Abstract
This work utilizes a realistic electro-optical coupled simulation to study the (i) impact of mesoporous TiO2 removal; (ii) the embedding of Ag@SiO2 and SiO2@Ag@SiO2 plasmonic nanoparticles; (iii) utilization of solution-processed inorganic p-type copper(I) thiocyanate (CuSCN) layer at the perovskite/carbon interface; and (iv) the increase of the work function of carbon electrodes (via incorporation of suitable additives/binders to the carbon ink) on the performance of carbon-based PSCs. Removal of mesoporous TiO2 increased the power conversion efficiency (PCE) of the device from 14.83 to 16.50% due to the increase in exciton generation rate and charge carriers’ mobility in the vicinity of the perovskite-compact TiO2 interface. Subsequently, variable mass ratios of Ag@SiO2 and SiO2@Ag@SiO2 plasmonic nanoparticles are embedded in the vicinity of the perovskite-compact TiO2 interface. In the optimum cases, the PCE of the devices increased to 19.72% and 18.92%, respectively, due to light trapping, scattering, and strong plasmonic fields produced by the plasmonic nanoparticles. Furthermore, adding the CuSCN layer remarkably increased the PCE of the device with a 0.93% mass ratio of Ag@SiO2 nanoparticles from 19.72 to 26.58% by a significant improvement of Voc and FF, due to the proper interfacial energy band alignment and the reduction of the recombination current density. Similar results were obtained by increasing the carbon work function, and the cell PCE was enhanced up to 26% in the optimal scenario. Our results pave the way to achieve high efficiencies in remarkably stable printable carbon-based PSCs.
Similar content being viewed by others
Introduction
Over the past decade, perovskite solar cells have become a promising candidate as a future source of energy. The unique optical and electrical properties of perovskites, such as high absorption coefficient, direct bandgap, long carrier diffusion length, and low exciton binding energy, have led to rapid growth and a significant increase in the device efficiency from 3.8 to > 25%1,2. However, most of the high-efficiency perovskite solar cells are fabricated with expensive organic materials (e.g., 2,2′,7,7′-tetrakis-(N,N-di-4-methoxyphenylamino)-9,9′-spirobifluorene (spiro-OMeTAD)) as the hole transfer layer (HTL) and noble metal (e.g., Au) as the electrode, which is deposited by thermal evaporation in a vacuum environment. This not only increases the cost of production but also reduces the stability of the device due to the degradation because of the mobile behavior of Au contact at 70 °C and the poor stability of Spiro-OMeTAD3,4.
In recent years, HTL-free carbon-based perovskite solar cells have attracted much academic and industrial attention for their outstanding stability with lower production costs5,6,7,8,9,10,11,12. In this case, when a carbon-based material is replaced with the metal contact and the HTL, the non-encapsulated device exhibits excellent stability in an ambient environment13. However, the efficiency of carbon-based devices is significantly lower than their counterparts equipped with HTL/metal back contact, where the highest efficiency of HTL-free carbon-based perovskite solar cells is ~ 15%14.
In general, the interface of the perovskite photoactive layer and the electron transfer layer (ETL) (which often includes the compact and mesoporous TiO2) plays a crucial role in achieving high efficiency15,16. The mesoporous structure acts as a pathway for electron transfer by increasing the contact surface between the perovskite and TiO2 17. However, the presence of isolated TiO2 nanoparticles can lead to charge accumulation and increased recombination due to the trapping of electron carriers and the low mobility of carriers in the perovskite-filled mesoporous structure. This, in turn, affects hysteresis behavior in measuring the efficiency and instability of the device due to the ion migration18. Therefore, providing a carbon-based planar structure by removing mesoporous TiO2 can not only reduce the device's hysteresis behavior19 but also take another step toward commercializing perovskite-based solar cells by reducing the cost of mass production.
