Abstract
In this paper, tri-phase Fe2O3–MgO–CuO nanocomposites (NCs) and pure CuO, Fe2O3 and MgO nanoparticles (NPs) were prepared using sol–gel technique. The physical properties of the prepared products were examined using SEM, XRD, and UV–visible. The XRD data indicated the formation of pure CuO, Fe2O3 and MgO NPs, as well as nanocomposite formation with Fe2O3 (cubic), MgO (cubic), and CuO (monoclinic). The crystallite size of all the prepared samples was calculated via Scherrer's formula. The energy bandgap of CuO, Fe2O3 and MgO and Fe2O3–MgO–CuO NCs were computed from UV–visible spectroscopy as following 2.13, 2.29, 5.43 and 2.96 eV, respectively. The results showed that Fe2O3–MgO–CuO NCs is an alternative material for a wide range of applications as optoelectronics devices due to their outstanding properties.
Similar content being viewed by others
Introduction
Due to their unique optical, electrical, thermal, photocatalytic, mechanical, adsorbent and structural properties, metal oxide (MO) nanocomposites (NCs) have attracted much attention in recent years1,2,3,4,5. The NCs are composed of two or more nano-oxides and possessing properties which depend on the concentration of each constituent oxide in the mixture6,7,8. They are useful in a variety of applications, including solar cells, photovoltaic instruments, battery materials, gas sensors, and fuel cells9,10,11,12,13,14,15. Copper oxide (CuO) is a p-type semiconductor with a narrow bandgap of 1.2 eV8. It has unique optical and structural properties with low-cost preparation. It has attracted considerable attention due to its potential applications in superconductivity, gas sensing, solar cell and supercapacitor16,17. Furthermore, it is a non-toxic and readily available semiconductor18,19. Magnesium oxide (MgO), with a direct bandgap of 5.2–7 eV, is an n-type semiconductor that displays noticeable structural, catalytic, optical, and chemical properties17,20,21,22. Iron(III) oxide (Fe2O3) is a narrow bandgap of nearly 2 eV. It is associated with certain features, like the low toxicity, low cost, magnetic behavior and high solubility23,24. Hence, it is engaged in various applications involving biomedicine, cosmetics, diagnostics, sensors, radiology, and vaccines9,23,25,26.
By combining the different metal oxides (MOs) to form new NCs, various properties of individual oxide could significantly enhanced and, consequently, open up a new avenue of research for optoelectronics, electrical, thermal, photo-catalysis, and biological applications26,27. Mixed metal oxide NCs can be fabricated via different approaches such as the co-precipitation28, sonochemical7, solution combustion29, microwave technique10, ultrasonic-assisted30 and green methods2,11.
In this work, tri-phase Fe2O3–MgO–CuO NCs and pure CuO, Fe2O3 and MgO NPs were prepared using sol–gel method. It has the advantages of being environmentally friendly, simple, cheap and fast to perform without any special equipment. Herein, the novelty lies in the designed combination of the three metal oxides in one NC, which supposedly could lead to enhanced properties and potential applications. The obtained oxides were characterized for their structural and optical properties using XRD, UV–visible, and SEM.
Materials and methods
Materials
Magnesium nitrate hexahydrate (Mg(NO3)2·6H2O; 97%), Iron nitrate nonahydrate (Fe(NO3)3·9H2O; 97%), copper nitrate trihydrate (Cu(NO3)2·3(H2O; 98%) and absolute ethanol were purchased from BDH and used as received without additional treatment.
