Abstract
A novel lead ferrite-magnetic activated carbon (lead ferrite-MAC) composite was developed using the chemical co-precipitation method. Instrumental analyses such as X-ray diffraction (XRD), scanning electron microscopy (SEM), Fourier-transform infrared spectroscopy (FTIR), and Brunauer–Emmett–Teller (BET) analysis were performed to characterize adsorbent. The uptake of phenol from aqueous solutions using the developed adsorbent was compared to that of pristine activated carbon. The maximum adsorption capacity of lead ferrite-MAC composite (145.708 mg/g) was more than that of pristine activated carbon (116.606 mg/g) due to the metal hydroxides coated on activated carbon since they improve the retention of phenol on the available active sites of adsorbent and create an additional electrostatic interaction with the phenol adsorbate. Regarding the high value of the coefficient of determination (R2) and adjusted determination coefficient (R2adj), coupled with the lower values of average relative error (ARE) and minimum squared error (MSE), it can be found that the isothermal data for the lead ferrite-MAC adsorbent were in agreement with the isotherm models of Redlich-Peterson and Langmuir. From the kinetic viewpoint, pseudo-second-order and linear driving force models explained the phenol adsorption data for both adsorbents. The reusability tests for lead ferrite-MAC composite revealed that after six cycles, 85% of the initial adsorption capacity was maintained. The developed adsorbent can be successfully applied to uptake phenol from aqueous solutions.
Similar content being viewed by others
Introduction
Phenols are the most common contaminants generated by petrochemical units, coal gasification sites, and oil refineries1, 2. They are mainly applied to produce phenolic resins, adhesives and epoxy resins, and polyamide for different uses3. However, phenolic compounds are extremely toxic4, lead to unpleasant odor and taste of drinking water, and endanger living organisms5, 6. Therefore, they should be removed from the aqueous solution. Different approaches are used for removing phenolic compounds from waste streams, including activated sludge7, bioremediation8, membrane filtration9, solvent extraction10, and adsorption11. Table 1 shows the advantages/disadvantages of mentioned techniques. Adsorption is the most efficient method for wastewater decontamination applications due to its efficiency, selectivity, low operating cost, and high adsorption capacity. Also, it does not produce toxic substances12, 13.
There are different adsorbents such as minerals14, biological materials15, polymer materials16, and activated carbon17 to uptake phenol from different aqueous solutions. Koduru et al.18 examined the uptake of bisphenol A (BPA) from water using goethite/activated carbon composite in a batch system. According to their report, the kinetic and equilibrium data were in agreement with the pseudo-second-order model and Freundlich isotherm, respectively. Park et al.19 powdered activated carbons impregnated with iron oxide nanoparticles and used it to uptake bisphenol A and natural organic matter from the aqueous solution. They found that the pH of the solution had no significant effect on BPA removal, but acidic conditions gave a slightly reduced sorption capacity, possibly because of weaker hydrogen bonding between iron oxide and BPA20. Lingamdinne et al.21 synthesized magnetic inverse spinel iron oxide nanoparticles using a biogenic methodology and used it for the removal of pollutants from the aqueous solutions. The batch adsorption studies concluded that the adsorption of Pb(II) and Cr(III) was the monolayer adsorptions on the homogenous surface of the developed adsorbent. In addition, the kinetic data were in agreement with the pseudo-second-order kinetic model.
Promisingly, the application of activated carbon for wastewater decontamination purposes is of great interest in recent years. In this way, the fabrication and improvement of the sorbent properties is the topic of research. Din et al.22 fabricated a type of activated carbon from coconut shell (MNAC) and studied its application for the uptake of phenol. The rate-limiting step of phenol adsorption onto MNAC was a chemical reaction because their kinetic data were consistent with the pseudo-second-order model. According to the Langmuir isotherm, the maximum adsorption capacity for this adsorbent was found to be 205.8 mg/g. Iron oxide/carbon nanocomposites are frequently used as effective adsorbent materials to uptake different pollutants from the aqueous solutions23. Ianoş et al.24 investigated the adsorption of the dyes Acid Blue 129, Methylene Blue, Rhodamine 6G, and Acid Orange 7 on iron oxide/carbon nanocomposites. However, the development of metal ferrite coated carbon composites has gained great interest as adsorbents to remove contaminants25, 26. In general, ferrite materials are of significant interest due to their high adsorption capacity, non-toxicity, and easy availability25. Regarding the favorable capacity of such adsorbents, it is important to develop an activated carbon-based composite and evaluate its possible application for wastewater treatment purposes. Spinel metal ferrites (MFe2O4, M = Zn, Mn, Co, Pb, Ba, Sr, and so on) have face-centered cubic structures and M2+ and Fe3+ cations fill the coordination places in tetrahedral and octahedral. The arrangement of spinel ferrites influences their structural, chemical, and magnetic properties. On the other hand, the use of lead (Pb), barium (Ba), and strontium (Sr) in the composition of ferrite materials could improve the magnetic properties of the material. Magnetically modified carbon materials have received great attention for wastewater treatment purposes. Many reports are available regarding the preparation of magnetic carbon material, and these materials were applied for the removal of different pollutions from the aqueous medium. These reported magnetically modified carbon materials have agreeable advantages of easy recovery and high adsorption efficiency. Yang et al.27 prepared magnetic Fe3O4/AC for the uptake of methylene blue from aqueous solutions. The application of MnFe2O4 and CuFe2O4 composites for the uptake of contaminants were reported by Zhang et al.28. Feng29 prepared NiFe2O4/AC composite for the uptake of methylene blue, rhodamine B, and malachite green. There is no systematic research on the synthesize and application of lead ferrite-activated carbon composite for the removal of pollutants from waste streams. Thus, the main aim of this research is to develop a new adsorbent namely lead ferrite-activated carbon composite for the uptake of phenol from waste streams.
In this research, a novel lead ferrite-activated carbon composite (lead ferrite-MAC) was prepared using chemical co-precipitation. The phenol uptake using the developed adsorbent was compared to that of pristine AC. The most influential factors like phenol concentration, contact time, and solution acidity on the performance of phenol adsorption onto both adsorbents were separately investigated. In addition, X-ray diffraction (XRD), scanning electron microscopy (SEM), Fourier-transform infrared spectroscopy (FTIR), Brunauer–Emmett–Teller (BET) analyses, and zeta potential measurement have confirmed physicochemical properties of the prepared adsorbent. Furthermore, the data were evaluated using different equilibrium and kinetic models to comprehend the mechanism of phenol adsorption onto both adsorbents. Finally, lead ferrite-MAC regeneration experiments were carried out to assess the prepared adsorbent from the practical aspect.