One of the most effective approaches to increase the efficiency of carbon-based perovskite solar cells is to use plasmonic nanoparticles within the device structure20,21,22,23,24,25,26. Under light illumination, metal nanoparticles create strongly localized fields around them during the coherent oscillations of their conducting electrons27,28, which can not only increase the photon flux inside the perovskite as a secondary light source but also increase the generation rate of free carriers by reducing the exciton binding energy29. In addition, the light scattering by resonated nanoparticles can increase the absorption via enlarging of the light pathway, even at thin perovskite thicknesses30. On the other hand, plasmonic nanoparticles generate hot electrons during plasmon resonance which can be directly injected into their surrounding perovskite environment and increase photo-generated electrons mobility by filling the trap states31.
To take advantage of local surface plasmon resonance (LSPR) effects, metal nanoparticles must be added to the photoactive layer. First, however, a capping layer over the metallic nanoparticles must be used due to the corrosive properties of CH3NH3PbI3, which lead to the decomposition of noble metals32. Then, heating the nanoparticles metallic surface during plasmon resonance will lead to the recombination of excitons at their surface33. Here, the usage of a thin layer of SiO2 as a capping layer not only stabilizes the noble metal nanoparticles against thermal and chemical interactions but also creates the slightest manipulation in the LSPR properties of the nanoparticles21. The presence of a dielectric coating layer can attenuate the plasmonic properties of the nanoparticles. In some studies21,34,35, to improve the plasmonic effects of nanoparticles, triple core–shell nanostructures with a metal nanoshell sandwiched between the dielectric outer shell and core (e.g. SiO2@Ag@SiO2 and TiO2@Au@TiO2) have been proposed, which can produce better LSPR properties thanks to their plasmon hybridization mechanism.
Herein, we improve the performance of HTL-free carbon-based planar perovskite solar cells using plasmonic NPs. Firstly, the photovoltaic measurements of the fabricated HTL-free carbon-based perovskite solar cell have been utilized for simulation and extract the electrical parameters via fitting its corresponding J-V characteristics. Then, a detailed analysis of the device performance in the presence of mesoporous TiO2 and planar structure has been presented using electro-optical coupled simulation regimes. Next, the effect of realistic embedding of Ag@SiO2 and SiO2@Ag@SiO2 nanoparticles with different concentrations on the optical and electrical performance of the carbon-based planar perovskite solar cell has been investigated. Finally, since the presence of a carbon electrode (work function of ~ 5 eV) as the non-ideal anode limits the device efficiency, for increasing the transfer rate of hole carriers and blocking the electron carriers, we investigate the effects of adding a copper(I) thiocyanate (CuSCN) interlayer at the perovskite-carbon interface and increasing the working function of carbon electrode on the performance of carbon-based perovskite solar cells.
Results and discussion
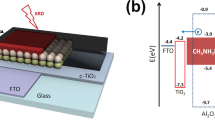
The structure of the fabricated and modeled device is Glass/FTO/Compact TiO2/Mesoporous TiO2/CH3NH3PbI3/Carbon (see Fig. 1a). Here, the mesoporous TiO2 is considered inside the perovskite photoactive layer. In other words, the pore sites of the mesoporous TiO2 are filled with perovskite material. In the simulated structures, mesoporous TiO2 is made of deformed TiO2 nanospheres with a size distribution of 7–10 nm that can overlap with each other and randomly distribute within an equivalent thickness of ~ 250 nm with a porosity of ~ 39% (Fig. 1a). This modeling is based on the results of FESEM and ellipsometry analyzes of the fabricated solar cell40. Accurate modeling of the structure of the mesoporous TiO2 is crucial because it plays a vital role in the distribution of the field profile within the perovskite layer. This directly affects the exciton distribution, generation, and recombination rates of charge carriers along with the photoactive layer. Essential details considered in this modeling have not been reported in any of the previously published works.
(a) Device configuration used for simulation and cross-section image of the experimental HTL-free carbon-based perovskite solar cell. (b) The photocurrent density–voltage (J-V) curves from experimental and simulation validation of the perovskite solar cell. J-V characteristics of the devices have been measured under one sun simulated illumination.
