Abstract
Immune checkpoint inhibitors (ICIs) have become the standard treatment for recurrent or metastatic head and neck cancer (RM-HNC). However, many patients fail to benefit from the treatment. Previous studies have revealed that tumor burden predicts the efficacy of ICIs, but this association remains unclear for RM-HNC. We retrospectively analyzed 94 patients with RM-HNC treated with ICI monotherapy. We estimated the tumor burden using the baseline number of metastatic lesions (BNML) and the baseline sum of the longest diameters of the target lesions (BSLD), and evaluated the association between BNML, BSLD, and standardized uptake value (SUV) and clinical outcomes. The median progression-free survival (PFS) was 7.1 and 3.1 months in the low-BNML and high-BNML groups, respectively (p = 0.010). The median PFS was 9.1 and 3.5 months in the low-BSLD and high-BSLD groups, respectively (p = 0.004). Moreover, patients with high SUVmax levels had worse overall survival (OS) and PFS. BNML, BSLD, and SUVmax are useful prognostic factors in patients with RM-HNC treated with ICIs. Imaging examinations before ICI treatment are recommended to predict the efficacy of ICIs. If the tumor burden is high, cytotoxic anticancer agents may be administered concomitantly with or prior to ICI monotherapy.
Similar content being viewed by others
Introduction
The development of immune checkpoint inhibitors (ICIs) has changed the treatment strategies for several cancers. The results of the CheckMate 141 trial1 and Keynote 048 trial2 suggest that nivolumab and pembrolizumab—anti-programmed death-1 (PD-1) antibodies—are available for treatment of unresectable recurrent or metastatic head and neck cancer (RM-HNC). In real-world settings, these drugs have contributed to improved survival in patients with RM-HNC. However, > 50% of the patients fail to receive any clinical benefit from these drugs3,4,5.
Several predictive or prognostic factors have been evaluated3,4,5,6,7,8, but optimal timing for administering ICIs and optimal patient selection remain controversial. Some studies suggest that tumor burden, such as tumor size or number of metastatic lesions, is associated with progression-free survival (PFS) in patients with advanced cancers treated with ICIs9,10,11,12. Therefore, based on these studies, tumor burden may indicate the utility of ICIs or cytotoxic agents. Thus, to clarify the impact of tumor burden on survival in patients with RM-HNC, we retrospectively investigated patient charts of patients treated with ICIs at our institute. Specifically, we evaluated the association between the baseline number of metastatic lesions (BNML), the baseline sum of the longest diameters of the target lesions (BSLD), and maximum standardized uptake value (SUVmax) and clinical outcomes such as survival and response to anti-PD-1 monotherapy.
Results
Patient characteristics and clinical outcomes of total population
Ninety-four patients treated with anti-PD-1 monotherapy were enrolled in this study. The median age of patients was 70 (37–90) years, and a majority were men (76 patients, 80.9%). Of these 94 patients, 65 and 29 were treated with nivolumab and pembrolizumab as the first ICI treatment, respectively. ICI was the first systemic therapy, 1st line, for RM-HNC in 64 patients, the 2nd line in 21 patients and the 3rd line or more in 9 patients. The best overall response (BOR) was complete response (CR) in one patient, partial response (PR) in 18 patients, stable disease (SD) in 42 patients, and progressive disease (PD) in 33 patients (Table 1).
The median overall survival (OS) and PFS of all patients were 14.0 months (95% confidence interval [CI]: 11.9–18.3) and 4.9 months (95% CI 3.2–6.9), respectively. One-year OS of all patients was 60.7% (95% CI 48.7–70.7%) and the estimated 6-months PFS was 43.5% (95% CI 33.1–53.4%). The overall response rate was 20.2% (95% CI 12.6–29.8%) and disease control rate was 64.9% (95% CI 54.4–74.5%).
Tumor burden and clinical outcomes
BNML was 1 in 42 cases, 2 in 31 cases, and ≥ 3 in 21 cases. The median BSLD was 43 (0–426) mm. By performing receiver operating characteristic (ROC) curve analysis, the cutoff value of BNML for predicting survival and classifying patients into low-BNML (BNML = 1; 42 patients [44.7%]) and high-BNML (BNML ≥ 2; 52 patients [55.3%]) groups was 1. The cutoff value of BSLD was 28 mm, determined by performing ROC curve analysis, and patients were classified into low-BSLD (BSLD ≤ 28; 33 patients [35.1%]) and high-BSLD (BSLD > 28; 61 patients [64.9%]) groups.