Experimental procedure
Materials and reagents
1000 mg/L stock phenol solution was prepared using a specific amount of phenol (Merck, Darmstadt, Germany) in double-distilled water and mixed on a magnetic stirrer at 300 rpm. Then, it was diluted to prepare the desired concentration of phenol solution. Activated carbon (P60, Hanil, Korea) was used with no further purification. All other chemical reagents [HCl, NaOH, FeCl3, PbCl2, and oleic acid (Sigma–Aldrich)] were analytical grade.
Lead ferrite-MAC preparation
0.4 mol/L (25 mL) of Fe3+ solution and 0.2 mol/L (25 mL) of Pb2+ solution were mixed in double-distilled water under magnetic stirring. 2.5 g of powdered activated carbon (PAC) was dispersed in 25 mL DD water under vigorous stirring. Then, the solution containing Fe3+ and Pb2+ was poured into 25 mL of the PAC solution26. After that, a solution of NaOH (25 mL) was added to the system with continuous magnetic stirring for 6 h until reaching the pH > 12. 3–4 drops (for 100 mL solution) of oleic acid were added with continuous magnetic stirring36. The obtained precipitate was then washed with DD water and ethanol to eliminate impurities. Finally, the obtained composite was separated and oven-dried at 60 °C. Finally, to complete the crystallization of the metals, the developed material was pyrolyzed in a muffle furnace at 700 °C for 1 h with a heating rate of 10 °C/min under an argon atmosphere. The formed material of lead ferrite-MAC was evaluated for phenol adsorption from the aqueous solutions.
Lead ferrite-MAC characterization
The XRD pattern was determined using an X-ray diffractometer 1140 using Cu Kα radiation. The surface chemical characterizations of the prepared adsorbent were scanned from 400 to 4000 cm−1 using FTIR spectroscopy (Shimadzu IR instrument). The samples were analyzed morphologically by scanning electron micrographs (SEM, Seron Technology, AIS2100). The N2 adsorption–desorption isotherms (Belsorp mini II model) were applied to obtain the specific surface area and pore size of the developed adsorbent. Magnetic characterization of the developed adsorbent was carried out by the vibrating-sample magnetometer (VSM) at ambient temperature. In addition, zeta potential measurements at an initial phenol concentration of 100 mg/L were carried out to determine the point of zero charges (pHpzc) (Zetasizer Nano ZS, Malvern Instruments Inc., UK). The Smoluchowski equation (Eq. 1) calculated the zeta potential (ξ):
where \(\mu\) is the electrophoretic mobility; \(\varepsilon\) and d are the dielectric constant of medium and the electrode separation, respectively; \(\eta\) and V are the viscosity of the suspension and the applied voltage, respectively37, 38.
Batch adsorption experiment
All adsorption tests were carried out in a glass vessel sealed with a rubber cover at ambient temperature. The vessels were compressed with an aluminum cover to avoid the phenol losses by volatilization39. Few drops of dilute NaOH and HCl solutions were applied to regulate the solution acidity. After equilibrium, the mixture was filtered, and the phenol concentrations in the aqueous solutions were measured using spectrophotometry at 271 nm. The tests were repeated three times, and their mean values were considered as outputs. All other experimental conditions are depicted in the figure or table captions. The adsorbed quantity of phenol (qe) was calculated as follows (Eq. 2):
C0 and Ce represent the equilibrium and initial phenol concentrations in solutions (mg/L), m indicates the adsorbent quantity (g), and V denotes the aqueous phase volume (L).
Adsorption isotherm experiments for phenol adsorption were conducted in a beaker containing various initial nitrate concentrations with the adsorbent dosage of 1.5 g/L at ambient temperature and pH 7.0. In this research, the adsorption of phenol onto the adsorbents was evaluated by Dubinin–Radushkevich, Redlich-Peterson, Temkin, Freundlich, and Langmuir models. The coefficient of determination (R2)40, 41, mean square error (MSE)42, and average relative error (ARE)43 were applied as statistical criteria to evaluate the best fit to the experimental data.
Desorption experiments were carried out to evaluate the reusability of the developed adsorbent. In this context, 0.15 g of the adsorbent was added to 100 mL of phenol solution (500 mg/L) at ambient temperature (25 °C) and stirred for 4 h. Then, the adsorbent was separated from the solution and treated with 1 mol/L NaOH solution. Afterward, the used adsorbent was dried in an oven at 100 °C for 30 min. The adsorption–desorption cycles were repeated 6 times by using a similar adsorbent and initial phenol concentration.
Results and discussion
Lead ferrite-MAC features
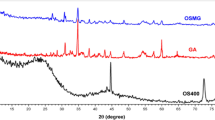
The XRD pattern of the adsorbent (lead ferrite-MAC) is shown in Fig. 1. The prominent peaks representing 23.71°, 36.22°, 52.33°, 62.25°, 65.34°, 75.27°, and 85.51° were assigned to the planes of (111), (220), (222), (400), (422), (440), and (620), respectively. These peak positions are related to the presence of the spinel of the cubic structure. The spectra are in close agreement with JCPDS card No.04-0705 and with the findings of previous reports44, 45.
Infrared spectroscopy provides information on the functional groups at the adsorbent surface. In this context, FTIR spectra of adsorbent before and after phenol adsorption were performed (Fig. 2). Two bands at 1601.65 cm−1 and 3402.32 cm−1 were related to the bending vibration of H–O and absorbed water on the sample surface, respectively. The bands that appeared at about 1110–1180 cm−1 were related to C–O and C–O–C bonds’ vibration for activated carbon46, 47. The weak absorption band at 2920 cm−1 is shown, allotted to the symmetrical and asymmetrical stretching vibration of the CH2 group. In addition, the bands at 2840–2940 cm−1 were related to the C–H stretch. Two bands at 643 and 687 cm−1 are related to asymmetric bending vibration of Pb–O-Pb and Fe metal oxide (M–O) bond, respectively48. These findings confirmed the presence of Pb and Fe on the adsorbent surface.
Some changes were observed in the spectrum of lead ferrite-MAC adsorbent after phenol adsorption (Fig. 2b). The vibration mode for Pb–O–Pb and Fe–O–Fe shifted from 643 and 687 to 584 and 588 cm−1. The new band at 1384 cm−1 indicated the phenolic structure presence. Moreover, the band appearing at about 3110–2938 cm−1 was related to the phenolic type C–H. A mono-substituted aromatic structure appears at 807 and 774 cm−1 in the fingerprint region after phenol adsorption.