Figure 1b shows the measured current–voltage curves from the experimental device compared to the simulation results, wherein the simulation results are in complete agreement with the experimental data. The power conversion efficiency (PCE) of 14.93% and 14.83% for the HTL-free carbon-based perovskite solar cell has been measured and obtained from the simulation, respectively. Furthermore, all the electrical characteristics obtained from the simulation (Voc, Jsc, FF, and PCE) are in good agreement with the experimental data, which shows that the modeling performed in this work can explain the experimental optical-electrical characteristics. Therefore, it provides a good description of the device performance.
The first perovskite solar cell manufacture was based on dye-sensitized solar cells in which the TiO2 mesoporous was used as the electron transfer layer. The main reason for using the mesoporous structure was to assume the short diffusion length of the carriers in the perovskite. Researchers later found that the perovskite diffusion length is at least more than 100 nm. Accordingly, TiO2 mesoporous is removed from the device structure, and its effect on the performance of the solar cell is investigated in Fig. 2 using the developed modeling. Embedding the mesoporous TiO2 in the perovskite-compact TiO2 interface can reduce the mobility of charge carriers in this region. This is the reason for obtaining the low charge carriers mobility during the fitting of the simulation results with those of the experimental data (see Fig. 1b and Table S1). Removal of TiO2 mesoporous has increased the power conversion efficiency (PCE) from 14.83 to 16.50% due to Jsc and FF improvement (Fig. 2a). Investigation of the exciton generation rate profile along the perovskite layer shows that the improved device performance is due to the increased exciton generation rate and charge carrier mobility near compact TiO2 (Fig. 2b).
To benefit from the plasmonic properties of metallic nanoparticles in improving the performance of carbon-based planer perovskite solar cells, Ag@SiO2 core–shell nanoparticles, and SiO2@Ag@SiO2 triple core–shell nanoparticles have been embedded at the interface of CH3NH3PbI3-compact TiO2 (Fig. 3). These plasmonic nanostructures have been designed and optimized in our previous studies and introduced as effective plasmonic nanoparticles21. Here, the deformed nanospheres capable of overlapping with each other are randomly distributed in a volumetric space with varying mass ratios (the volume of distributed plasmonic nanoparticles/perovskite layer volume × 100) using a realistic simulation. The overall dimensions of the nanoparticles, the thicknesses of Ag inner shell and SiO2 outer shell are 32 nm, 2.5 nm, and 1 nm, respectively.
The device efficiency has increased to 19.72% and 18.20% after adding a 0.93% mass ratio of Ag@SiO2 and SiO2@Ag@SiO2 NPs, respectively, due to the Jsc increment shown in Fig. 3. Further increment of the mass ratio of embedded Ag@SiO2 nanoparticles decreases the efficiency stemming from the Jsc drop (Table 1). This could be due to the enhancement of the reflectivity behavior of nanoparticles, which prevents light from penetrating deep into the perovskite layer. This, in turn, can weaken the performance of the device by reducing the optical absorption. However, the increase in the mass ratio of SiO2@Ag@SiO2 nanoparticles has increased the device efficiency, and the maximum power conversion efficiency of 18.92% is obtained for the devices incorporated with the mass ratios of 2.79% (Fig. 3b and Table 1). Removal of mesoporous TiO2 and adding 0.93% mass ratio of Ag@SiO2 NPs between perovskite and compact-TiO2 have increased the device efficiency by 33%. The presence of mesoporous TiO2 and the addition of NPs within the mesoporous structure could only increase the device efficiency by 17% (Fig. 3c and Table 2). This suggests that the presence of TiO2 mesoporous as the surrounding environment of doped plasmonic nanoparticles can weaken their plasmonic enhancement.
