Abstract
Reduced salinity is a major factor that causes macrobenthic degradation in reclaimed wetlands. We investigated populations of the sesarmid crab Chiromantes dehaani in reclaimed and natural wetlands. Then, in the laboratory, we exposed male and female crabs to four salinity levels (0, 6, 12 and 18) for 96 h to analyse the effects of reduced salinity on osmoregulatory enzyme activities in the posterior gills and digestive and immune enzyme activities in the hepatopancreas of C. dehaani. The results revealed a significant positive correlation between the number of crabs and salinity. In the laboratory, we found that the isosmotic point of C. dehaani was close to 16 ppt. The crabs showed strong hyper-osmotic regulation when exposed to 0–6 ppt salinities. Moreover, in this salinity range, amylase activities were significantly inhibited. Under low-salinity stress, the immune enzyme activities were significantly activated. However, phenoloxidase and lysozyme activities were inhibited in the freshwater environment. The male and female crabs showed no significant differences in most of the enzyme activities. Thus, reduced salinity can adversely affect the digestive and immune functions of C. dehaani, which may cause population degradation in reclaimed wetlands. Our findings can provide new insights into the effects of reclamation on macrobenthos.
Similar content being viewed by others
Introduction
Shanghai is one of the most prosperous cities in China; however, the development of this city has been restricted by land scarcity. Wetland reclamation in the Yangtze Estuary has been considered as one of the main solutions to land scarcity. From the 1960s to 2015, the area of reclaimed land in the Yangtze Estuary has increased by 543.9 km21. A major risk associated with reclamation is the loss of macrobenthic diversity. Previous studies have shown that reclamation projects can cause water salinity changes in intertidal wetlands2. Thus, many scholars have speculated that the reduced salinity caused by reclamation may be one of the main reasons for the degradation of macrobenthic communities3,4.
The sesarmid crab Chiromantes dehaani is a dominant crab in the mudflats of the Yangtze Estuary5. This crab plays ecologically important roles as a secondary consumer in intertidal ecosystems6. C. dehaani prefers to live in brackish water to meet its salinity requirements for reproduction and larval development7. However, like other macrobenthos, C. dehaani populations have been seriously disturbed by reclamation8. Currently, there is limited information on why C. dehaani cannot survive in low-salinity reclaimed areas because few studies have investigated the crab physiological traits altered by salinity changes in reclaimed wetlands.
Usually, estuarine species can regulate osmotic pressure, which can contribute to maintaining the osmotic concentration of their body fluids during salinity challenges9,10,11. Many studies have shown that euryhaline crabs mainly rely on the posterior gills to transport ions and maintain haemolymph osmotic pressure and membrane potential balance12,13,14,15. In the gill epithelium, Na+ and Cl− enter ionocytes via Na+/H+ and Cl−/HCO3− exchangers, respectively, at the apical membrane of ionocytes. In this process, the activity of Na+/K+-ATPase plays an important role for NaCl absorption via the gills16. Thus, the occurrence of osmoregulationis based on efficient ionic regulation (mainly of Na+ and CI−) and increased levels of Na+/K+-ATPase activity17.
In euryhaline aquatic animals, osmoregulation during salinity acclimation may cause changes in digestive enzyme activities18. A possible explanation is that aquatic animals undergo metabolic reorganization to meet the increasing energy requirements due to salinity changes19. Digestive enzymes play an important role in the metabolic regulation mechanism of organisms. Because many crustaceans are omnivorous, the digestive enzymes associated with carbohydrates (e.g. amylase and cellulase), lipids (e.g. lipase) and proteins (e.g. pepsin and trypsin) have been systematically studied19,20. Previous studies have demonstrated that salinity acclimation can promote digestive enzyme activities in euryhaline decapods. Moreover, the effect of salinity change on digestive enzyme activity is usually related to the intensity and mode of acclimation21.