To study the morphology of lead ferrite-MAC composite and pristine activated carbon, scanning electron microscopy analysis was applied. Figure 3a,c show the SEM photographs of the lead ferrite-MAC composite at different magnifications. Furthermore, by comparing Fig. 3a,b, it can be seen that the lead ferrite-MAC composite has more spherical particles than pristine activated carbon. In fact, pristine activated carbon has sharp-edged particles. It can be seen that the precipitates of lead and iron were aggregated together on the surface of AC. X-ray mapping in the SEM analyses can be used to identify the elemental distribution on the sample's surface. As shown in Fig. 3d,e, only elements Pb and Fe were found. It can be found that they were homogeneously distributed in the lead ferrite-MAC composite.
Figure 4 demonstrates the N2 adsorption and desorption isotherm for both adsorbents. Based on the IUPAC classification, both adsorbents have the pores of Type IV with an H3 hysteresis loop, representing that the adsorbents have a mesoporous structure with some microporous. The hysteresis loop at high relative pressure signifies the possible mesopores' nature. Furthermore, a small number of micropores within the developed adsorbent (lead ferrite-MAC) was seen at a pressure of around 0 to around 0.2. The pore size distribution plot of the adsorbent is shown in Fig. 4b. This curve indicates that the pores of the lead ferrite-MAC sample are generally distributed within multi-scales. For lead ferrite-MAC composite, two peaks at 14 and 25 nm and the peak ranging from 1 to 2 nm imply mesopore and micropore regions, respectively. Also, it can be found that pristine activated carbon and lead ferrite-MAC composite have a specific surface areas of 1023.9 and 774.53 m2/g and pore sizes of 4.013 and 11.89 nm, respectively. The total pore volume of lead ferrite-MAC adsorbent decreased a little, which may attribute to the blockage of these pores by metal ferrites after precipitation of lead and iron on the surface of activated carbon. However, the pore size of both adsorbents is very bigger than the phenol molecular size enabling its transference inside the adsorbent.
The relative magnetization curve of lead ferrite-MAC was presented in Fig. 5. This curve was used to investigate the magnetic feature of the adsorbent. As a result, it was verified that the saturation magnetization of the developed adsorbent was found to be 37.9 emu/g. Furthermore, it was found that the permanent magnet could separate the developed adsorbent from the aqueous solutions. The saturation magnetization of lead ferrite-MAC composite is the range of saturation magnetization reported for other metal ferrites27, 29. The magnetic metal ferrites when compared with the magnetic iron oxide particles have several important advantages. These materials may have a narrow size distribution, whereas direct production of iron oxide nanoparticles leads to a widespread size range. In addition, they exhibit a higher specific loss power than the iron oxide nanoparticles, which makes them interesting as potential candidates for wastewater treatment purposes.
Figure 6 shows the zeta potential and pHPZC on the surface of lead ferrite-MAC and pristine AC. The values of zero point charge for lead ferrite-MAC and pristine activated carbon were about 6.7 and 7.1, respectively. Thus, the surface of the adsorbent presents negative sites to adsorb cations when pHPZC < pH, whereas in the case of pH < pHPZC the positive sites are presented to adsorb anions49, 50.
Given the toxicity of Pb and Fe elements, it is necessary to evaluate the leaching of Pb and Fe into water. According to Environmental Protection Agency (EPA), the permissible levels of lead and iron in drinking water are 0.05 and 0.1 mg/L, respectively. In this regard, a series of experiments were carried out in a glass vessel sealed with a rubber cover at ambient temperature. The adsorbent and water (without phenol) were mixed with constant stirring using a magnetic stirrer at a fixed value of pH (2–14) for 24 h. Then, the concentrations of Pb and Fe were measured with an Agilant ICP-AES spectrometer. It can be found that at different values of pH, the concentration of Pb and Fe are well below the permissible limit based on EPA. So, this high stable adsorbent can be successfully used for wastewater treatment purposes.
Effect of pH on the phenol adsorption
Solution acidity is the most important variable influencing the process. Indeed, solution acidity affects the surface charge of the sorbent surface and the state of phenol that existed in the solution. The adsorption capacity of phenol onto lead ferrite-MAC and pristine AC was investigated at an initial pH range of 2.0–12.0 (Fig. 7). The extent of phenol adsorbed onto lead ferrite-MAC increased from 36.23 to 62.56 mg/g by increasing the pH of the solution from 2.0 to 7.0, respectively. In addition, the amount of adsorption capacity for pristine AC increased from 23.45 to 43.41 mg/g by increasing the pH of the solution from 2.0 to 7.0, respectively. When the solution pH decreases from about 7.0 to 2.0, the H+ concentration increases. The presence of more H+ in the solution makes the condition unfavorable for adsorption of phenol, as emerges a competition between H+ and phenol for occupying the adsorption sites. Albeit, further increase in pH, beyond the value of 7.0, decreased the adsorption capacity of both adsorbents. As mentioned, the zero-point charge values of lead ferrite-MAC and pristine AC were about 6.7 and 7.1, respectively, above which the adsorbent surface presents negative charges. Besides, at the higher values of pH, the presence of higher OH− in the solution cause the dissociation of H+ from the phenol, leaving them in the anionic dominant form. Therefore, a repulsive electrostatic interaction prevailed that resulted in a reduction in the capacity of adsorption. Thus, there would be an optimum pH condition (about 7 for the current study) around which the maximum uptake is usually expected.
Equilibrium studies
The relationship between the quantity of phenol adsorbed onto lead ferrite-MAC and pristine AC, and the phenol concentration in the equilibrium state at a fixed temperature presents an adsorption isotherm. Adsorption isotherm provides essential information about the capability of the adsorption process for wastewater decontamination applications13. According to Fig. 8, the extent of phenol absorbed onto both adsorbents enhances by increasing the concentration of phenol until it reaches a plateau area, representing the maximum adsorption capacity of lead ferrite-MAC and pristine AC. This shape is a favorable type of isotherm and indicates that lead ferrite-MAC and pristine AC are good adsorbents for phenol.
