Silveira, L. F., Develey, P. F., Pacheco, J. F. & Whitney, B. M. Avifauna of the Serra das Lontras-Javi montane complex, Bahia, Brazil. Cotinga 24, 45–54 (2005).
Srbek-Araujo, A. C. & Chiarello, A. G. Registro recente de harpia, Harpia harpyja (Linnaeus) (Aves, Accipitridae), na Mata Atlântica da Reserva Natural Vale do Rio Doce, Linhares, Espírito Santo e implicações para a conservação regional da espécie. Rev. Bras. Zool. 23, 1264–1267. https://doi.org/10.1590/S0101-81752006000400040 (2006).
Novaes, T. D. et al. Registros recentes de Harpia harpyja e espécies de Spizaetus (Falconiformes: Accipitridae) na Reserva Biológica Augusto Ruschi, Santa Teresa, Espírito Santo, Brasil. Bol. Mus. Biol. Mello Leitão 28, 143–166 (2010).
Sánchez-Lalinde, C., García-Vélez, F., Cornélio, A. C., Silveira, L. F. & Alvarez, M. R. Records of the Happy Eagle (Harpia harpyja) in the Serra Bonita reserves complex, Camacan, Bahia, with evidence of breeding. Rev. Bras. Ornitol. 19, 436–438 (2011).
Banhos, A., Hrbek, T., Sanaiotti, T. M. & Farias, I. P. Reduction of genetic diversity of the Harpy Eagle in Brazilian tropical forests. PLoS One 11, e0148902. https://doi.org/10.1371/journal.pone.0148902 (2016).
Meller, D. A. & Guadagnin, D. L. Rediscovery of the Harpy Eagle Harpia harpyja (Accipitriformes: Accipitridae) for Rio Grande do Sul state, Brazil. Rev. Bras. Ornitol. 24, 53–57. https://doi.org/10.1007/BF03544329 (2016).
Anfuso, J., Suarez, M. & Chebez, J. Nuevo registro de nidificación de la Harpía (Harpia harpyja) en la Provincia de Misiones, Argentina y consideraciones dobre du conservación. Nót. Fauníst. 21, 1–13 (2008).
Aguiar-Silva, F. H. et al. Harpy Eagle sightings, traces and nesting records at the “Reserva Natural Vale”, a Brazilian Atlantic Forest remnant in Espírito Santo, Brazil. Rev. Bras. Ornitol. 20, 148–155 (2012).
Luz, B. B., Sanaiotti, T. M., Oliveira, D. B. & Cotes, M. Registro de ninho é esperança de manutenção de harpias (Harpia harpyja) na Mata Atlântica (2005).
Pires, M. M., Benchimol, M., Cruz, L. R. & Peres, C. A. Terrestrial food web complexity in Amazonian forests decays with habitat loss. Curr. Biol. 33, 389–396. https://doi.org/10.1016/j.cub.2022.11.066 (2023).
Aguiar-Silva, F. et al. Resource availability and diet in Harpy Eagle breeding territories on the Xingu River, Brazilian Amazon. Braz. J. Biol. 75, 181–189. https://doi.org/10.1590/1519-6984.00914BM (2015).
Miranda, E. B. P., Peres, C. A. & Downs, C. T. Landowner perceptions of livestock predation: Implications for persecution of an Amazonian apex predator. Anim. Conserv. 25, 110–124. https://doi.org/10.1111/acv.12727 (2022).
Naples, V. L. Cranial osteology and function in the tree sloths, Bradypus and Choloepus. Am. Mus. Nat. Hist. 2739, 1–41 (1982).
Gregorin, R. Taxonomia e variação geográfica das espécies do gênero Alouatta Lacépède (Primates, Atelidae) no Brasil. Rev. Bras. Zool. 23, 64–144. https://doi.org/10.1590/S0101-81752006000100005 (2006).