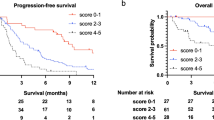
The median PFS was 7.1 months (95% CI 3.7–11.1) and 3.1 months (95% CI 2.3–4.9) in the low-BNML and high-BNML groups, respectively (p = 0.008). The median OS was 31.5 months (95% CI 13.5–NA) and 10.7 months (95% CI 7.7–17.7) in the low-BNML and high-BNML groups, respectively (p = 0.002) (Fig. 1A). The median PFS was 9.1 months (95% CI 4.9–14.2) and 3.5 months (95% CI 2.4–5.2) in the low-BSLD and high-BSLD groups, respectively (p = 0.003). The median OS was 31.5 months (95% CI 16.0–NA) and 11.8 months (95% CI 8.0–14.0) in the low-BSLD and high-BSLD groups, respectively (p < 0.001) (Fig. 1B).
Progression-free and overall survival stratified by the baseline number of metastatic lesions (A), the baseline sum of the longest diameters of the target lesions (B), and maximum standardized uptake value (C). (A) Kaplan–Meier curves of Progression-free survival (PFS) and Overall survival (OS) stratified by the baseline number of metastatic lesions (BNML). Patients with BNML was one (N = 42) had significantly better PFS than those with BNML was more than one (N = 52) (6-months PFS: 55.0% [95% CI 38.2–69.0%] vs. 34.0% [95% CI 21.5–47.0%], p = 0.008). Patients with BNML was one (N = 42) had significantly better OS than those with BNML was more than one (N = 52) (1-year OS: 78.6% [95% CI 59.8–89.4%] vs. 47.0% [95% CI 31.5–60.9%], p = 0.002). (B) Kaplan–Meier curves of PFS and OS stratified by baseline sum of the longest diameters of the target lesions (BSLD). Patients with BSLD ≤ 28 mm (low, N = 33) had significantly better PFS than those with BSLD > 28 (high, N = 61) (6-months PFS: 62.6% [95% CI 43.5–76.8%] vs. 33.2% [95% CI 21.6–45.3%], p = 0.003). Patients with BSLD ≤ 28 mm (low, N = 33) had significantly better OS than those with BSLD > 28 (high, N = 61) (1-year OS: 86.9% [95% CI 63.7–95.7%] vs. 47.8% [95% CI 33.8–60.6%], p < 0.001). (C) Kaplan–Meier curves of PFS and OS stratified by maximum standardized uptake value (SUVmax) of PET. Patients with SUVmax ≤ 12.93 (low, N = 23) had significantly better PFS than those with SUVmax > 12.93 (high, N = 25) (6-months PFS: 59.2% [95% CI 35.9–76.5%] vs. 28.0% [95% CI 12.4–46.0%], p = 0.014). Patients with SUVmax ≤ 12.93 (low, N = 23) had significantly better OS than those with SUVmax > 12.93 (high, N = 25) (1-year OS: 75.0% [95% CI 49.7–88.9%] vs. 48.1% [95% CI 25.6–67.5%], p = 0.003).
In univariate analysis, patients with worse BOR (hazard ratio [HR]: 15.20, 95% CI 7.49–30.84, p < 0.001), high-BNML (HR: 1.91, 95% CI 1.17–3.12, p = 0.010), or high BSLD (HR: 2.17, 95% CI 1.27–3.69, p = 0.004) had worse PFS (Table 2). Additionally, patients with worse Eastern Cooperative Oncology Group (ECOG) performance status (PS) (HR: 3.24, 95% CI 1.70–6.17, p < 0.001), worse BOR (HR: 3.51, 95% CI 1.95–6.32, p < 0.001), high-BNML (HR: 2.58, 95% CI 1.39–4.80, p = 0.003), or high-BSLD (HR: 4.47, 95% CI 1.99–10.03, p < 0.001) had worse OS (Table 2). Multivariate analysis revealed that patients with high-BNML (HR: 1.98, 95% CI 1.19–3.29, p = 0.008) or high-BSLD (HR: 2.57, 95% CI 1.44–4.58, p = 0.001) had worse PFS (Table 2). Furthermore, patients with worse ECOG PS (HR: 2.80, 95% CI 1.45–5.37, p = 0.002), high-BNML (HR: 2.30, 95% CI 1.21–4.36, p = 0.011), or high-BSLD (HR: 4.62, 95% CI 1.99–10.71, p < 0.001) had worse OS after adjustment for potential confounders (Table 2).