Synthesis
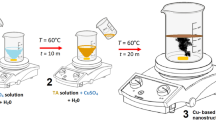
The sol–gel method20,31 was used to fabricate the Fe2O3–MgO–CuO NCs, which involves the following steps: Cu(NO3)2·3(H2O) (3.382 g in 20 mL ethanol), Fe(NO3)3·9(H2O) (5.65 g in 20 mL ethanol) and Mg(NO3)2·6(H2O) (3.589 g in 20 mL ethanol) with constant molar ratio (1:1:1) were synthesized as three separate solutions. Each solution was stirred for 10 min at 23 ± 2 °C to obtain a homogeneous solution. The solutions were mixed under constant stirring for 70 min at 80 °C until gel was obtained. After that, the gel burns to create xerogel, which grinded to fine powder and annealed at 800 °C for 90 min. The individual pure oxides (Fe2O3, CuO, and MgO) were separately prepared following similar steps as composite, using the corresponding salt.
Instruments
The optical properties of the synthesized materials were investigated using UV–Vis spectrophotometer (Hitachi U3900 with a software of Varian Cary 50). The structural properties were investigated by X-ray diffraction (XRD) using a Shimadzu EDX-720 (China) with CuKα radiation (λ = 0.154 nm). Morphological properties were assessed using SEM machine from JEOL (Jeol Ltd., Tokyo, Japan).
Results and discussion
The structural integrity of the synthesized metal oxides is confirmed via powder X-ray crystallography. The targeted substances were obtained via sol–gel route followed by calcination at 800 °C. The annealing temperature of 800 °C suggests high crystalline products as reported elsewhere32. However, such high temperature could stimulate production of pure substances with better performance.
X-ray diffraction
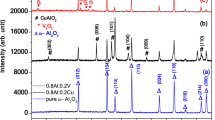
The crystalline arrangements and phase of the prepared nanopowder are estimated by XRD. Figure 1 shows the XRD pattern of the fabricated Fe2O3–MgO–CuO NCs. The observed diffraction peaks of pure oxides are close to the diffraction patterns reported in the X-ray database of JCPDS CuO (45-0937), Fe2O3 (33-0664) and MgO (45-0946). Similarly, in Fe2O3–MgO–CuO NCs, the diffraction patterns of CuO (48-1548), Fe2O3 (39-1346), and MgO (45-0946) match well with their respective standard reference cards. The diffracted peaks in composite were assigned for MgO (cubic), Fe2O3 (cubic) and CuO (monoclinic) phases. The characteristic diffraction peaks of CuO, Fe2O3 and MgO are well specified with no peaks relating to secondary or impurity segments or hydroxide in the sample, confirming the successful growth of Fe2O3–MgO–CuO NCs. The crystalline nature of the sample is assessed based on the sharp and strong diffraction peaks in Fig. 1. The cell volume (v), lattice constants (a, b, c) and d-spacing for pure CuO monoclinic, MgO cubic and Fe2O3 hexagonal phase and Fe2O3–MgO–CuO nanocomposite were calculated22,33,34,35,36 and listed in Table 1.
The Scherrer equation37 was utilized to compute the crystallite size (D) of CuO, Fe2O3, MgO and Fe2O3–MgO–CuO NCs. Then, their dislocation density was also calculated1,38,39, Table 2. As can be seen, the average D values of CuO, and MgO were larger than in the NCs as compared with individual oxides, due to the agglomeration of particles caused by the presence of Fe2O3. The Fe2O3 particles act as nucleation sites for the CuO and MgO particles, resulting in aggregation into larger clusters. This phenomenon is known as the Ostwald ripening effect, where smaller particles dissolve and re-deposit on larger particles, resulting in an increase in their size. Hence, the presence of Fe2O3 in the NCs leads to an increase in the particle size of CuO and MgO.
SEM analysis
Figure 2 represents the SEM images of grown pure CuO, Fe2O3, MgO and Fe2O3–MgO–CuO NCs. It is seen that the formed nanostructures have spherical shapes with hardly distinct morphology. Furthermore, due to the low resolution of the presented SEM images, the non-size and thus, particle sizes and distribution are difficult to be counted. Nevertheless, the XRD data supported the claimed nanostructures. To improve the seen, and thus the suggested nanostructures, a higher magnification of the SEM image was presented as an insert within the corresponding image. The resulting magnified view is simply support that the particles are in nanometer range. In addition, some nanoparticles are well separated and thus could be counted. For example, by counting of the obviously countable particles of the composite image (Fig. 2D), it is found that the averaged particle size is 153 ± 30 nm, which is higher than that calculated from XRD (56 ± 4 nm) shown in Table 2. According to literature40,41, the SEM-based particle size is often larger than those measured by other techniques like XRD, the case that can be seen herein.