Hayssen, V. Bradypus variegatus (Pilosa: Bradypodidae). Mamm. Species 42, 19–32. https://doi.org/10.1644/850.1 (2010).
Hayssen, V. Tamandua tetradactyla (Pilosa: Myrmecophagidae). Mamm. Species 43, 64–74. https://doi.org/10.1644/875.1 (2011).
Caldara, V. J. & Leite, Y. L. R. Geographic variation in hairy dwarf porcupines of Coendou from eastern Brazil (Mammalia: Erethizontidae). Zoologia 29, 318–336. https://doi.org/10.1590/S1984-46702012000400005 (2012).
Young, J. W., Danczak, R., Russo, G. A. & Fellmann, C. D. Limb bone morphology, bone strength, and cursoriality in lagomorphs. J. Anat. 225, 403–418. https://doi.org/10.1111/joa.12220 (2014).
Chahud, A. Uma coleção de Carnivora derivada de atividades de caça da Sociedade Awá-Guajá do Estado do Maranhão, Brasil. Biota Amaz. 10, 34–37 (2020).
Brandão, M. V. & Hingst-Zaher, E. Atlas Craniano Mamíferos da Mata Atlântica e lista de espécies (TIJD Edições, 2021).
Miranda, G. H. B., Rodrigues, F. H. G. & Paglia, A. P. Guia de identificação de pelos de mamíferos brasileiros (Ciência Forenses, 2014).
Ingberman, B. & Monteiro-Filho, E. L. A. Identificação microscópica dos pelos das espécies brasileiras de Alouatta Lacépéde, 1799 (Primates, Atelidae, Alouattinae). Arq. Mus. Nac. 64, 61–71 (2006).
Quadros, J. & Monteiro-Filho, E. L. A. Identificação dos mamíferos de uma área de floresta atlântica utilizando a microestrutura de pelos-guarda de predadores e presas. Arq. Mus. Nac. 68, 47–66 (2010).
Falcão, F. D. C., Guanaes, D. H. A. & Paglia, A. Medium and large-sized mammals of RPPN Estação Veracel, southernmost Bahia, Brazil. Check List 8, 929–934. https://doi.org/10.15560/8.5.929 (2012).
Srbek-Araujo, A. C., Rocha, M. F. & Peracchi, A. L. A mastofauna da Reserva Natural Vale, Linhares, Espírito Santo, Brasil. Ciênc. Ambient. 1, 153–167 (2014).
Srbek-Araujo, A. C. & Kierulff, M. C. M. Mamíferos de médio e grande porte das florestas de Tabuleiro do norte do Espírito Santo: Grupos funcionais e principais ameaças. In Floresta Atlântica de Tabuleiro: Diversidade e endemismos na Reserva Natural Vale (eds Rolim, S. G. et al.) 469–479 (Rona Editora, 2016).
Sánchez-Lalinde, C., Ribeiro, P., Vélez-García, F. & Alvarez, M. R. Medium-and large-sized mammals in a protected area of Atlantic Forest in the northeast of Brazil. Oecol. Aust. 23, 234–245. https://doi.org/10.4257/oeco.2019.2302.04 (2019).
Emmons, L. & Feer, F. Neotropical Rainforest Mammals: A Field Guide (University of Chicago Press, 1997).
Reis, N. R., Perachi, A. L., Pedro, W. A. & Lima, I. P. Mamíferos do Brasil (Technical Books Editora, 2011).
Srbek-Araujo, A. C. & Chiarello, A. G. Armadilhas fotográficas na amostragem de mamíferos: Considerações metodológias e comparação de equipamentos. Rev. Bras. Zool. 24, 647–656. https://doi.org/10.1590/S0101-81752007000300016 (2007).
Wilman, H. et al. EltonTraits 1.0: Species-level foraging attributes of the world’s birds and mammals. Ecology 95, 2027. https://doi.org/10.1890/13-1917.1 (2014).