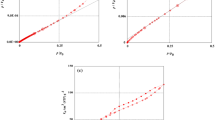
There are different isotherm models in the literature to represent the equilibrium curves and extract information about the system13, 51. In this research, Freundlich, Langmuir, Redlich-Peterson, and Tempkin isotherms were applied to explain the equilibrium adsorption (Fig. 9). The Langmuir isotherm assumes that all adsorption sites have the same sorption activation energy and have no transmigration in the plane of the surface52. The Freundlich isotherm was derived from the assumption that adsorption occurs on a heterogeneous surface through a multilayer adsorption mechanism53. The Tempkin isotherm considers the adsorbate–adsorbent interactions54. These interactions decrease the heat of molecular adsorption in the layer. The distribution of binding energies determines the adsorption process54. Redlich-Peterson55 behaves like the Freundlich isotherm at high concentrations of sorbate, and at a low concentration, it approximates Henry’s law. Their nonlinear equations are presented as follows:
where qm is the theoretical saturation capacity (mg/g); KL is the Langmuir constant (L/mg); KF is the constant of the Freundlich model indicating the adsorbent’s adsorption capacity (mg/g)(mg/L)−1/n; 1/n is the Freundlich exponent; B (defined as RT/bT) (mg/g) is the Tempkin constant corresponding to the adsorption heat; KT is a constant related to maximum binding energy (L/mg); T is temperature; R denotes the universal gas constant; KRP (L/g), aRP (L/mg)β), and β are constants in Redlich-Peterson isotherm.
Table 2 shows the equilibrium parameters for the systems of phenol/lead ferrite-MAC and phenol/pristine AC, according to Eqs. (3–6).
The parameter estimations were performed using proper script programming on Matlab 2017. Regarding the higher values of coefficient of determination (R2) and adjusted determination coefficient (R2adj), coupled with the lower values of average relative error (ARE) and minimum squared error (MSE) (Table 2), it can be found that the isothermal data for Pristine AC were in agreement with the isotherm models of Tempkin and Redlich-Peterson. As the number of surface groups and the phenol uptake are high for activated carbon, adsorbate–adsorbate interactions are of major importance, justifying the relevant application of the Tempkin equation. In addition, the isothermal data for lead ferrite-MAC were in agreement with the isotherm models of Redlich-Peterson and Langmuir. These results indicate that the phenol molecules are adsorbed by specific sites of the lead ferrite-MAC adsorbent, undergoing adsorption in a monolayer form. It has been found that the presence of metal hydroxides on the surface of AC increases the adsorption capacity of phenol on lead ferrite-MAC (Table 2). This can be explained in terms of the molecular coordination between phenol and metal hydroxides18 along with the active sites of AC. The Freundlich constants and 1/n values also confirmed that the present adsorption results for phenol on both adsorbents stemmed from physical and chemical interactions between the adsorbent and adsorbate.
According to isotherm parameters, the maximum adsorption capacity of the lead ferrite-MAC composite was higher than that of pristine AC, which was in agreement with adsorption experimental trends. This difference is due to the difference in the structure of the adsorbent because the composite of lead ferrite-MAC contains additional metal hydroxides.
The Dubinin–Radushkevich (D–R) model also performed the analysis of the equilibrium data. This analysis was realized to determine whether the adsorption process is chemical or physical56. This model assumes that ionic species binding follows by multilayer adsorption. D–R equation is as follows:
Being qs is the isotherm saturation capacity, and KDR is the D–R constant. The mean free energy is defined as follows:
The plot of [lnqe] vs. [ln2(1 + 1/Ce)] for the phenol adsorption onto lead ferrite-MAC and pristine AC presented coefficients of determination of 0.9917 and 0.9979, respectively. The E value for both adsorbents was relatively 4 kJ/mol. According to the D–R model, chemical adsorption is favored at mean free energy (E) between 8 and 16 kJ/mol, while for E < 8 kJ/mol, adsorption proceeds physically. Therefore, the adsorption of phenol onto both adsorbents may be conducted by a physical mechanism.
Adsorption kinetics
To identify the necessary time for equilibrium, the phenol uptake onto lead ferrite-MAC and pristine AC at different initial phenol concentrations was examined versus contact time. The amount of phenol absorbed on both adsorbents was measured at 0, 30, 60, 90, 120, 150, 180, 240, 360, and 480 min. Figure 10 shows the phenol adsorption profiles onto lead ferrite-MAC and pristine AC at various initial phenol concentrations. The adsorption of phenol at the initial time increased drastically and reached approximately a constant value after 200 min. Therefore, the contact time of 180 min is enough to attain equilibrium. However, a contact time of 300 min was selected to ensure completed equilibrium.
The reaction models of pseudo-first-order (PFO) (Eq. 10), pseudo-second-order (PSO) (Eq. 11), and Elovich (Eq. 12)55, 57 were applied to comprehend better the adsorption mechanism according to the kinetic curves. All these models consider adsorption as a reaction. It is noted that this fact is not true, but sometimes, these models can complement the information about the adsorption process.
where k1 (1/min) and k2 (g/mg min) are the kinetics constants of PFO and PSO, respectively; q1 and q2 are the theoretical values for the adsorption capacity (mg/g); b is the initial velocity (mg/g min); a is the desorption constant of Elovich model (g/mg).
In addition to the reaction models, the linear driving force model (LDF)58, which is based on the mass transfer phenomena, was applied in this research. LDF model is given by:
where \(\stackrel-{\text{q}}\) is the average phenol adsorption capacity (mg/g), \({\text{q}}^{*}\) is the phenol adsorption capacity related to the concentration in the liquid phase at equilibrium (mg/g−1), and kLDF is the LDF mass transfer coefficient (1/min). In the case of this work, the Langmuir model presented the best fit for the equilibrium, so q* in Eq. (14) was substituted by Eq. (3). In addition, the global mass balance of phenol in the batch (Eq. 2) was inserted in Eq. (14), leading to:
The parameter kLDF can be related to the diffusivity inside the adsorbent (Dh) and the particle radius (R) by58:
The kinetic parameters of the systems phenol/lead ferrite-MAC and phenol/pristine AC, estimated by the fitting of Eqs. (10), (11), (12), and (15) to the experimental data (Fig. 10), are depicted in Table 3. Among the reaction models, the PSO model was the best to represent the data, with higher values of coefficient of determination (R2) and adjusted determination coefficient (R2adj), and lower values of average relative error (ARE) and minimum squared error (MSE) (Tables 3, 4). The parameter q2 of such model for lead ferrite-MAC and pristine AC increased from 47.397 and 35.177 mg/g to 121.963 and 82.395 mg/g, respectively when the initial phenol concentration increased from 100 to 500 mg/L. However, the rate of this increase was successively lower. Both facts are related to the progressive increase in the surface coverage.
In the same way, the parameter k2 for lead ferrite-MAC and pristine AC raised from 8.531 × 10–4 and 4.365 × 10–4 g/mg min to 6.706 × 10–4 and 1.160 × 10–3 g/mg min, respectively with the increase in initial phenol concentration (Tables 3, 4). This behavior shows that the adsorption phenomenon was progressively faster when the initial concentration increased. This fact is explained by the external and internal concentration gradients that are higher at higher phenol concentrations.