UV–Vis spectroscopy
The optical properties of the Fe2O3–MgO–CuO NCs were studied by UV–visible spectroscopy. Figure 3 displays the absorption spectrum of Fe2O3–MgO–CuO NCs within 200–1000 nm. The absorption spectrum of the scattering radiation is observed in the longer wavelength region, and a larger-tail is seen due to the mixing of different oxides.
The transmission spectra of all the synthesized materials showed almost an opposite behavior to that seen in Fig. 4. Obviously, the optical transmission increased in the visible region for all the synthesized materials and possesses maximum value for Fe2O3–MgO–CuO NCs. The absorption coefficient (α) value can be computed via the following equation \(\mathrm{\alpha }=\frac{2.303A}{t}\)34.
The change in α (λ) for CuO, Fe2O3, MgO and Fe2O3–MgO–CuO NCs is presented in Fig. 5. From this Figure, it can be seen that α decreases as the wavelength (\(\lambda \)) of the incident photon increases. The extinction coefficient (α) value can be calculated via the following equation \(k=\frac{\mathrm{\alpha }\lambda }{4\pi }\)42,43.
The change in \(k\) (λ) for CuO, Fe2O3, MgO and Fe2O3–MgO–CuO NCs is presented in Fig. 6. It can be observed that \(k\) increases as the wavelength of the incident photon increases.
The energy bandgap (Eg) values of CuO, Fe2O3, MgO and Fe2O3–MgO–CuO NCs for the direct electronic transition between the valence band (VB) and conduction band (CB) can be computed via Tauc's relation38,44,45 as shown in Fig. 7. The \({E}_{g}\) values of CuO, Fe2O3, MgO NPs were calculated to about 2.13, 2.29, and 5.43 eV, respectively. However, Fe2O3–MgO–CuO NCs displayed the \({E}_{g}\) of 2.96 eV. In comparison to individual CuO, Fe2O3, MgO NPs, Fe2O3–MgO–CuO displayed significant increased absorbance in the visible region due to incorporation of three metal oxide. The reason for the change in the bandgap energy of the NCs compared to the individual metal oxides is likely due to the formation of new energy states at the interfaces between the different metal oxides. This can result in a shift in the electronic structure and a change in the bandgap energy. Additionally, the presence of multiple metal oxides in the NCs can also lead to increased electron–hole separation and improved charge transport properties, which can further affect the bandgap energy. This result was in good agreement with the literature46, which showed the possibility of using the prepared materials in some optical application.
Conclusion
In Summary, tri-phase Fe2O3–MgO–CuO NCs and pure CuO, Fe2O3 and MgO NPs were successfully fabricated using a sol–gel approach. The XRD emphasized the formation of pure CuO, Fe2O3 and MgO NPs and CuO–Fe2O3–MgO NCs. The variation in the average crystallite size (D) and lattice constant were observed due to the interaction of the corresponding metal oxides. The optical bandgap was reached 2.13, 5.43, 2.29 and 2.96 eV for CuO, MgO, Fe2O3 and Fe2O3–MgO–CuO NCs, respectively.
Data availability
The datasets generated and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Rajeh, A., Ragab, H. M. & Abutalib, M. M. Co doped ZnO reinforced PEMA/PMMA composite: Structural, thermal, dielectric and electrical properties for electrochemical applications. J. Mol. Struct. 1217, 128447 (2020).
Yulizar, Y., Apriandanu, D. O. B. & Jabbar, J. L. A. Facile one-pot preparation of V2O5–Fe2O3 nanocomposites using Foeniculumvulgare extracts and their catalytic property. Inorg. Chem. Commun. 123, 108320 (2021).