We also evaluated the association between tumor burden and response to anti-PD-1 monotherapy. Univariate logistic regression analyses identified high-BNML (odds ratio [OR]: 2.54, 95% CI 1.04–6.22, p = 0.042) and high-BSLD (OR: 3.57, 95% CI 1.29–9.90, p = 0.014) as risk factors for worse disease control rate. After adjusting for potential confounders, high-BNML (OR: 2.81, 95% CI 1.10–7.20, p = 0.031) and high-BSLD (OR: 4.71, 95% CI 1.53–14.50, p = 0.007) were validated as risk factors for worse disease control rate (Table 3).
Maximum standardized uptake value (SUVmax) and clinical outcomes
Of the 94 patients, 48 underwent positron emission tomography (PET) between the appearance of recurrent or metastatic lesions and initial administration of nivolumab or pembrolizumab. Median SUVmax of these 48 patients was 13.07 (range: 3.50–31.70). The cutoff value of SUVmax determined by performing ROC curve analysis was 12.93. When we compared survival outcomes between patients with high SUVmax (> 12.93; 25 patients) and those with low SUVmax (≤ 12.93; 23 patients), patients with high SUVmax had worse PFS and OS. The median PFS was 9.0 months (95% CI 3.2–14.2) and 3.1 months (95% CI 1.87–5.40) for patients with low SUVmax and high SUVmax, respectively (p = 0.014). The median OS was 38.1 months (95% CI 11.8–NA) and 11.9 months (95% CI 7.7–16.0) for patients with low SUVmax and high SUVmax, respectively (p = 0.003) (Fig. 1C). In univariate analysis, high SUVmax was associated with worse PFS (HR: 2.34, 95% CI 1.16–4.70, p = 0.017) and OS (HR: 3.65, 95% CI 1.46–9.16, p = 0.006) (Table 2). In the multivariate analysis, patients with high SUVmax had worse PFS (HR: 3.85, [95% CI 1.72–8.65], p = 0.001) (Table 2). We also evaluated the association between SUVmax and response to anti-PD-1 monotherapy. In univariate (OR: 4.28, 95% CI 1.16–16.60, p = 0.030) and multivariate (OR: 18.60, 95% CI 1.95–178.00, p = 0.011) logistic regression analyses, high SUVmax was found to be a risk factor for worse disease control rate (Table 3).
Discussion
In the present study, we evaluated the impact of BNML, BSLD, and SUVmax on clinical outcomes in patients with RM-HNC treated with ICIs; the analyses identified high BNML, high BSLD, and high SUVmax as risk factors for worse survival rates and worse disease control rates.
To the best of our knowledge, this is the first study to clarify that high BNML, high BSLD, and high SUVmax are associated with worse clinical outcomes in patients with RM-HNC treated with nivolumab or pembrolizumab. A study suggested that pretreatment tumor size may affect response to nivolumab in patients with head and neck squamous cell carcinoma10; but, we analyzed about both tumor size and number of lesions, as well as both nivolumab and pembrolizumab, to evaluate the impact of tumor burden on the clinical outcomes of ICIs.
The impact of tumor burden on survival has been reported for several cancers9,11,12. Miyawaki et al. reported that tumor burden can predict the efficacy of PD-1/PD-L1 inhibitor monotherapy against non-small cell lung cancer9. In this study, the values of BNML and BSLD were commonly available in clinical settings and were strongly associated with the clinical outcomes of PD-1/PD-L1 inhibitor monotherapy. Therefore, we adopted these parameters to evaluate tumor burden.
The mechanism by which a high tumor burden diminishes ICI efficacy remains unclear. Huang et al. demonstrated that a lower proportion of reinvigorated CD8+ T cells and tumor burden was associated with worse clinical outcomes in patients with melanoma treated with anti-PD-1 therapy13. Immune phenotypes of immune-inflamed, immune-excluded, and immune-deserted tumors have been described to be correlated with response to immunotherapy14. Therefore, the infiltration of immune cells may be regulated by a large tumor volume. However, tumor volume failed to correlate with immune phenotypes in one study15; thus, further investigation is needed.