Salinity acclimation may also affect the immune systems of aquatic animals. Previous studies have demonstrated that the activity of immune enzymes such as phenoloxidase, alkaline phosphatase and lysozyme in crustaceans is modified in response to salinity stress. The nonspecific immune system of crustaceans includes cellular and humoral immunity. Phenoloxidase is associated with the phenoloxidase activating system, which plays an important role in humoral immunity. When this system is activated, the released phenoloxidase can inhibit and kill pathogens to achieve the effect of immunity22. Phosphatase is an important part of the lysosomal enzyme in macrophages, which can cleave and eliminate foreign bodies by forming the hydrolase system23. Lysozyme in bactericidal phagocytes breaks down the cell wall of gram-positive bacteria by hydrolysis of the β-1,4-glycosidic bond24.
In this study, we investigated the number of C. dehaani in different habitats in reclaimed wetlands in the Yangtze Estuary. We simulated the changes in water salinity in reclaimed wetlands and measured the activities of osmoregulatory, digestive and immune enzymes. The primary objective of this study was to detect the effects of reduced salinity caused by reclamation on the population and physiological characteristics of C. dehaani.
Results
Water salinity and population characteristics
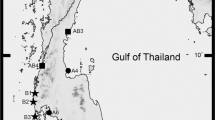
The changes in water salinity and numbers of female and male crabs are shown in Fig. 1A,B. The water salinities were significantly higher at VT (3.5–5.0 ppt) and BT (3.0–5.1 ppt) sites than at VN (0.1–0.9 ppt) and BN (0.2–1.7 ppt) sites (P < 0.05). However, the water salinities at VT and BT sites were significantly lower than those at VC (1.7–12.8 ppt) and BC (1.5–12.9 ppt) sites (P < 0.05).
Water salinity (A) and number of C. dehaani crabs (B) in six sampling areas. Values are mean ± SE; N = 4. The correlations between water salinity and numbers of male (C) and female (D) crabs. The abbreviations represent sampling sites: VT and BT, vegetated and bald sites in tidal-affected region; VN and BN, vegetated and bald sites in non–tidal-affected region; VC and BC, vegetated and bald sites BC in natural tidal flat. * indicates that the number of crabs was 0.
The numbers of male and female crabs were significantly higher at the BT site (8.7 ± 0.3, 10.3 ± 3.4 individuals) than other sites in the reclaimed zone (P < 0.05). In contrast, the BT site had significantly lower numbers of male and female crabs than VC (35.9 ± 11.3, 27.1 ± 6.4 individuals) and BC (24.5 ± 5.9, 20.7 ± 6.8 individuals) sites (P < 0.05). No significant differences were observed in the numbers of male and female crabs at all sites (P > 0.05), and no crabs were found at the VN and BN sites.
The correlation between water salinity and numbers of female (regression analysis, y = 3.1619x − 0.6417, r = 0.669, P < 0.001, Fig. 1C) and male (regression analysis, y = 2.1535x + 1.5028, r = 0.601, P = 0.002, Fig. 1D) crabs was significant.
Haemolymph osmolality and Na+/K+ ATPase activity in the posterior gills
No mortality was observed in all treatments during the experiment period. There were significant differences in haemolymph osmolality and Na+/K+ ATPase activities in the posterior gills between the female and male crabs (P > 0.05; Fig. 2A). After 96 h of exposure, haemolymph osmolality of the crabs was significantly higher in the groups exposed to 12 and 18 ppt salinity than in those exposed to 0 and 6 ppt salinity (P < 0.05). The crabs were hyper-osmoregulators at salinities ranging from 0 to 12 ppt, but the crabs acted as hypo-osmoregulators at 18 ppt. The isosmotic point was calculated at ∼16 ppt salinity.
Haemolymph osmolality (A) and gill Na+/K+ ATPase activities (B) of C. dehaani crabs after exposure to salinity changes for 96 h. Values are mean ± SE, N = 4. Different letters above the data indicate significant differences between the salinity treatments, as assessed using Tukey’s tests (P < 0.05).
The activities of Na+/K+ ATPase in the posterior gills of the female and male crabs were significantly higher at 0–6 ppt salinities than at 12–18 ppt salinities (P < 0.05; Fig. 2B). However, no significant difference in Na+/K+ ATPase activity was detected when the crabs were exposed to 0–6 ppt and 12–18 ppt salinities (P < 0.05).