Sun, M. et al. Mg doped CuO–Fe2O3 composites activated by persulfate as highly active heterogeneous catalysts for the degradation of organicpollutants. J. Alloys Compd. 825, 154036 (2020).
Batool, S. et al. Green synthesis of Cordia myxa incubated ZnO, Fe2O3, and Co3O4 nanoparticle: Characterization, and their response as biological and photocatalytic agent. Adv. Powder Technol. 33, 103780 (2022).
Alnehia, A., Al-Sharabi, A., Al-Hammadi, A., Al-Odayni, A.-B., Alramadhan, S.A., Alodeni, R.M. Phyto-mediated synthesis of silver-doped zinc oxide nanoparticles from Plectranthus barbatus leaf extract: Optical, morphological, and antibacterial properties. Biomass Convers. Bioref. 1–13 (2023).
Xiang, Y. et al. Co3O4/α-Fe2O3 catalyzed oxidative degradation of gaseous benzene: Preparation, characterization and its catalytic properties. Solid State Sci. 93, 79–86 (2019).
Deepthi, N. H. et al. Optical, electrical and luminescent studies of CuO/MgO nanocomposites synthesized via sonochemical method. J. Alloy. Compd. 786, 855–866 (2019).
Alp, E., Eşgin, H., Kürşat Kazmanlı, M. & Genç, A. Synergetic activity enhancement in 2D CuO–Fe2O3 nanocomposites for the photodegradation of rhodamine B. Ceram. Int. 45, 9174–9178 (2019).
Lei, Y., Huo, J. & Liao, H. Fabrication and catalytic mechanism study of CeO2–Fe2O3–ZnO mixed oxides on double surfaces of polyimide substrate using ion-exchangetechnique. Mater. Sci. Semicond. Process 74, 154–164 (2018).
Revathi, V. & Karthik, K. Microwave assisted CdO–ZnO–MgO nanocomposite and its photocatalytic and antibacterial studies. Mater. Sci. Mater. Electron. 29, 18519–18530 (2018).
Amanulla, A. M. et al. Antibacterial, magnetic, optical and humidity sensor studies of b-CoMoO4-Co3O4 nanocomposites and its green synthesis and characterization. J. Photochem. Photobiol. B 183, 233–241 (2018).
Vignesh, S., Muppudathi, A. L. & Sundar, J. K. Multifunctional performance of gC3N4–BiFeO3–CuO2 hybrid nanocomposites for magnetic separable photocatalytic and antibacterial activity. Mater. Sci. Mater. Electron. 31, 13998–13999 (2020).
Kanwal, A., Sajjad, S., Leghari, S. A. K. & Yousaf, Z. Cascade electron transfer in ternary CuO/α-Fe2O3/γ-Al2O3 nanocomposite as an effective visible photocatalyst. J. Phys. Chem. Solids. 151, 109899 (2021).
Hammood, S. A. et al. Synthesis and characterization of PVA–Fe2O3–CuO hybrid structure for biomedical application. Int. J. Nanosci. 21, 2250030 (2022).
Rahmah, M. I. Novel triple hydrothermal method for preparation of CuO/Fe2O3/Ag2O nanocomposite with antimicrobial application. Inorg. Nano-Metal Chem. (2023).
Hong, Z., Cao, Y. & Deng, J. A convenient alcohothermal approach for low temperature synthesis of CuO nanoparticles. Mater. Lett. 52, 34–38 (2002).
Selvi, K. T., Mangai, K. A., Priya, M. & Sagadevan, S. Enhanced electrical and magnetic properties of CuO/MgO nanocomposites. Chem. Phys. Lett. 765, 138320 (2021).
Grigore, M. E., Biscu, E. R., Holban, A. M., Gestal, M. C. & Grumezescu, A. M. Methods of synthesis properties and biomedical applications of CuO nanoparticles. Pharmaceuticals 9, 75 (2016).