The LDF mass transfer model could also explain the phenol adsorption on both adsorbents. In this sense, it can be found that the phenol adsorption on lead ferrite-MAC and pristine activated carbon occurs according to the diffusion in a homogeneous adsorbent from the mass transfer aspect. This mechanism was also assumed by Franco et al.59, studying the phenol adsorption on activated carbon prepared from fruit wastes of the Ceiba speciosa. The values of kLDF and Dh corroborated the same trend of k2. Besides, Dh for lead ferrite-MAC and pristine activated carbon ranged from 4.41 × 10–9 and 1.41 × 10–9 to 2.06 × 10–8 and 8.43 × 10–8 cm2/s, respectively. These values are in the same range as those found by Franco et al.59 (activated carbon) and Ocampo-Pérez et al.60 (bituminous coal).
Proposed mechanism of phenol adsorption
According to isotherm parameters, the maximum adsorption capacity of lead ferrite-MAC composite was more than that of pristine activated carbon, which was in agreement with adsorption experimental trends. This difference is due to the metal hydroxides coated on activated carbon since they improve the retention of phenol on the available active sites of the adsorbent and create an additional electrostatic interaction with the phenol adsorbate. Electrostatic attraction between metal hydroxides and the acidic –OH of phenol can be one of the possible mechanisms of phenol adsorption onto lead ferrite-MAC composite61. Some changes in the bending vibration of M–O before and after phenol adsorption (FTIR analysis) confirmed the electrostatic interaction of phenol with lead ferrite-MAC. Another possible mechanism of phenol adsorption onto the developed adsorbent is hydrogen-bonding interactions between the functional groups of activated carbon (CO and OH) with phenolic –OH. In addition, π–π electron donor–acceptor interactions between aromatic rings of phenol and the surface of the adsorbent affect the adsorption process62.
Comparison with other adsorbents and reusability of lead ferrite-MAC
Selective phenol adsorption using different adsorbents like activated carbon22, biological materials31, and minerals14 has been investigated. Table 5 indicates the comparison of phenol removal using different adsorbents. The adsorption capacity of the lead ferrite-MAC adsorbent in this research is not maximum. However, it is better than some adsorbents such as magnetic activated carbon cobalt nanoparticles; tithonia diversifolia activated carbon, biochar produced from an oil palm frond, diethylenetriamine-modified activated carbon, activated carbon obtained from black wattle bark waste, NiFe2O4-powdered activated carbon, and biochar made of the pine fruit shells (BC550). Therefore, it can be concluded that the adsorbent prepared here is promising and competitive.
Another attribute of lead ferrite-MAC that makes it a promising material is reusability. The reusability of an adsorbent is a crucial factor from the practical aspect, making it a commercially attractive and green method. Different methods include heating regeneration, solvent washing, and chemical and petrochemical regeneration for regenerating adsorbents63, 64. Among them, solvent washing is a well-known method for recovering and reusing the adsorbent. So, the solvent washing method was applied to regenerate the developed adsorbent. The regeneration experiments of lead ferrite-MAC were conducted for six cycles using 1 mol/L NaOH. When washing with NaOH, sodium phenolate is formed because of the reaction of sodium hydroxide with phenol. This product can be easily desorbed and dissolved in the solution of solvent65, 66. The obtained results are presented in Fig. 11. The adsorption capacity of phenol onto the adsorbent decreased with the number of cycles. This phenomenon have resulted from the saturation of active sites on the surface of the adsorbent, the protonation of available functional groups on the surface of the adsorbent, and/or the destruction of adsorbent particles during successive cycles of adsorption/desorption. However, the adsorbent maintained 85% of this maximum potential after these cycles. It can be found that lead ferrite-MAC has interesting adsorption potential after six cycles of regeneration. The results confirmed the practical usefulness of the lead ferrite-MAC composite.
Phenol removal from the real industrial wastewater
To evaluate the practical application of the lead ferrite-MAC adsorbent for the removal of phenol from the real industrial wastewater, 0.15 g of the lead ferrite-MAC composite and pristine activated carbon was stirred with 100 ml of wastewater sample taken from the nearby industrial area of Parsian, Bandarabbas, Iran. To remove solid particles and impurities, the wastewater sample was filtered. The wastewater sample was analyzed for the contents of pollutants. The major pollutants of this sample were phenol, copper, chromium, nickel, zinc, cobalt, manganese, and arsenic as presented in Table 6. It is noted that the original sample spiked with 90 μg/L of phenol. The experiment was carried out at an ambient temperature, stirring speed of 300 rpm, and a stirring time of 400 min.
The developed adsorbent (lead ferrite-MAC) showed significant adsorption efficiency for several pollutants. Results indicated that the lead ferrite-MAC composite could efficiently remove phenol from waste streams and the removal was relatively 96%. Furthermore, the developed adsorbent removed other pollutants like copper, chromium, nickel, zinc, cobalt, manganese, and arsenic with efficiencies of relatively 90%. Therefore, the developed adsorbent can be successfully applied with high efficiency for the practical removal of phenol from waste streams to produce healthy water using a stable and efficient adsorbent.
Conclusions
In this research, a novel lead ferrite-activated carbon composite (lead ferrite-MAC) was developed and compared with pristine AC for the uptake of phenol, where the most influential operating parameters were analyzed. Instrumental analyses such as XRD, SEM, FTIR, zeta potential, and BET analysis were carried out to characterize lead ferrite-MAC adsorbent. lead ferrite-MAC was preponderantly a mesoporous adsorbent, with some microporous, and presented a surface area of 747.53 m2/g and pore size of 11.89 nm. The zeta potential was 6.7, and a saturation magnetization of 37.9 emu/g proved the magnetic character. Concerning the phenol adsorption potential for both adsorbents, the best pH was 7.0. Results indicated that the maximum adsorption capacities for lead ferrite-MAC composite and pristine activated carbon were 145.708 and 116.606 mg/g, respectively. Regarding the high value of the coefficient of determination (R2) and adjusted determination coefficient (R2adj), coupled with the lower values of average relative error (ARE) and minimum squared error (MSE), it can be found that the isothermal data for lead ferrite-MAC adsorbent were in agreement with the isotherm models of Redlich-Peterson and Langmuir. According to isotherm parameters, the maximum adsorption capacity of lead ferrite-MAC composite was more than that of pristine activated carbon, which was in agreement with adsorption experimental trends. This difference is due to the metal hydroxides coated on activated carbon since they improve the retention of phenol on the available active sites of the adsorbent and create an additional electrostatic interaction with the phenol adsorbate. The mean free energy for the systems of phenol/lead ferrite-MAC and phenol/pristine AC was relatively 4 kJ/mol, indicating a physical mechanism. Among the kinetic models, the PSO model was the best to represent the data, with higher values of coefficient of determination (R2) and adjusted determination coefficient (R2adj), and lower values of average relative error (ARE) and minimum squared error (MSE). The phenol adsorption onto lead ferrite-MAC and pristine AC was relatively fast and explained by the diffusion in a homogeneous solid. The diffusion coefficient values, Dh, for lead ferrite-MAC and pristine AC ranged from 4.41 × 10–9 and 1.41 × 10–9 to 2.06 × 10–8 and 8.43 × 10–8 cm2/s, respectively. In addition, the adsorption capacity of lead ferrite-MAC is within the literature range, but the material can be reused 6 times. The results presented in this work point out that lead ferrite-MAC is a promising and competitive adsorbent to uptake phenol from aqueous media.