In Figs. 4 and 5, the LSPR properties of SiO2@Ag@SiO2 and Ag@SiO2 nanoparticles embedded in the perovskite environment are investigated, respectively, as single-particle and mass during plasmon resonance. Compared to the single nanoparticle, mass nanoparticles show a larger absorption cross-section due to the production of new plasmonic peaks and their increase in spectral width due to plasmonic interactions of neighboring nanoparticles (Fig. 4a and c). This is a reason for further improvement in the efficiency of the device incorporated with SiO2@Ag@SiO2 nanoparticles by increasing the mass ratio of embedded nanoparticles (Fig. 3b). Plasmon resonance of SiO2@Ag@SiO2 nanoparticles occurs due to the plasmon hybridization mechanism at higher wavelengths than Ag@SiO2 nanoparticles (Figs. 4 and 5), where the photon flux produced by the nanoparticle is unable to transfer electrons from the valance band to the conduction band of perovskite. This results in less plasmonic improvement of the device in the presence of SiO2@Ag@SiO2 nanoparticles compared to Ag@SiO2 nanoparticles. In addition, Ag@SiO2 nanoparticles generate stronger plasmonic fields in the perovskite environment compared to SiO2@Ag@SiO2 nanoparticles. Figure S2 shows the absorption spectrum of perovskite in the presence of a 0.93% mass ratio of Ag@SiO2 and SiO2@Ag@SiO2 nanoparticles compared to the reference device (without nanoparticles). As expected, perovskite absorption in the presence of Ag@SiO2 nanoparticles shows further improvement.
The presence of a carbon electrode (work function of ~ 5 eV) as the non-ideal anode and the absence of an electron blocking layer at the perovskite-carbon interface limits the device efficiency by enhancing the probability of electron recombination at the interface. Adding an appropriate interlayer at the perovskite-carbon interface and/or adequate increment of the working function of carbon by using suitable additives/binders in carbon ink to increase the transfer rate of hole carriers while blocking electron carriers are other approaches to improve the performance of carbon-based perovskite solar cells. In metal electrode-based perovskite solar cells, spiro-OMeTAD has been widely used as a representative of hole transport material41,42. However, there are significant limitations to the use of this material (and generally organic and conductive polymer-based molecules) in terms of cost and stability43,44. In addition, previous studies have shown that spiro-OMeTAD-equipped carbon electrode-based perovskite solar cells offer lower efficiencies than a metal electrode-based device45. This is due to the incompatibility of some organic solvents in carbon paste with spiro-OMeTAD, which leads to its degradation46. Therefore, the effect of the inorganic CuSCN layer with excellent semiconducting properties such as high hole mobility, wide bandgap, optical transparency, thermal and chemical stability, and solution processability44,47 on the performance of the device has been investigated. It should be noted that CuSCN can be deposited at low temperatures using various techniques including spin and spray coatings, doctor Blade, and electro-deposition44,48,49,50.
Figure 6a shows the J–V characteristic calculated under AM 1.5G irradiation for the device comprised of FTO/TiO2/Ag@SiO2 NPs: CH3NH3PbI3/CuSCN/Carbon. The optimized plasmonic cell in the absence of the hole transport layer of CuSCN delivers Jsc of 28.05 mA/cm2, Voc of 0.918 V, FF of 76.57%, and PCE of 19.72%. On the other hand, the used solution-processed inorganic p-type CuSCN layer with a thickness of 30 nm at the perovskite-carbon interface has increased the device PCE by ~ 35% to 26.58% thanks to the increased Voc and FF to 1.156 V and 81.64%, respectively (Table 3). The remarkable improvement of the Voc is attributed to better interfacial energy alignment between the perovskite and CuSCN (see Figure S3). Figure 6b shows the recombination current density for the device equipped with a CuSCN layer compared to the reference device. Adding the CuSCN layer not only has reduced the SRH recombination by producing a back surface field and blocking electrons toward the TiO2 ETL but also has reduced the current density of the back surface recombination down to ~ 10−22 mA/cm2, even at high voltages, by appropriately aligning the interfacial energy.
Furthermore, Figs. 6c and d show the performance of the optimized plasmonic device as a function of the back contact work function. As the working function of the carbon electrode increases, which is possible by using suitable additives/binders in the carbon ink, Voc and FF have increased thanks to the excellent alignment of the interfacial energy and the reduction of the recombination current density. As a result, a PCE above 26% is obtained for the work function of 5.4 eV (Table 3).