Munawar, T. et al. Sunlight-induced photocatalytic degradation of various dyes and bacterial inactivation using CuO–MgO–ZnO nanocomposite. Environ. Sci. Pollut. Res. 28, 42243–42260 (2021).
Khodair, Z. T., Al-Saadi, T. M., Abed, A. H. Effect of fuel on the structural and optical properties of MgO nanoparticles prepared by auto-combustion of sol–gel method. Adv. Phys. Theories Appl. 60 (2016).
Dinga, E. et al. Phyto-me diate d synthesis of MgO nanoparticles using Melia azedarach seed extract: Larvicidal and antioxidant activities. Sci. Afr. 17, e01366 (2022).
Al-Sharabi, A., Sada’a, K. S., Al-Osta, A. & Abd-Shukor, R. Structure, optical properties and antimicrobial activities of MgO–Bi2− x Cr x O3 nanocomposites prepared via solvent-deficient method. Sci. Rep. 12, 10647 (2022).
Ramesh, R. et al. Biogenic synthesis of α-Fe2O3 nanoparticles using Plectranthus amboinicus leaf extrac. Mater. Today Proc. 36, 453–458 (2020).
Alam, M. M. et al. Fabrication of ascorbic sensor acid with Co3O4 Fe2O3 nanosphere materials by electrochemical technique. Surf. Interfaces 20, 100607 (2020).
Bin Ren, J. M. et al. A grape-like N-doped carbon/CuO-Fe2O3nanocomposite as a highly active heterogeneous Fenton-like catalyst in methylene blue degradation. J. Clean. Prod. 240, 118143 (2019).
Shekoohiyan, S. et al. A novel CuO/Fe2O3/ZnO composite for visible-light assisted photocatalytic oxidation of Bisphenol A: Kinetics, degradation pathways, and toxicity elimination. Sep. Purif. Technol. 242, 116821 (2020).
Zhuang, Y. et al. Co3O4–CuO hollow nanocage hybrids with high oxidase-like activity for biosensing of dopamine. Mater. Sci. Eng. C C94, 858–866 (2019).
Subhan, M. A., Uddin, N., Sarker, P., Azad, A. K. & Begum, K. Photoluminescence, photocatalytic and antibacterial activities of CeO2–CuO–ZnO nanocomposite fabricated by co-precipitation method. Spectrochim. Acta 149, 839–850 (2015).
Kulkarni, J. et al. Structural optical and photocatalytic properties of MgO/CuO nanocomposite prepared by a solution combustion method. Mater. Today Proc. 4, 11756–11763 (2017).
Karthik, K., Dhanuskodi, S., Gobinath, C., Prabukumar, S. & Sivaramakrishnan, S. Ultrasonic-assisted CdO–MgO nanocomposite for multifunctional applications. Mater. Technol. 34, 403–414 (2019).
Alnahari, H., Al-Sharabi, A., Al-Hammadi, A., Al-Odayni, A.-B. & Alnehia, A. Synthesis of glycine-mediated CuO–Fe2O3–MgO nanocomposites: Structural, optical, and antibacterial properties. Compos. Adv. Mater. 32, 1–13 (2023).
Lermusiaux, L., Mazel, A., Carretero-Genevrier, A., Sanchez, C. & Drisko, G. L. Metal-induced crystallization in metal oxides. Acc. Chem. Res. 55, 171–185 (2022).
Munawar, T., Iqbal, F., Yasmeen, S., Mahmood, K. & Hussain, A. Multi metal oxide NiO–CdO–ZnO nanocomposite-synthesis, structural, optical, electrical properties and enhanced sunlight driven photocatalytic activity. Ceram. Int. 46, 2421–2437 (2020).
Alnehia, A., Al-Hammadi, A., Al-Sharabi, A. & Alnahari, H. Optical, structural and morphological properties of ZnO and Fe+ 3 doped ZnO-NPs prepared by Foeniculum vulgare extract as capping agent for optoelectronic applications. Inorg. Chem. Commun. 143, 109699 (2022).