Digestive enzyme activities in the hepatopancreas
Figure 3 shows digestive enzyme activities in the hepatopancreas of C. dehaani after the salinity treatments for 96 h. Trypsin activity in both female and male crabs showed a significant decrease as the salinity level increased (P < 0.05; Fig. 3A). Moreover, trypsin activity of the female crabs was significantly higher than that of the males at 18 ppt salinity (P < 0.05). Amylase activities of the female crabs were significantly higher at 12–18 ppt than at 0–6 ppt salinities (P < 0.05; Fig. 3B). However, no significant differences were detected in the amylase activities of male crabs in the four salinity treatment groups (P > 0.05). The female crabs exposed to 12–18 ppt salinities showed significantly higher amylase activities than the males (P < 0.05). Lipase activities of the crabs were not significantly different with respect to sex and salinity treatments (P > 0.05; Fig. 3C).
Effects of salinity exposure on the activities of trypsin (A), amylase (B) and lipase (C) in the hepatopancreas of male and female C. dehaani crabs. Values are mean ± SE, N = 4. Different letters above the data indicate significant differences between the salinity treatments, as assessed using Tukey’s tests (P < 0.05). * indicates significant differences between female and male crabs, as assessed using Tukey’s tests (P < 0.05).
Immune enzyme activities in the hepatopancreas
Immune enzyme activities in the hepatopancreas of C. dehaani after 96 h of exposure are shown in Fig. 4. Alkaline phosphatase activities in the female and male crabs were significantly higher at 0–6 ppt salinities than at 12–18 ppt salinities (P < 0.05; Fig. 4A). Moreover, the female crabs showed significantly higher alkaline phosphatase activity than the male crabs at 0 ppt salinity (P < 0.05). Phenoloxidase activities in the male and female crabs decreased significantly from 6 to 12 ppt salinities after a significant increase at 0–6 ppt salinities (P < 0.05; Fig. 4B). The female crabs exposed to 6 ppt salinity showed significant higher phenoloxidase activity than the male crabs (P < 0.05). Lysozyme activities in the female and male crabs were significantly higher at 18 ppt salinity than at 0 ppt salinity, but significantly lower at 6–12 ppt salinities (P < 0.05; Fig. 4C). No significant differences in lysozyme activities were observed between the male and female crabs (P > 0.05).
Effects of salinity exposure on the activities of alkaline phosphatase (A), phenoloxidase (B) and lysozyme (C) in the hepatopancreas of male and female C. dehaani crabs. Values are mean ± SE, N = 4. Different letters above the data indicate significant differences between the salinity treatments, as assessed using Tukey’s tests (P < 0.05). * indicates significant differences between female and male crabs, as assessed using Tukey’s tests (P < 0.05).
Discussion
In this study, C. dehaani crabs could not be collected from the non-tidal-affected zone, where the water salinity was extremely low (0.1–1.7 ppt). In contrast, in the nearby tidal-affected area, C. dehaani crabs were found at 3–5.1 ppt water salinity. The correlation analysis showed a significant positive correlation between the number of female and male crabs and water salinity. Therefore, water salinity is the key factor that affects the sustainable development of C. dehaani population. However, the number of crabs in the tidal-affected area was significantly lower than that in the natural tidal flat. This may be because of the adverse effects of reclamation on tidal salinity, which may cause long-term osmotic stress in C. dehaani.
The simulated experiments showed that the haemolymph osmolality of C. dehaani increased significantly as the salinity increased. Moreover, the osmoregulatory pattern of the crabs changed from hyper-osmoregulation to hypo-osmoregulation at salinities ranging from 12 to 18 ppt. These results indicate that C. dehaani has a strong ability to regulate osmolality during short periods of salinity exposure (96 h). In addition, the isosmotic point of C. dehaani was close to 16 ppt. This salinity level is higher than that observed in the natural tidal flat (1.5–13.0 ppt). Crustaceans may increase the isosmotic point of the haemolymph during gonadal development, which may contribute to the absorption of ions from the surrounding environment25. In this study, the crabs were collected in October, when they were in the gonadal development stage.