Conclusions
In summary, we have investigated the effects of the removal of mesoporous TiO2, plasmonic nanoparticle embedding, and utilization of solution-processed inorganic p-type CuSCN as an HTL on the performance of carbon-based PSCs in an electro-optical study. We have shown that removing mesoporous TiO2 can increase the device PCE by increasing the exciton generation rate and charge carriers' mobility in the vicinity of the CH3NH3PbI3-compact TiO2 interface. Furthermore, we have shown that the embedding of Ag@SiO2 and SiO2@Ag@SiO2 plasmonic nanoparticles can increase the device PCE from 16.50% to 19.72% and 18.92% (in the optimum mass ratio), respectively, thanks to benefiting from the light scattering, trapping, and strong plasmonic fields produced by the nanoparticles. On the other hand, non-ideal carbon electrode has limited the device efficiency by increasing the interface recombination. Adding a solution-processed inorganic p-type CuSCN layer at the perovskite-carbon interface increased the device PCE to 26.58% by significantly improving the Voc and FF thanks to the proper alignment of the interfacial energy and the reduction of the recombination current density. The same results were obtained by increasing the carbon working function (through incorporating suitable additives/binders to the carbon ink), and the cell PCE was enhanced up to 26% in the optimal case. These results pave the ways to achieve high efficiencies in carbon-based PSCs.
Materials and methods
Perovskite device fabrication
Firstly, a cleaned FTO glass substrate was treated in TiCl4 (40 mM) at 70 °C for 30 min and washed with deionized water. Next, TiO2 mesoporous scaffold has been prepared from the mixed TiO2 precursor solution including Titanium(IV) ethoxide (Sigma-Aldrich, 12.7 g), concentrated HCl (Merck, 9.7 g), Pluronic P123 (Sigma-Aldrich, 4.0 g), and butyl alcohol (Sigma-Aldrich, 36.3 g). The solution has been dip-coated on the substrate (withdrawing speed; 30 mm/min) and has been subsequently annealed at 450 °C for 1 h. Then, the prepared PbI2 solution (1.4 M) via solving PbI2 in DMSO/DMF (1:9) has been spin-coated on TiO2 mesoporous coated substrate at 2000 rpm for 20 s (during the process, the temperature is kept at 80 °C). To convert the PbI2 film to CH3NH3PbI3, CH3NH3I solution (1 mg mL−1) was prepared in a mixed solvent of isopropanol/cyclohexane (1:9), and then, PbI2 films were immersed into the CH3NH3I solution for 12 h, and the as-prepared methylammonium lead triiodide (MAPbI3) films have been heated at 100 °C for 15 min36,37. Finally, a commercial carbon paste composed of carbon black and graphite has been used for preparing the carbon back electrode. The carbon paste has been painted on the perovskite film followed by spraying an ethanol solution of 1H, 1H,2H,2H-perfluouorodecyltriethoxysilane (Sigma-Aldrich, 5% v/v) on the paste and heating at 100 °C for 1 h7.
Numerical calculations
Optical-electrically coupled simulation regimes have been used to investigate the plasmonic nanoparticles embedding effects on the performance of carbon-based perovskite solar cells. The rate of exciton generation dependent on the absorption profile of the cell was calculated using FDTD software based on solving Maxwell equations38. The complex refractive indices obtained from the ellipsometric analyzes of the fabricated device were utilized to simulate and calculate the exciton generation rate profile optically. Perfectly matched layer (PML) boundary conditions are set at the top and bottom of the unit cell along the Z direction. Periodic boundary conditions are located along x and y directions. A plane wave light source propagated along the Z direction is used; the photon flux density is calculated based on standard solar spectrum AM1.5. Experimental data of the materials' complex refractive indices used for optical simulation are shown in Figure S1. To model the electrical response of the cell, the exciton generation rate profile is directly imported into the Solar Cell Capacitance Simulator (SCAPS-1D) software. Photovoltaic measurements of the fabricated devices were utilized to extract electrical parameters by fitting their corresponding J–V characteristics. The software uses the finite difference approach to solve electron and hole drift–diffusion equations to describe the motion of charge carriers inside the photoactive layer. Here Shockley–Read–Hall (SRH) model has been used to describe recombination current density39. The electrical parameters used for the simulation, which are extracted by fitting the simulation results with experimental data, are shown in Table S1.