IUCN—International Union for Conservation of Nature. The IUCN Red List of Threatened Species. Version 2022-2. https://www.iucnredlist.org/ (2022).
Costa, L. P. et al. Mamíferos Ameaçados de extinção no estado no Espírito Santo. In Fauna e flora ameaçadas de extinção no estado do Espírito Santo (eds Fraga, C. N. et al.) 314–341 (Instituto Nacional da Mata Atlântica, 2019).
Eisenberg, J. F. & Redford, K. H. Mammals of the Neotropics: The Central Neotropics: Ecuador, Peru, Bolivia, Brazil Vol. 3 (University of Chicago Press, 1999).
Colwell, R. & Coddington, J. Estimating terrestrial biodiversity through extrapolation. Philos. Trans. R. Soc. London. Ser. B Biol. Sci. 345, 101–118. https://doi.org/10.1098/rstb.1994.0091 (1994).
Gotelli, N. J. & Colwell, R. K. Estimating species richness. In Biological Diversity: Frontiers in Measurement and Assessment (eds Magurran, A. E. & McGill, B. J.) 39–44 (Oxford University Press Inc, 2011).
Colwell, R. K. EstimateS: Statistical estimation of species richness and shared species from samples. https://www.robertkcolwell.org/ (2019).
R Core Team. R: A language and environment for statistical computing. R Foundation for Statistical Computing. https://www.r-project.org/ (2022).
Peixoto, A. L. et al. Tabuleiro Forests north of the Rio Doce: Their representation in the Vale do Rio Doce Natural Reserve, Espírito Santo, Brazil. In The Atlantic Coastal Forest of Northeastern Brazil (ed. Thomas, W. W.) 369–372 (New York Botanical Garden Press, 2008).
Dean, W. With Broadax and Firebrand: The Destruction of the Brazilian Atlantic Forest (University of California Press, 1997).
UNESCO. Discovery Coast Atlantic Forest Reserves. https://whc.unesco.org/en/list/892/ (1999).
Galetti, M. et al. Priority areas for the conservation of Atlantic forest large mammals. Biol. Conserv. 142, 1229–1241. https://doi.org/10.1016/j.biocon.2009.01.023 (2009).
Rolim, S. G., Menezes, L. F. T. & Srbek-Araujo, A. C. Floresta Atlântica de Tabuleiro: Diversidade e Endemismo na Reserva Natural Vale (Editora Rona, 2016).
Gotelli, N. J. Ecologia (Planta, 2009).
Touchton, J. M., Hsu, Y. C. & Palleroni, A. Foraging ecology of reintroduced captive-bred subadult Harpy Eagles (Harpia harpyja) on Barro Colorado Island, Panama. Ornitol. Neotrop. 13, 365–379 (2002).
Miranda, E. B. P. Prey composition of Harpy Eagles (Harpia harpyja) in Raleighvallen. Suriname. Trop. Conserv. Sci. https://doi.org/10.1177/1940082918800789 (2018).
Miranda, E. B. P., Campbell-Thompson, E., Muela, A. & Vargas, F. H. Sex and breeding status affect prey composition of Harpy Eagles Harpia harpyja. J. Ornithol. 159, 141–150. https://doi.org/10.1007/s10336-017-1482-3 (2018).
Aguiar-Silva, F. H. Uso e seleção de recursos por Harpia em múltiplas escalas espaciais: persistência e vulnerabilidade (Instituto Nacional de Pesquisas da Amazônia, 2016).
Suscke, P. et al. Predatory threat of Harpy Eagles for yellow-breasted capuchin monkeys in the Atlantic Forest. Primates 58, 141–147. https://doi.org/10.1007/s10329-016-0557-8 (2017).
Schiavetti, A., Fonseca, M., Bedê, L. & Pinto, L. P. Plano de Manejo: Reserva Particular do Patrimônio Natural Estação Veracel (2007).