We also evaluated the impact of SUVmax on clinical outcomes. In other cancers, SUVmax has been reported as a prognostic factor or predictor in patients treated with ICIs16,17. Ichiki et al. demonstrated that aggressive cancers, such as those with high SUVmax, may not be suitable for ICIs16. Approximately 50% of patients in the present study were not included in the SUVmax analysis, but the results indicate an association between SUVmax and clinical outcomes in patients with RM-HNC treated with ICIs.
This study has several strengths. First, the number of patients who received anti-PD-1 monotherapy and were included in the study (n = 94) was relatively large. We found statistically significant differences in clinical outcomes between the high and low tumor burden groups. Second, we used both BNML and BSLD to evaluate the tumor burden. The BSLD is a useful marker, but unmeasurable lesions could not be evaluated. Therefore, we used both BNML and BSLD to minimize the potential variation from the actual tumor burden. Third, we evaluated the impact of SUVmax. The analysis suggests the potential use of SUVmax as a marker for predicting ICI efficacy. As a high SUVmax value may indicate tumor aggressiveness, SUVmax is a potential tool for evaluating tumor burden.
This study has some limitations. First, it was a retrospective study conducted at a single institution. Second, we could not evaluate all metastatic lesions. The unmeasurable lesions, such as those with unclear borders or small lesions, were excluded from analyses. Third, the follow-up duration was too modest to evaluate long-term survival outcomes. Forth, although we analyzed both survival outcomes and the best overall response, it is difficult to strictly define the tumor burden as a predictive factor or prognostic factor for ICI treatment, because this is not a comparison study between ICIs and other treatment18. Therefore, a prospective, multicenter study is warranted to evaluate the impact of clinical tumor burden on survival in patients with RM-HNC treated with ICIs.
In conclusion, the study suggests that BNML, BSLD, and SUVmax may be prognostic factors in patients with RM-HNC treated with nivolumab or pembrolizumab monotherapy. We recommend to perform imaging examinations, including computed tomography (CT), magnetic resonance imaging (MRI), and PET before administration of ICIs to assess tumor spread and predict efficacy of ICIs. The study also indicates that a high tumor burden may qualify for chemotherapy with cytotoxic agents to be administered with or prior to ICI monotherapy.
Methods
Patients
We retrospectively analyzed 94 patients with RM-HNC who received nivolumab or pembrolizumab monotherapy at the Nagoya City University Hospital between July 2017 and September 2021.
We defined measurable tumor lesions as those with the longest diameter > 10 mm on CT or MRI. Measurable neck lymph nodes were defined as those with maximum minor axis diameter > 10 mm. We estimated the tumor burden using BNML and BSLD9. We defined BNML as both measurable and unmeasurable lesions, and multiple metastases in the same region were counted as single lesions.
We also measured SUVmax using PET and assessed its impact on survival. Patients who did not undergo PET in the duration between the appearance of recurrent/metastatic lesions and initial administration of nivolumab or pembrolizumab were excluded from analyses of SUVmax.
Treatment and follow-up
Between July 2017 and January 2020, nivolumab was administered at a dosage of 3 mg/kg every two weeks. Between February 2020 and April 2021, the dosage was 240 mg/body every two weeks. In stable cases, nivolumab was administered at a dosage of 480 mg/body every four weeks since September 2020. Pembrolizumab was administered at a dosage of 200 mg/body every three weeks in standard cases and 400 mg/body every six weeks in stable cases. The response to these therapies was evaluated according to the Response Evaluation Criteria in Solid Tumor (RECIST) version 1.119, using CT or MRI, every 8–12 weeks. Patients with clinically obvious disease progression were diagnosed with PD even when images were not evaluated. Chemotherapy with cytotoxic agents was administered to patients diagnosed with PD. Follow-up was continued until death or the cutoff date (November 30, 2021).
PET image acquisition
The image acquisition methods were same as previous studies20,21. Patients received fasting at least 4 h and then administered a standardized dose of 3.5 MBq 18-fluorodeoxyglucose (FDG) per kilogram body weight. After FDG injection, patients were kept in a lying position for 60 min prior to image acquisition. FDG-uptake parameters were evaluated using Advantage Workstation 4.6 software program the PET VCAR (GE Healthcare, Chalfont, UK). SUVmax was calculated automatically using a standard formula [maximum activity in region of interest ÷ (injected dose × body weight)].