Alnahari, H., Al-Hammadi, A. H., Al-Sharabi, A., Alnehia, A. & Al-Odayni, A.-B. Structural, morphological, optical, and antibacterial properties of CuO–Fe2O3–MgO–CuFe2O4 nanocomposite synthesized via auto-combustion route. J. Mater. Sci. Mater. Electron. 34, 682 (2023).
Al-Ariki, S. et al. Synthesis and comparative study on the structural and optical properties of ZnO doped with Ni and Ag nanopowders fabricated by sol gel technique. Sci. Rep. 11, 11948 (2021).
Alnehia, A. et al. Garlic extract-mediated synthesis of ZnS nanoparticles: Structural optical, antibacterial and hemolysis studies. Nanomaterials 2023, 1–9 (2023).
Al-Sharabi, A., Alnehia, A., Al-Hammadi, A. H., Alhumaidha, K. A. & Al-Osta, A. The effect of Nigella sativa seed extract concentration on crystal structure, band gap and antibacterial activity of ZnS-NPs prepared by green route. J. Mater. Sci. Mater. Electron. 33, 20812–20822 (2022).
Abutalib, M. M. & Rajeh, A. Enhanced structural, electrical, mechanical properties and antibacterial activity of Cs/PEO doped mixed nanoparticles (Ag/TiO2) for food packaging applications. Polym. Test. 93, 107013 (2021).
Zhou, W. & Greer, H. F. What can electron microscopy tell us beyond crystal structures?. Eur. J. Inorg. Chem. 2016, 941–950 (2016).
Jensen, H. et al. Determination of size distributions in nanosized powders by TEM, XRD, and SAXS. J. Exp. Nanosci. 1, 355–373 (2006).
Al-Osta, A., Alnehia, A., Qaid, A. A., Al-Ahsab, H. T. & Al-Sharabi, A. Structural, morphological and optical properties of Cr doped ZnS nanoparticles prepared without any capping agent. Optik Int. J. Light Electron Opt. 214, 164831 (2020).
Al-Sharabi, A., Alnehia, A., Al-Osta, A. & Yahya, N. A. A. Effect of copper doping on structural and optical properties of zinc sulfide (ZnS) nanoparticles. Al-Baydha Univ. J. Res. 1, 224–234 (2019).
Alwany, A. B. et al. Structural, optical and radiation shielding properties of ZnS nanoparticles. QDs Optik Int. J. Light Electron Opt. 260, 169124 (2022).
Mukhtar, F. et al. Dual S-scheme heterojunction ZnO–V2O5–WO nanocomposite with enhanced photocatalytic and antimicrobial activity. Mater. Chem. Phys. 263, 124372 (2021).
Rahman, A. et al. Structural, optical and photocatalytic studies of trimetallic oxides nanostructures prepared via wet chemical approach. Synth. Met. 259, 116228 (2020).
Acknowledgements
The authors are grateful to Mr. Ahmed Al-Khalaki for the help with optical experiments.
Author information
Authors and Affiliations
Contributions
Conceptualization: H.A., A.A.-S. and A.H.A.-H. Methodology: A.A. and H.A. Formal analysis and investigation: A.A., H.A., A.A.-S. and A.H.A.-H. Writing—original draft: H.A., A.A. and A.A.-S. Writing-review and editing: A.-B.A.-O., A.A., and A.A.-S. Visualization: A.A., H.A. and A.-B.A.-O. Supervision: A.A.-S. and A.H.A.-H.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Al-Hammadi, A.H., Alnehia, A., Al-Sharabi, A. et al. Synthesis of trimetallic oxide (Fe2O3–MgO–CuO) nanocomposites and evaluation of their structural and optical properties. Sci Rep 13, 12927 (2023). https://doi.org/10.1038/s41598-023-39845-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-023-39845-5
This article is cited by
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.