Bérnils, R. S. et al. Répteis na Reserva Natural Vale, Linhares, Espírito Santo, Brasil. Ciênc. Ambient. 1, 193–210 (2014).
Muñiz-López, R. Harpy Eagle (Harpia harpyja) mortality in Ecuador. Stud. Neotrop. Fauna Environ. 52, 81–85. https://doi.org/10.1080/01650521.2016.1276716 (2017).
Srbek-Araujo, A. C. et al. A avifauna da Reserva Natural Vale, Linhares, Espírito Santo, Brasil. Ciênc. Ambient. 1, 169–192 (2014).
Bowler, M. et al. Harpy eagles (Harpia harpyja) nesting at Refugio Amazonas, Tambopata, Peru feed on abundant disturbance-tolerant species. Food Webs 24, e00154. https://doi.org/10.1016/j.fooweb.2020.e00154 (2020).
Presley, S. J. Eira barbara. Mamm. Species 2000, 1–6. https://doi.org/10.1644/1545-1410(2000)636%3c0001:EB%3e2.0.CO;2 (2000).
Aguiar-Silva, F. H. et al. Camera trapping at Harpy Eagle nests: Interspecies interactions under predation risk. J. Raptor Res. 51, 72–78. https://doi.org/10.3356/JRR-15-58.1 (2017).
Miranda, E. B. P. et al. High moon brightness and low ambient temperatures affect sloth predation by Harpy Eagles. PeerJ. 8, e9756. https://doi.org/10.7717/peerj.9756 (2020).
Miranda, E. Conservation implications of Harpy Eagle Harpia harpyja predation patterns. Endanger. Species Res. 29, 69–79. https://doi.org/10.3354/esr00700 (2015).
de Almeida-Rocha, J. M., Monsalvo, J. A. B. & Oliveira, L. D. Diet specialisation reduces the occupancy of cocoa agroforests by diurnal raptors. Bird Conserv. Int. 29, 558–574. https://doi.org/10.1017/S0959270919000017 (2019).
Junior, A. A. B. et al. Late Pleistocene and Holocene vegetation, climate dynamics, and Amazonian taxa in the Atlantic Forest, Linhares-ES, Brazil. Radiocarbon 55, 1747–1762. https://doi.org/10.1017/S0033822200048669 (2013).
Ribeiro, M. C. et al. The Brazilian Atlantic Forest: A shrinking biodiversity hotspot. In Biodiversity Hotspots (eds Zachos, F. E. & Habel, J. C.) 405–434 (Springer, Berlin, 2011).
Martins, W. P. et al. Sapajus robustus. The IUCN Red List of Threatened Species. https://doi.org/10.2305/IUCN.UK.2021-1.RLTS.T42697A192592444.en (2021).
Mota, F. M. M., Leite, M. R. & Martins, W. P. Fragmentation dynamics and loss of area of potential occupancy within the distribution limits of the endangered crested capuchin monkey (Sapajus robustus). Am. J. Primatol. 80, 1–13. https://doi.org/10.1002/ajp.22906 (2018).
Benchimol, M. & Venticinque, E. M. Harpy Eagle (Harpia harpyja) predation on an infant brown capuchin monkey (Cebus apella) in the Brazilian Amazon. Rev. Bras. Ornitol. 18, 352–354 (2010).
Canale, G. R. et al. Sapajus xanthosternos. The IUCN Red List of Threatened Species. https://doi.org/10.2305/IUCN.UK.2021-1.RLTS.T4074A192592138.en (2021).
Mittermeier, R. A. et al. Primates in peril: The world’s 25 most endangered primates, 2004–2006. Primate Conserv. 2006, 1–28. https://doi.org/10.1896/0898-6207.20.1.1 (2006).
Canale, G. R., Alonso, A. C. & Martins, W. P. Sapajus xanthosternos (Wied-Neuwied, 1826). In Livro Vermelho da Fauna Brasileira Ameaçada de Extinção Vol. 2 275–279 (ICMBio/MMA, 2018).