References
Kojima, A., Teshima, K., Shirai, Y. & Miyasaka, T. Organometal halide perovskites as visible-light sensitizers for photovoltaic cells. J. Am. Chem. Soc. 131, 6050–6051 (2009).
Jeong, M. et al. Stable perovskite solar cells with efficiency exceeding 24.8% and 0.3-V voltage loss. Science (80-) 369, 1615–1620 (2020).
Domanski, K. et al. Not all that glitters is gold: Metal-migration-induced degradation in perovskite solar cells. ACS Nano 10, 6306–6314 (2016).
Liang, L., Cai, Y., Li, X., Nazeeruddin, M. K. & Gao, P. All that glitters is not gold: Recent progress of alternative counter electrodes for perovskite solar cells. Nano Energy 52, 211–238 (2018).
Mei, A. et al. A hole-conductor–free, fully printable mesoscopic perovskite solar cell with high stability. Science (80-) 345, 295–298 (2014).
Chen, J. et al. Solvent effect on the hole-conductor-free fully printable perovskite solar cells. Nano Energy 27, 130–137 (2016).
Keshavarzi, R., Molabahrami, N., Afzali, N. & Omrani, M. Improving efficiency and stability of carbon-based perovskite solar cells by a multifunctional triple-layer system: Anti-reflective, UV-protective, superhydrophobic and self-cleaning. Sol. RRL 4, 202000491. https://doi.org/10.1002/solr.202000491 (2020).
Vijayaraghavan, S. N. et al. Low-temperature processed highly efficient hole transport layer free carbon-based planar perovskite solar cells with SnO2 quantum dot electron transport layer. Mater. Today Phys. 13, 100204 (2020).
Zhang, X. et al. Efficient and carbon-based hole transport layer-free CsPbI 2 Br planar perovskite solar cells using PMMA modification. J. Mater. Chem. C 7, 3852–3861 (2019).
Chen, R. et al. Carbon-based HTL-free modular perovskite solar cells with improved contact at perovskite/carbon interfaces. J. Mater. Chem. C 8, 9262–9270 (2020).
Liang, L., Cai, Y. & Gao, P. A facile gas-driven ink spray (GDIS) deposition strategy toward hole-conductor-free carbon-based perovskite solar cells. Emergent Mater. https://doi.org/10.1007/s42247-021-00247-w (2021).
Cai, Y., Liang, L. & Gao, P. Promise of commercialization: Carbon materials for low-cost perovskite solar cells. Chin. Phys. B 27, 018805 (2018).
Lee, K. et al. A highly stable and efficient carbon electrode-based perovskite solar cell achieved via interfacial growth of 2D PEA 2 PbI 4 perovskite. J. Mater. Chem. A 6, 24560–24568 (2018).
Yang, Y. et al. Ultrasound-spray deposition of multi-walled carbon nanotubes on NiO nanoparticles-embedded perovskite layers for high-performance carbon-based perovskite solar cells. Nano Energy 42, 322–333 (2017).
Abdi-Jalebi, M. et al. Maximizing and stabilizing luminescence from halide perovskites with potassium passivation. Nature 555, 497–501 (2018).
Abdi-Jalebi, M. et al. Impact of a mesoporous titania–perovskite interface on the performance of hybrid organic–inorganic perovskite solar cells. J. Phys. Chem. Lett. 7, 3264–3269 (2016).
Lu, H. et al. Efficient perovskite solar cells based on novel three-dimensional TiO2 network architectures. Sci. Bull. 61, 778–786 (2016).
Calado, P. et al. Evidence for ion migration in hybrid perovskite solar cells with minimal hysteresis. Nat. Commun. 7, 1–10 (2016).
Choi, J., Song, S., Hörantner, M. T., Snaith, H. J. & Park, T. Well-defined nanostructured, single-crystalline TiO2 electron transport layer for efficient planar perovskite solar cells. ACS Nano 10, 6029–6036 (2016).