Data availability
The datasets used and/or analyzed during the current study available from the corresponding author on reasonable request.
References
El-Bery, H. M., Saleh, M., El-Gendy, R. A., Saleh, M. R. & Thabet, S. M. High adsorption capacity of phenol and methylene blue using activated carbon derived from lignocellulosic agriculture wastes. Sci. Rep. 12, 1–17 (2022).
Song, M. et al. Incorporation of humic acid into biomass derived carbon for enhanced adsorption of phenol. Sci. Rep. 9, 1–8 (2019).
Yahia, M. B. et al. Modeling and interpretations by the statistical physics formalism of hydrogen adsorption isotherm on LaNi4.75Fe0.25. Int. J. Hydrog. Energy 38, 11536–11542 (2013).
Kumar, A. et al. Phenolic compounds degradation: Insight into the role and evidence of oxygen vacancy defects engineering on nanomaterials. Sci. Total Environ. 800, 149410. https://doi.org/10.1016/j.scitotenv.2021.149410 (2021).
Bhadra, B. N., Lee, J. K., Cho, C.-W. & Jhung, S. H. Remarkably efficient adsorbent for the removal of bisphenol A from water: Bio-MOF-1-derived porous carbon. Chem. Eng. J. 343, 225–234. https://doi.org/10.1016/j.cej.2018.03.004 (2018).
Al Bsoul, A. et al. Efficient removal of phenol compounds from water environment using Ziziphus leaves adsorbent. Sci. Total Environ. 761, 143229. https://doi.org/10.1016/j.scitotenv.2020.143229 (2021).
Aksu, Z. & Gönen, F. Biosorption of phenol by immobilized activated sludge in a continuous packed bed: Prediction of breakthrough curves. Process Biochem. 39, 599–613 (2004).
Liu, Y. et al. Phenol biodegradation by Acinetobacter radioresistens APH1 and its application in soil bioremediation. Appl. Microbiol. Biotechnol. 104, 427–437 (2020).
Rosly, M. B. et al. Stability of emulsion liquid membrane using bifunctional diluent and blended nonionic surfactant for phenol removal. Chem. Eng. Process. Process Intensification 148, 107790 (2020).
Cañadas, R., González-Miquel, M., González, E. J., Díaz, I. & Rodríguez, M. Hydrophobic eutectic solvents for extraction of natural phenolic antioxidants from winery wastewater. Sep. Purif. Technol. 254, 117590 (2021).
Mishra, S., Yadav, S. S., Rawat, S., Singh, J. & Koduru, J. R. Corn husk derived magnetized activated carbon for the removal of phenol and para-nitrophenol from aqueous solution: Interaction mechanism, insights on adsorbent characteristics, and isothermal, kinetic and thermodynamic properties. J. Environ. Manage. 246, 362–373. https://doi.org/10.1016/j.jenvman.2019.06.013 (2019).
Rezai, B. & Allahkarami, E. In Soft Computing Techniques in Solid Waste and Wastewater Management (eds Rama, R. K. et al.) 75–93 (Elsevier, 2021).
Allahkarami, E. & Rezai, B. Removal of cerium from different aqueous solutions using different adsorbents: A review. Process Saf. Environ. Prot. 124, 345–362. https://doi.org/10.1016/j.psep.2019.03.002 (2019).
Dehmani, Y. et al. Kinetic, thermodynamic and mechanism study of the adsorption of phenol on Moroccan clay. J. Mol. Liq. 312, 113383 (2020).
Dong, R. et al. Removal of phenol from aqueous solution using acid-modified Pseudomonas putida-sepiolite/ZIF-8 bio-nanocomposites. Chemosphere 239, 124708 (2020).
Qu, Y., Qin, L., Liu, X. & Yang, Y. Reasonable design and sifting of microporous carbon nanosphere-based surface molecularly imprinted polymer for selective removal of phenol from wastewater. Chemosphere 251, 126376 (2020).
Sellaoui, L. et al. Adsorption of phenol on microwave-assisted activated carbons: Modelling and interpretation. J. Mol. Liq. 274, 309–314. https://doi.org/10.1016/j.molliq.2018.10.098 (2019).
Koduru, J. R., Lingamdinne, L. P., Singh, J. & Choo, K.-H. Effective removal of bisphenol A (BPA) from water using a goethite/activated carbon composite. Process Saf. Environ. Prot. 103, 87–96 (2016).
Park, H.-S., Koduru, J. R., Choo, K.-H. & Lee, B. Activated carbons impregnated with iron oxide nanoparticles for enhanced removal of bisphenol A and natural organic matter. J. Hazard. Mater. 286, 315–324 (2015).
Lingamdinne, L. P., Koduru, J. R. & Karri, R. R. A comprehensive review of applications of magnetic graphene oxide based nanocomposites for sustainable water purification. J. Environ. Manage. 231, 622–634. https://doi.org/10.1016/j.jenvman.2018.10.063 (2019).
Lingamdinne, L. P. et al. Biogenic reductive preparation of magnetic inverse spinel iron oxide nanoparticles for the adsorption removal of heavy metals. Chem. Eng. J. 307, 74–84. https://doi.org/10.1016/j.cej.2016.08.067 (2017).
Din, A. T. M., Hameed, B. H. & Ahmad, A. L. Batch adsorption of phenol onto physiochemical-activated coconut shell. J. Hazard. Mater. 161, 1522–1529 (2009).
Fröhlich, A. C., Foletto, E. L. & Dotto, G. L. Preparation and characterization of NiFe2O4/activated carbon composite as potential magnetic adsorbent for removal of ibuprofen and ketoprofen pharmaceuticals from aqueous solutions. J. Clean. Prod. 229, 828–837. https://doi.org/10.1016/j.jclepro.2019.05.037 (2019).