Canale, G. R. Ecology and behaviour of the yellow-breasted capuchin monkey Cebus xanthosternos in the northern Atlantic Forest (University of Cambridge, 2010).
Kierulff, M. C. M. et al. Avaliação das populações do macaco-prego-do-peito-amarelo (Cebus xanthosternos) e propostas de estratégias para manejo e conservação da espécie (2005).
Lernould, J. M., Kierulff, M. C. M. & Canale, G. Yellow-breasted capuchin Cebus xanthosternos: Support by zoos for its conservation—a success story. Int. Zoo Yearb. 46, 71–79. https://doi.org/10.1111/j.1748-1090.2012.00169.x (2012).
Suscke, P., Presotto, A. & Izar, P. The role of hunting on Sapajus xanthosternos’ landscape of fear in the Atlantic Forest, Brazil. Am. J. Primatol. 83, e23243. https://doi.org/10.1002/ajp.23243 (2021).
Neves, L. G., Jerusalinsky, L. & Melo, F. R. Alouatta guariba (Humboldt, 1812). In Livro Vermelho da Fauna Brasileira Ameaçada de Extinção Vol. 2 162–166 (ICMBio/MMA, 2018).
Vale, C. A. & Prezoto, F. A culpa não é do macaco: os primatas e a febre amarela. Multiverso 2, 1–12 (2017).
Printes, R., Jerusalinsky, L., Melo, F. R. & Mittermeier, R. A. Callicebus melanochir. The IUCN Red List of Threatened Species. https://doi.org/10.2305/IUCN.UK.2020-3.RLTS.T39930A17975106.en (2020).
Melo, F. R. et al. Callicebus personatus. IUCN Red List of Threatened Species. https://doi.org/10.2305/IUCN.UK.2021-1.RLTS.T3555A191700126.en (2021).
Melo, F. R., Quadros, S., Oliveira, L. C. & Jerusalinsky, L. Callicebus personatus (É. Geoffroy, 1812). In Livro Vermelho da Fauna Brasileira Ameaçada de Extinção Vol. 2 298–302 (ICMBio/MMA, 2018).
Printes, R. C. & Jerusalinsky, L. Callicebus melanochir Wied-Neuwied, 1820. In Livro Vermelho da Fauna Brasileira Ameaçada de Extinção Vol. 2 293–297 (ICMBio/MMA, 2018).
Ferreguetti, A. C., Tomas, W. M. & Bergallo, H. G. Abundância e densidade de mamíferos de médio e grande porte na Reserva Natural Vale. In Floresta Atlântica de Tabuleiro: diversidade e endemismos na Reserva Natural Vale 473–467 (Editora Rona, 2016).
Costa-Araújo, R. et al. Occurrence and conservation of the Vulnerable titi monkey Callicebus melanochir in fragmented landscapes of the Atlantic Forest hotspot. Oryx 55, 916–923. https://doi.org/10.1017/S0030605319001522 (2021).
Faria, D., Giné, G. A. F. & Reis, M. L. Plano de Ação Nacional para a Conservação do Ouriço-preto (ICMBio/MMA, 2010).
Catzeflis, F., Patton, J., Percequillo, A., Bonvicino, C. R. & Weksler, M. Chaetomys subspinosus. The IUCN Red List of Threatened Species. https://doi.org/10.2305/IUCN.UK.2017-2.RLTS.T4366A22213335.en (2017).
Ruedas, L. & Smith, A. T. Sylvilagus brasiliensis. The IUCN Red List of Threatened Species. https://doi.org/10.2305/IUCN.UK.2019-2.RLTS.T87491102A45191186.en (2019).
Maxwell, S. L., Fuller, R. A., Brooks, T. M. & Watson, J. E. M. Biodiversity: The ravages of guns, nets and bulldozers. Nature 536, 143–145. https://doi.org/10.1038/536143a (2016).