Statistical analysis
OS was calculated from the start of anti-PD-1 antibody monotherapy until death from any cause. PFS was calculated from the start of anti-PD-1 antibody monotherapy until disease progression or death from any cause. The median OS and PFS were evaluated using the Kaplan–Meier method and log-rank test. The impact of BNML, BSLD, and SUVmax on survival was assessed using univariate and multivariate analyses with Cox proportional hazards models. The cutoff value for each factor was determined by performing ROC curve analyses. The correlations between BNML, BSLD, and response to anti-PD-1 monotherapy were assessed using univariate and multivariate logistic regression analyses. A p < 0.05 was considered to be statistically significant. Age, sex, ECOG PS, and BOR for anti-PD-1 antibody monotherapy were defined as potential confounders in multivariate analysis of OS. Whereas, age, sex, and ECOG PS were defined as confounders in multivariate analysis of PFS.
All analyses were performed using EZR (Saitama Medical Center, Jichi Medical University, Saitama, Japan), a graphical user interface for R (The R Foundation for Statistical Computing, Vienna, Austria, version 3.5.0). EZR, a modified version of the R commander (version 2.7–1), was designed to incorporate statistical functions frequently used in biostatistics.
Ethical approval
This study was approved by the Institutional Review Board of the Nagoya City University Graduate School of Medical Sciences (Accession No. 60-21-0001). As this was a retrospective, non-intervention study, patients could reject participation by opting out to an announcement on the Nagoya City University Hospital’s website, and the requirement of written informed consent was waived. This study was conducted in accordance with the principles of the Declaration of Helsinki.
Data availability
The datasets generated and/or analyzed during the study are available from the corresponding author upon reasonable request.
References
Ferris, R. L. et al. Nivolumab for recurrent squamous-cell carcinoma of the head and neck. N. Engl. J. Med. 375, 1856–1867. https://doi.org/10.1056/NEJMoa1602252 (2016).
Burtness, B. et al. Pembrolizumab alone or with chemotherapy versus cetuximab with chemotherapy for recurrent or metastatic squamous cell carcinoma of the head and neck (KEYNOTE-048): A randomised, open-label, phase 3 study. Lancet 394, 1915–1928. https://doi.org/10.1016/S0140-6736(19)32591-7 (2019).
Matsuo, M. et al. Relationship between immune-related adverse events and the long-term outcomes in recurrent/metastatic head and neck squamous cell carcinoma treated with nivolumab. Oral. Oncol. 101, 104525. https://doi.org/10.1016/j.oraloncology.2019.104525 (2020).
Hanai, N. et al. Effectiveness and safety of nivolumab in patients with head and neck cancer in Japanese real-world clinical practice: A multicenter retrospective clinical study. Int. J. Clin. Oncol. 26, 494–506. https://doi.org/10.1007/s10147-020-01829-0 (2021).
Ferris, R. L. et al. Nivolumab vs investigator’s choice in recurrent or metastatic squamous cell carcinoma of the head and neck: 2-year long-term survival update of CheckMate 141 with analyses by tumor PD-L1 expression. Oral Oncol. 81, 45–51. https://doi.org/10.1016/j.oraloncology.2018.04.008 (2018).
Matsuki, T. et al. Hematological predictive markers for recurrent or metastatic squamous cell carcinomas of the head and neck treated with nivolumab: A multicenter study of 88 patients. Cancer Med. 9, 5015–5024. https://doi.org/10.1002/cam4.3124,Pubmed:32441463 (2020).
Yarchoan, M., Hopkins, A. & Jaffee, E. M. Tumor mutational burden and response rate to PD-1 inhibition. N. Engl. J. Med. 377, 2500–2501. https://doi.org/10.1056/NEJMc1713444 (2017).
Nishikawa, D. et al. Eosinophil prognostic scores for patients with head and neck squamous cell carcinoma treated with nivolumab. Cancer Sci. 112, 339–346. https://doi.org/10.1111/cas.14706 (2021).
Miyawaki, T. et al. Association Between clinical tumor burden and efficacy of immune checkpoint inhibitor monotherapy for advanced non-small-cell lung cancer. Clin. Lung Cancer 21, e405–e414. https://doi.org/10.1016/j.cllc.2020.02.012 (2020).
Inoue, H. et al. Pre-treatment tumor size impacts on response to nivolumab in head and neck squamous cell carcinoma. Auris Nasus Larynx 47, 650–657. https://doi.org/10.1016/j.anl.2020.01.003 (2020).
Joseph, R. W. et al. Baseline tumor size is an independent prognostic factor for overall survival in patients with melanoma treated with Pembrolizumab. Clin. Cancer Res. 24, 4960–4967. https://doi.org/10.1158/1078-0432.CCR-17-2386 (2018).