The activities of Na+/K+-ATPase are crucial for osmoregulation in decapod crustaceans. In this study, Na+/K+-ATPase activities in the posterior gills of C. dehaani were significantly activated by 0–6 ppt salinity. This suggests that C. dehaani crabs can actively reabsorb Na+ and Cl- to replace the lost NaCl and maintain osmotic balance when entering a low-salinity or freshwater environment. In addition, in this study, no significant differences in Na+/K+-ATPase activity were found between the posterior gills of the male and female crabs, which is similar to the results of the freshwater crab Eriocheir sinensis26. Similar to our results, another study showed that female and male of the euryhaline crab Neohelice (Chasmagnathus) granulata have strong hypo-osmoregulatory abilities27. However, the differences in the osmoregulatory ability of euryhaline crabs due to sex may be affected by season and development stage27,28. In the Yangtze Estuary, the biomass of C. dehaani was usually the highest in autumn. During this season, fewer environmental conditions were not conducive to the survival of this crab. Thus, both female and male crabs may have enough energy to regulate osmotic pressure.
This study revealed that trypsin activities in the hepatopancreas of both male and female crabs were activated in the freshwater environment. This may be a physiological adjustment of C. dehaani to cope with low salt conditions. In crustaceans, proteins can be hydrolysed by proteases to form free amino acids, which may also contribute to the regulation of osmotic balance28. Trypsin is a proteolytic enzyme that cleaves peptide bonds in the carboxylic groups of arginine and lysine29. Previous studies have confirmed that arginine and lysine contents increased in aquatic animals under hyper-osmotic or hypo-osmotic stress30,31. However, there is a lack of definitive evidence to demonstrate the general correlation between trypsin and osmoregulation, so further studies are required to analyse whether the increasing trypsin activity could reflect a higher amino acid metabolism in attempt of the crab to obtain osmotic effectors under low salinity conditions.
The digestive enzyme activity was generally consistent with the feeding habitats32. In this study, we found relatively high amylase activities in the hepatopancreas of C. dehaani after 96 h of exposure. This result may seem contradictory, as mussel meat was the only food in the experiment. However, C. dehaani is a typical herbivorous macrobenthos33. In the tidal flat of the Yangtze Estuary, this crab mainly lives in the growing areas of Phragmites australis and Spartina alterniflora and feeds on the leaves of these plants. Thus, the high activity of amylase enzyme could be rather attributed to the previous secretion in the tidal flats where they fed mainly on hydrophytes. However, we also found extremely low lipase activity, which may be due to the low fat content of the diets or the substrate used in the experiment19. In addition, the digestive enzyme activities of the female crabs were generally higher than those of the male crabs, which is similar to the results of Ye34. The crabs were collected in October, which is a typical mating season35. This shows the possibility of deriving extra food energy to compensate for the energy loss of reproduction.
This study also revealed that the immune enzymes (i.e. alkaline phosphatase, phenoloxidase and lysozyme) in the male and female crabs were activated at 6 ppt salinity, which suggests that reduced salinity may trigger a temporary immune response in C. dehaani. Similar results have been found in Penaeus japonicus36, Penaeus chinensis37 and Penaeus vannamei37. In contrast, phenoloxidase and lysozyme activities were inhibited in the crabs exposed to 0 ppt salinity. Decapods usually exhibit an immune stress response from they move from an isotonic to non-isotonic environment, and it mainly manifests as an emergency response, metabolic adaptation or changes to the body’s immune capacity38,39,40. According to the intensity and time of exogenous stimulus and immune regulation characteristics of the animal body, stress response can be manifested as stress adaptation and stress injury. In this study, phenoloxidase and lysozyme activities of C. dehaani were inhibited in the freshwater environment, which indicates that the crabs may have been maladaptive and their immune resistance will continue to decline in the non–tidal-affected area of the reclaimed wetland.
Alkaline phosphatase, phenoloxidase and lysozyme activities in the hepatopancreas were higher in the female crabs than in the male crabs. Differences in the immune defence ability of decapods due to sex may be related to gonadal development and reproductive behaviour41. Female crabs may need to maintain a higher immune defence than male crabs to cope with the threat of constant changes in environmental salinity during reproduction. Gonadal development of the female crabs was relatively delayed when compared with that of the male crabs. The hepatopancreas is the main energy storage tissue of C. dehaani, and energy transfer during gonadal development may influence the activities of immune-related enzymes in the different sexes of C. dehaani.