Ceballos, G., Ehrlich, P. R. & Raven, P. H. Vertebrates on the brink as indicators of biological annihilation and the sixth mass extinction. Proc. Natl. Acad. Sci. 117, 13596–13602. https://doi.org/10.1073/pnas.1922686117 (2020).
Trinca, C. T., Ferrari, S. F. & Lees, A. C. Curiosity killed the bird: Arbitrary hunting of Harpy Eagles Harpia harpyja on an agricultural frontier in southern Brazilian Amazonia. Cotinga 30, 12–15 (2008).
Gusmão, A. C. et al. Records of the occurrence, nesting, and hunting of the Harpy Eagle (Harpia harpyja) (Aves: Accipitridae) in Rondônia, southwestern Brazilian Amazonia. Atual. Ornitol. 190, 19–23 (2016).
Acknowledgements
We thank all the colleagues who helped or facilitated the realization of the Harpy Eagle Project—Atlantic Forest (Projeto Harpia—Mata Atlântica) field activities: Jailson de Souza, Olivier Jaudoin, Gabriel Bonfá, Gustavo Magnago, Carlos Hartur Ribeiro Noia, Frederico Pereira, Sayonara Cometti, Maxwell Banhos, Ednilson Santos, Iran Leal, Ronoaldo Santos, José Everildo, Luis F. O. P. Gonzaga, Genilson Tadeu, Sávio Augusto Machado, Kézia Catein dos Santos, Tomas de Lima Rocha, Damiani Paolo Gomes, Ronielio Teixeira de Souza, Eliton Lima, Jackeceli Falqueto, Idelbrando de Jesus, Nira Fialho, Camila Santana, Justiniano Magnago, Letícia Magnago, Leonardo Merçon, Gustavo Diniz, Virginia Londe de Camargos, Gildevanio Pereira do Santos, Vitor Becker, Márcio Santos Ferreira, Priscilla Sales Gomes, Yara Barros and Romulo Silva. We thank Dra. Cristiane Vergílio for authorizing the use of the Laboratory of Animal Morphology at CCENS/UFES, Dra. Maria Nazareth Ferreira da Silva authorizing the use of the access to the Mammal Collection of the INPA and Dr. Eduardo de Sá Mendonça for supporting data collection in the field. We thank Vitor Roberto Schettino for having made the map of Figure 2. This work was developed in the Institutional Program of Scientific Initiation of UFES and as part of the graduation in Biological Sciences by Mylena Kaizer. This is publication #16 of the Harpy Eagle Project (Projeto Harpia).
Funding
We thank the Fundação de Amparo à Pesquisa e Inovação do Espírito Santo (FAPES), Beauval Nature Association, Fundação Espírito-santense de Tecnologia (FEST), Instituto Últimos Refúgios, Vale S.A. and Veracel Celulose for funding support. We thank managers of Sooretama Biological Reserve (Reserva Biológica de Sooretama/Instituto Chico Mendes de Conservação da Biodiversidade—ICMBio), Serra Bonita Private Natural Heritage Reserve (Reserva Serra Bonita/Instituto Uiraçu), Vale Natural Reserve (Reserva Natural Vale) and Veracel Station Private Natural Heritage Reserve (RPPN Estação Veracel), for allowing access to reserve areas, support for field activities and use of accommodation to carry out research activities.
Author information
Authors and Affiliations
Contributions
M.K., F.H.A-S., T.M.S. and A.B. conceived of the idea. All authors collected the data. M.K., B.F., F.H.A-S. and A.B. analyzed the data and wrote the paper. M.K., B.F., F.H.A-S., T.M.S. and A.B. reviewed and edited the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kaizer, M., Fabres, B., Aguiar-Silva, F.H. et al. The prey of the Harpy Eagle in its last reproductive refuges in the Atlantic Forest. Sci Rep 13, 18308 (2023). https://doi.org/10.1038/s41598-023-44014-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-023-44014-9
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.