Ianoş, R. et al. Combustion synthesis of iron oxide/carbon nanocomposites, efficient adsorbents for anionic and cationic dyes removal from wastewaters. J. Alloy. Compd. 741, 1235–1246. https://doi.org/10.1016/j.jallcom.2018.01.240 (2018).
Heo, J. et al. Enhanced adsorption of bisphenol A and sulfamethoxazole by a novel magnetic CuZnFe2O4–biochar composite. Biores. Technol. 281, 179–187 (2019).
Karthikeyan, P., Vigneshwaran, S., Preethi, J. & Meenakshi, S. Preparation of novel cobalt ferrite coated-porous carbon composite by simple chemical co-precipitation method and their mechanistic performance. Diam. Relat. Mater. 108, 107922 (2020).
Yang, N., Zhu, S., Zhang, D. & Xu, S. Synthesis and properties of magnetic Fe3O4-activated carbon nanocomposite particles for dye removal. Mater. Lett. 62, 645–647. https://doi.org/10.1016/j.matlet.2007.06.049 (2008).
Zhang, G., Qu, J., Liu, H., Liu, R. & Wu, R. Preparation and evaluation of a novel Fe–Mn binary oxide adsorbent for effective arsenite removal. Water Res. 41, 1921–1928. https://doi.org/10.1016/j.watres.2007.02.009 (2007).
Feng, L. Bifurcations of limit cycles in a quintic Lyapunov system with eleven parameters. Chaos Solitons Fractals 45, 1417–1422. https://doi.org/10.1016/j.chaos.2012.07.012 (2012).
Dąbrowski, A., Podkościelny, P., Hubicki, Z. & Barczak, M. Adsorption of phenolic compounds by activated carbon—a critical review. Chemosphere 58, 1049–1070 (2005).
Tengku-Mazuki, T. A. et al. Optimization of phenol degradation by Antarctic bacterium Rhodococcus sp.. Antarct. Sci. 32, 486–495 (2020).
Barros, F., Dykes, L., Awika, J. M. & Rooney, L. W. Accelerated solvent extraction of phenolic compounds from sorghum brans. J. Cereal Sci. 58, 305–312 (2013).
Lazarova, Z. & Boyadzhieva, S. Treatment of phenol-containing aqueous solutions by membrane-based solvent extraction in coupled ultrafiltration modules. Chem. Eng. J. 100, 129–138 (2004).
Li, D. et al. Preparation and characterization of PFTS grafted alumina supported zirconia (ASZ) membrane for removal of phenol from aqueous solution. Appl. Surf. Sci. 505, 144608 (2020).
Lin, S.-H. & Juang, R.-S. Adsorption of phenol and its derivatives from water using synthetic resins and low-cost natural adsorbents: A review. J. Environ. Manage. 90, 1336–1349 (2009).
Maaz, K., Mumtaz, A., Hasanain, S. K. & Ceylan, A. Synthesis and magnetic properties of cobalt ferrite (CoFe2O4) nanoparticles prepared by wet chemical route. J. Magn. Magn. Mater. 308, 289–295 (2007).
Dehghan Monfared, A. & Ghazanfari, M. H. Wettability alteration of oil-wet carbonate porous media using silica nanoparticles: Electrokinetic characterization. Ind. Eng. Chem. Res. 58, 18601–18612. https://doi.org/10.1021/acs.iecr.9b03060 (2019).
Jung, C. et al. Hexavalent chromium removal by various adsorbents: Powdered activated carbon, chitosan, and single/multi-walled carbon nanotubes. Sep. Purif. Technol. 106, 63–71 (2013).
Hao, Z., Wang, C., Yan, Z., Jiang, H. & Xu, H. Magnetic particles modification of coconut shell-derived activated carbon and biochar for effective removal of phenol from water. Chemosphere 211, 962–969 (2018).
Allahkarami, E., Igder, A., Fazlavi, A. & Rezai, B. Prediction of Co (II) and Ni (II) ions removal from wastewater using artificial neural network and multiple regression models. Physicochem. Probl. Miner. Process. 53, 25 (2017).
Igder, A., Fazlavi, A., Allahkarami, E. & Dehghanipour, A. Optimization of Ni(II) & Co(II) removal from wastewater and statistical studies on the results of experimental designs. Geosyst. Eng. 22, 91–100. https://doi.org/10.1080/12269328.2018.1477072 (2019).
Paledi, U., Allahkarami, E., Rezai, B. & Aslani, M. R. Selectivity index and separation efficiency prediction in industrial magnetic separation process using a hybrid neural genetic algorithm. SN Appl. Sci. 3, 351. https://doi.org/10.1007/s42452-021-04361-6 (2021).
Karri, R. R., Tanzifi, M., Yaraki, M. T. & Sahu, J. N. Optimization and modeling of methyl orange adsorption onto polyaniline nano-adsorbent through response surface methodology and differential evolution embedded neural network. J. Environ. Manage. 223, 517–529 (2018).
Smitha, P., Pandey, P. K., Kurian, S. & Gajbhiye, N. S. ICAME 2007 543–548 (Springer, 2008).
Zhou, Y. et al. New insight into the mechanism of peroxymonosulfate activation by nanoscaled lead-based spinel for organic matters degradation: A singlet oxygen-dominated oxidation process. J. Colloid Interface Sci. 572, 318–327. https://doi.org/10.1016/j.jcis.2020.03.116 (2020).
Li, H. et al. Facile preparation of zeolite-activated carbon composite from coal gangue with enhanced adsorption performance. Chem. Eng. J. 390, 124513. https://doi.org/10.1016/j.cej.2020.124513 (2020).
Luo, J. et al. Preparation and characterization of benzoic acid-modified activated carbon for removal of gaseous mercury chloride. Fuel 160, 440–445. https://doi.org/10.1016/j.fuel.2015.08.002 (2015).
Zhang, Z., Wang, T., Zhang, H., Liu, Y. & Xing, B. Adsorption of Pb(II) and Cd(II) by magnetic activated carbon and its mechanism. Sci. Total Environ. 757, 143910. https://doi.org/10.1016/j.scitotenv.2020.143910 (2021).
Zhang, Y. et al. Utilization of wheat bran for producing activated carbon with high specific surface area via NaOH activation using industrial furnace. J. Clean. Prod. 210, 366–375 (2019).
Allahkarami, E. & Rezai, B. A literature review of cerium recovery from different aqueous solutions. J. Environ. Chem. Eng. 9, 104956. https://doi.org/10.1016/j.jece.2020.104956 (2021).