Omrani, M. K. & Fallah, H. Improving light trapping of polymer solar cell via doping a new array of triple core-shell spherical nanoparticles utilizing realistic modeling. Sol. Energy 163, 600–609 (2018).
Omrani, M., Fallah, H. & Hajimahmoodzadeh, M. High efficiency metallic nanoshells for improving polymer solar cells performance: An opto-electrical study. Sol. Energy 207, 409–418 (2020).
Cui, X. et al. Tailoring carrier dynamics in perovskite solar cells via precise dimension and architecture control and interfacial positioning of plasmonic nanoparticles. Energy Environ. Sci. 13, 1743–1752 (2020).
Li, Y.-F., Kou, Z.-L., Feng, J. & Sun, H.-B. Plasmon-enhanced organic and perovskite solar cells with metal nanoparticles. Nanophotonics 9, 3111–3133 (2020).
Gao, Y. et al. Plasmon-enhanced perovskite solar cells with efficiency beyond 21%: The asynchronous synergistic effect of water and gold nanorods. ChemPlusChem 86, 291–297 (2021).
Zhao, D.-W. et al. Enhanced efficiency and stability of planar perovskite solar cells using a dual electron transport layer of gold nanoparticles embedded in anatase TiO2 films. ACS Appl. Energy Mater. 3, 9568–9575 (2020).
Juan, F. et al. Plasmonic Au nanooctahedrons enhance light harvesting and photocarrier extraction in perovskite solar cell. ACS Appl. Energy Mater. 4, 3201–3209 (2021).
Omrani, M., Mohammadi, H. & Fallah, H. Ultrahigh sensitive refractive index nanosensors based on nanoshells, nanocages and nanoframes: Effects of plasmon hybridization and restoring force. Sci. Rep. 11, 2065 (2021).
Sovizi, M. & Omrani, M. K. Design and simulation of high sensitive cylindrical nanogear shell sensors according to localized surface plasmon resonance. Optik (Stuttg). 144, 300–307 (2017).
Zhang, W. et al. Enhancement of perovskite-based solar cells employing core–shell metal nanoparticles. Nano Lett. 13, 4505–4510 (2013).
Atwater, H. A. & Polman, A. Plasmonics for improved photovoltaic devices. Nat. Mater. 9, 205–213 (2010).
Siavash Moakhar, R. et al. Recent advances in plasmonic perovskite solar cells. Adv. Sci. 7, 1902448 (2020).
He, Z. et al. Influence of Ag@ SiO2 with Different Shell Thickness on Photoelectric Properties of Hole-Conductor-Free Perovskite Solar Cells. Nanomaterials 10, 2364 (2020).
Lee, D. S. et al. Self-position of Au NPs in perovskite solar cells: Optical and electrical contribution. ACS Appl. Mater. Interfaces 8, 449–454 (2016).
Dang, X. et al. Tunable localized surface plasmon-enabled broadband light-harvesting enhancement for high-efficiency panchromatic dye-sensitized solar cells. Nano Lett. 13, 637–642 (2013).
Omrani, M., Fallah, H., Choy, K.-L. & Abdi-Jalebi, M. Impact of hybrid plasmonic nanoparticles on the charge carrier mobility of P3HT:PCBM polymer solar cells. Sci. Rep. 11, 19774 (2021).
Chen, H. et al. Solvent engineering boosts the efficiency of paintable carbon-based perovskite solar cells to beyond 14%. Adv. Energy Mater. 6, 1502087 (2016).
Chang, X. et al. Colloidal precursor-induced growth of ultra-even CH3NH3PbI3 for high-performance paintable carbon-based perovskite solar cells. ACS Appl. Mater. Interfaces 8, 30184–30192 (2016).
OptiFDTD. http://www.optiwave.com/. OptiFDTD Software.
Burgelman, M., Nollet, P. & Degrave, S. Modelling polycrystalline semiconductor solar cells. Thin Solid Films 361, 527–532 (2000).
Amini, M. et al. From dense blocking layers to different templated films in dye sensitized and perovskite solar cells: Toward light transmittance management and efficiency enhancement. J. Mater. Chem. A 6, 2632–2642 (2018).