Katsurada, M. et al. Baseline tumor size as a predictive and prognostic factor of immune checkpoint inhibitor therapy for non-small cell lung cancer. Anticancer Res. 39, 815–825 (2019). https://doi.org/10.21873/anticanres.13180
Huang, A. C. et al. T-cell invigoration to tumour burden ratio associated with anti-PD-1 response. Nature 545, 60–65. https://doi.org/10.1038/nature22079 (2017).
Hegde, P. S., Karanikas, V. & Evers, S. The where, the when, and the how of immune monitoring for cancer immunotherapies in the era of checkpoint inhibition. Clin. Cancer Res. 22, 1865–1874. https://doi.org/10.1158/1078-0432.CCR-15-1507 (2016).
Sun, R. et al. A radiomics approach to assess tumour-infiltrating CD8 cells and response to anti-PD-1 or anti-PD-L1 immunotherapy: An imaging biomarker, retrospective multicohort study. Lancet Oncol. 19, 1180–1191. https://doi.org/10.1016/S1470-2045(18)30413-3 (2018).
Ichiki, Y. et al. Prognostic factors of advanced or postoperative recurrent non-small cell lung cancer targeted with immune check point inhibitors. J. Thorac. Dis. 11, 1117–1123 (2019). https://doi.org/10.21037/jtd.2019.04.41.
Tabei, T. et al. Early assessment with 18F–2-fluoro-2-deoxyglucose positron emission tomography/computed tomography to predict short-term outcome in clear cell renal carcinoma treated with nivolumab. BMC Cancer 19, 298. https://doi.org/10.1186/s12885-019-5510-y (2019).
Ballman, K. V. Biomarker: predictive or prognostic?. J. Clin. Oncol. 33, 3968–3971. https://doi.org/10.1200/JCO.2015.63.3651 (2015).
Eisenhauer, E. A. et al. New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1). Eur. J. Cancer 45 228–247 (2009). https://doi.org/10.1016/j.ejca.2008.10.026
Werner, J., Strobel, K., Lehnick, D. & Rajan, G. P. Overall neutrophil-to-lymphocyte ratio and SUV(max) of nodal metastases predict outcome in head and neck cancer before chemoradiation. Front. Oncol. 11, 679287. https://doi.org/10.3389/fonc.2021.679287 (2021).
Suzuki, H. et al. Peak of standardized uptake value in oral cancer predicts survival adjusting for pathological stage. In Vivo 32, 1193–1198 (2018). https://doi.org/10.21873/invivo.11363.
Acknowledgements
We would like to thank Editage (www.editage.com) for English language editing.
Funding
This work was financially supported by Grants-in-Aid for Young Scientists (B) to Dr. D. Kawakita (No. 17K18006) and Dr. T. Matoba (No. 19K18779), and Grant-in-Aid for Scientific Research (C) to Dr. D. Kawakita (No. 20K10508) from the Japan Society for the Promotion of Science (JSPS).
Author information
Authors and Affiliations
Contributions
Study conceptualization and design, T.M., D.K., and S. Iwasaki; data acquisition, T.M., K.M., D.K., G.T., K.O., A. Murashima, K.N., S. Iwaki, H.T., N.T., S. Imaizumi, W.H., A. Matsumura, K.T., S.E., and S. Iwasaki; Data analysis and interpretation, T.M. and D.K.; statistical analysis, T.M. and D.K.; writing—original draft, T.M., D.K., and S. Iwasaki; writing—review and editing, All authors.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Matoba, T., Minohara, K., Kawakita, D. et al. Impact of tumor burden on survival in patients with recurrent or metastatic head and neck cancer treated with immune checkpoint inhibitors. Sci Rep 12, 14319 (2022). https://doi.org/10.1038/s41598-022-18611-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-022-18611-z
This article is cited by
-
Survival impact of sequential chemotherapy following pembrolizumab for recurrent or metastatic head and neck squamous cell carcinoma
International Journal of Clinical Oncology (2024)
-
Nivolumab and ipilimumab in recurrent or refractory cancer of unknown primary: a phase II trial
Nature Communications (2023)
-
Site of distant metastasis affects the prognosis with recurrent/metastatic head and neck squamous cell carcinoma patients treated with Nivolumab
International Journal of Clinical Oncology (2023)
-
Clinical implications of T cell exhaustion for cancer immunotherapy
Nature Reviews Clinical Oncology (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.