Dehghan Monfared, A., Ghazanfari, M. H., Jamialahmadi, M. & Helalizadeh, A. Adsorption of silica nanoparticles onto calcite: Equilibrium, kinetic, thermodynamic and DLVO analysis. Chem. Eng. J. 281, 334–344. https://doi.org/10.1016/j.cej.2015.06.104 (2015).
Langmuir, I. The constitution and fundamental properties of solids and liquids. Part I. Solids. J. Am. Chem. Soc. 38, 2221–2295 (1916).
Freundlich, H. M. F. Over the adsorption in solution. J. Phys. Chem 57, 1100–1107 (1906).
Tempkin, M. I. & Pyzhev, V. Kinetics of ammonia synthesis on promoted iron catalyst. Acta Phys. Chim. USSR 12, 327 (1940).
Rezai, B. & Allahkarami, E. In Soft Computing Techniques in Solid Waste and Wastewater Management (eds Rama, R. K. et al.) 35–53 (Elsevier, 2021).
Allahkarami, E., Soleimanpour Moghadam, N., Jamrotbe, B. & Azadmehr, A. Competitive adsorption of Ni (II) and Cu (II) ions from aqueous solution by vermiculite-alginate composite: Batch and fixed-bed column studies. J. Dispersion Sci. Technol. 20, 1–11 (2021).
Allahkarami, E., Azadmehr, A., Noroozi, F., Farrokhi, S. & Sillanpää, M. Nitrate adsorption onto surface-modified red mud in batch and fixed-bed column systems: Equilibrium, kinetic, and thermodynamic studies. Environ. Sci. Pollut. Res. https://doi.org/10.1007/s11356-022-19311-x (2022).
Georgin, J. et al. Efficient removal of naproxen from aqueous solution by highly porous activated carbon produced from Grapetree (Plinia cauliflora) fruit peels. J. Environ. Chem. Eng. 9, 106820. https://doi.org/10.1016/j.jece.2021.106820 (2021).
Franco, D. S. et al. Highly effective adsorption of synthetic phenol effluent by a novel activated carbon prepared from fruit wastes of the Ceiba speciosa forest species. J. Environ. Chem. Eng. 9, 105927. https://doi.org/10.1016/j.jece.2021.105927 (2021).
Ocampo-Pérez, R., Leyva-Ramos, R., Sanchez-Polo, M. & Rivera-Utrilla, J. Role of pore volume and surface diffusion in the adsorption of aromatic compounds on activated carbon. Adsorption 19, 945–957 (2013).
Mohan, D., Sarswat, A., Singh, V. K., Alexandre-Franco, M. & Pittman, C. U. Jr. Development of magnetic activated carbon from almond shells for trinitrophenol removal from water. Chem. Eng. J. 172, 1111–1125 (2011).
Bandosz, T. J. Activated Carbon Surfaces in Environmental Remediation (Elsevier, 2006).
Mirbagheri, N. S. & Sabbaghi, S. A natural kaolin/γ-Fe2O3 composite as an efficient nano-adsorbent for removal of phenol from aqueous solutions. Microporous Mesoporous Mater. 259, 134–141 (2018).
Mohammadi, S. Z., Darijani, Z. & Karimi, M. A. Fast and efficient removal of phenol by magnetic activated carbon-cobalt nanoparticles. J. Alloy. Compd. 832, 154942 (2020).
Singh, D. K. & Srivastava, B. Removal of phenol pollutants from aqueous solutions using various adsorbents. (2002).
Gaber, D., Haija, M. A., Eskhan, A. & Banat, F. Graphene as an efficient and reusable adsorbent compared to activated carbons for the removal of phenol from aqueous solutions. Water Air Soil Pollut. 228, 1–14 (2017).
Sharma, G. & Naushad, M. Adsorptive removal of noxious cadmium ions from aqueous medium using activated carbon/zirconium oxide composite: Isotherm and kinetic modelling. J. Mol. Liq. 310, 113025 (2020).
Supong, A. et al. Experimental and theoretical insight into the adsorption of phenol and 2, 4-dinitrophenol onto Tithonia diversifolia activated carbon. Appl. Surf. Sci. 529, 147046 (2020).
Lawal, A. A. et al. Adsorption mechanism and effectiveness of phenol and tannic acid removal by biochar produced from oil palm frond using steam pyrolysis. Environ. Pollut. 269, 116197 (2021).
Saleh, T. A., Adio, S. O., Asif, M. & Dafalla, H. Statistical analysis of phenols adsorption on diethylenetriamine-modified activated carbon. J. Clean. Prod. 182, 960–968 (2018).
Lütke, S. F. et al. Preparation of activated carbon from black wattle bark waste and its application for phenol adsorption. J. Environ. Chem. Eng. 7, 103396 (2019).
Changmai, M. & Purkait, M. K. Kinetics, equilibrium and thermodynamic study of phenol adsorption using NiFe2O4 nanoparticles aggregated on PAC. J. Water Process Eng. 16, 90–97 (2017).
Mohammed, N. A. S., Abu-Zurayk, R. A., Hamadneh, I. & Al-Dujaili, A. H. Phenol adsorption on biochar prepared from the pine fruit shells: Equilibrium, kinetic and thermodynamics studies. J. Environ. Manage. 226, 377–385 (2018).
Author information
Authors and Affiliations
Contributions
E.A.: conceptualization, methodology, software, investigation, data curation, writing- original draft preparation. A.D.M.: conceptualization, methodology, data curation, investigation, supervision, visualization, investigation, writing- reviewing and editing. L.F.O.S.: software, validation, writing- reviewing and editing. G.L.D.: writing- reviewing and editing, visualization, software, validation.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Allahkarami, E., Dehghan Monfared, A., Silva, L.F.O. et al. Lead ferrite-activated carbon magnetic composite for efficient removal of phenol from aqueous solutions: synthesis, characterization, and adsorption studies. Sci Rep 12, 10718 (2022). https://doi.org/10.1038/s41598-022-15077-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-022-15077-x
This article is cited by
-
Magnetic-based adsorbing platforms for water and wastewater purification
International Journal of Environmental Science and Technology (2024)
-
Excess secondary sludge reuse by H2O2 thermal dehydration
Environmental Science and Pollution Research (2024)
-
Enhancing the efficiency of Ni(II), Cd(II), and Cu(II) adsorption from aqueous solution using schist/alginate composite: batch and continuous studies
Environmental Science and Pollution Research (2023)
-
Recent progress in the preparation, characterization, and applications of modified halloysite nanotubes as adsorbents for wastewater treatment
Korean Journal of Chemical Engineering (2023)
-
Porous design of molecularly imprinted polymers for improved drug loading and organized release properties
Journal of Materials Science (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.