Yao, D. et al. Dimensionality-controlled surface passivation for enhancing performance and stability of perovskite solar cells via triethylenetetramine vapor. ACS Appl. Mater. Interfaces 12, 6651–6661 (2020).
Yang, Y. et al. Ammonium-iodide-salt additives induced photovoltaic performance enhancement in one-step solution process for perovskite solar cells. J. Alloys Compd. 684, 84–90 (2016).
Chen, W.-Y. et al. Low-cost solution-processed copper iodide as an alternative to PEDOT: PSS hole transport layer for efficient and stable inverted planar heterojunction perovskite solar cells. J. Mater. Chem. A 3, 19353–19359 (2015).
Qin, P. et al. Inorganic hole conductor-based lead halide perovskite solar cells with 12.4% conversion efficiency. Nat. Commun. 5, 1–6 (2014).
Yang, Y. et al. Spiro-OMeTAD or CuSCN as a preferable hole transport material for carbon-based planar perovskite solar cells. J. Mater. Chem. A 8, 12723–12734 (2020).
Wei, H. et al. Free-standing flexible carbon electrode for highly efficient hole-conductor-free perovskite solar cells. Carbon N. Y. 93, 861–868 (2015).
Wijeyasinghe, N. et al. Copper (I) thiocyanate (CuSCN) hole-transport layers processed from aqueous precursor solutions and their application in thin-film transistors and highly efficient organic and organometal halide perovskite solar cells. Adv. Funct. Mater. 27, 1701818 (2017).
Murugadoss, G., Thangamuthu, R. & Kumar, S. M. S. Fabrication of CH3NH3PbI3 perovskite-based solar cells: Developing various new solvents for CuSCN hole transport material. Sol. Energy Mater. Sol. Cells 164, 56–62 (2017).
Yang, I. S. et al. Formation of pristine CuSCN layer by spray deposition method for efficient perovskite solar cell with extended stability. Nano Energy 32, 414–421 (2017).
Zhao, K. et al. Solution-processed inorganic copper (I) thiocyanate (CuSCN) hole transporting layers for efficient p–i–n perovskite solar cells. J. Mater. Chem. A 3, 20554–20559 (2015).
Acknowledgements
R.K. acknowledges Iran National Science foundation (INSF) for the financial support under project No. 4000748. Activity of P.G. is funded by CAS-VPST Silk Road Science Fund 2022. M.A.‐J. acknowledges the Royal Society (RGS\R1\211068), European Commission under the Horizon 2020 (ERA-NET ACT 2021, NEXTCCUS project), Cambridge Materials Limited, and Wolfson College, the University of Cambridge for their funding and technical support.
Author information
Authors and Affiliations
Contributions
M.K.O. Writing-Original Draft, Data analysis, Formal analysis, Validation, Methodology. R.K. Writing-Review & Editing, Validation, Data analysis. M.A.J. Writing-Review & Editing, Validation, Supervision. P.G. Writing-Review & Editing, Validation.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Omrani, M., Keshavarzi, R., Abdi-Jalebi, M. et al. Impacts of plasmonic nanoparticles incorporation and interface energy alignment for highly efficient carbon-based perovskite solar cells. Sci Rep 12, 5367 (2022). https://doi.org/10.1038/s41598-022-09284-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-022-09284-9
This article is cited by
-
Plasmonic Au nanoparticles enhanced photovoltaic characteristics of perovskite BiFeO3 nanostructures
Journal of Materials Science: Materials in Electronics (2024)
-
A novel natural scaffold layer improving efficiency, stability and reproducibility of Perovskite solar cells
Scientific Reports (2023)
-
Optimization of Hollow Materials-Based Electron Transport Layer for Enhanced Performance of Perovskite Solar Cell
Plasmonics (2023)
-
An optimized hybrid nanostructure for tunable radiation and filter characteristics in optical circuits
Journal of Optics (2023)
-
Molecular engineering of several butterfly-shaped hole transport materials containing dibenzo[b,d]thiophene core for perovskite photovoltaics
Scientific Reports (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.