On the basis of these results, C. dehaani cannot be well adapted to the reduced salinity in a reclaimed area, which may cause a decline in its population. In fact, a vast majority of the native macrobenthos will gradually disappear after the reclamation of tidal flats8. According to previous studies, the number of macrobenthos has decreased from 63 species in 1998–2000 to 40 species in 2011–2013 in the east shoal of Chongming Island42. The south bank of the Yangtze Estuary, another reclaimed wetland, lost 33 species of macrobenthos during the same period42. The Yangtze Estuary offers an overwintering habitat for thousands of migratory birds between East Asia and Australia43. The loss of macrobenthos, which are major secondary producers, is bound to have a negative impact on overwintering birds. Moreover, the reclamation may cause the loss of spawning and reproduction grounds for many commercial and protected species, such as E. sinensis44 and Acipenser sinensis45. The decrease in macrobenthic diversity could reduce the efficiency of material cycling and energy flow in the reclaimed wetlands. From the perspective of ecological security, the intensity of wetland reclamation in the Yangtze Estuary should be reduced appropriately. Natural tides can be introduced to regulate water salinity in reclaimed areas and promote the self-restoration of macrobenthic diversity.
Conclusions
In this comprehensive study, we used both field and laboratory data to evaluate the effects of reduced salinity caused by reclamation on the population and physiological characteristics of C. dehaani. The results of the field investigation revealed that the C. dehaani population decline in reclaimed areas is closely related to decreased salinity. In the laboratory, the isosmotic points of female and male C. dehaani were detected at ∼16 ppt salinity. The crabs acted as hyper-osmoregulators in low-salinity and freshwater environments (0–6 ppt). Their ability to digest carbohydrates was impaired, but their ability to digest proteins was enhanced. Under low-salinity stress, the immune indices of C. dehaani were activated within a short period. However, some immune enzymes of the crabs were inhibited when they were exposed to the freshwater environment. The activities of digestive and immune enzymes were generally higher in the female crabs than in the male crabs. Thus, reduced salinity may adversely affect the digestive and immune processes of C. dehaani, which could explain the effects of reduced salinity on essential crab species in reclaimed wetlands.
Materials and methods
Field investigation
In this study, we investigated the number of C. dehaani and water salinity in a reclaimed area in the east shoal of Chongming Island. This wetland was enclosed in 2013, and several sluices were constructed in the seawalls during the project. The main functions of these seawalls were to regulate water level and salinity.
C. dehaani crabs were collected from reclaimed and natural wetlands once a month in August and November in 2020 and January and March in 2021. A total of four sampling sites were distributed across the two survey regions: vegetated site (VT) and bald site (BT) in a tidal-affected region and vegetated site (VN) and bald site (BN) in a non–tidal-affected region. Two control sites were selected in a natural tidal flat: vegetated site (VC) and bald site (BC) (Fig. 5A,B). The catch-per-unit-effort (CPUE) method was used to collect C. dehaani. CPUE is defined as crab collection for 30 min by a person. In each sampling area, CPUE was repeated four times. The water salinity at each sampling area was measured using a Multi 350i water analyzer (WTW, MUC, Germany). The samples were transferred to the laboratory, where the crabs were counted and their sex was identified.
Physiological experiment
C. dehaani adults in the intermoult stage (about 15–25 g) were purchased from Zhengyu farmers’ markets in October 2020. The experimental crabs were acclimated at Zhuanghang Experiment Station in Shanghai Academy of Agriculture Science (Shanghai, China) for one week. During the acclimation, all crabs were reared in cement pools with aerated artificial seawater at 12.0 ± 0.1 ppt salinity. Artificial seawater was prepared using marine salt dissolved in dechlorinated tap water. Water salinity was measured with the Multi 350i water analyzer. Throughout the acclimation period, all crabs were fed with freshwater mussels once a day. The water temperature and pH were maintained at 24 ± 1 ℃ and 8.2 ± 0.2, respectively.
After the acclimation period, female and male crabs were separately exposed to 0, 6, 12 and 18 ppt salinity treatments (four replications per treatment) in a stepwise manner (6 ppt every 3 h) to prevent physiological shock due to sudden changes. Each treatment group of 32 crabs (16 females and 16 males) was randomly placed in eight plastic containers, i.e. four crabs in each container. After 96 h, one crab from each container were anesthetized in the ice bath for 15 min. The haemolymph samples were extracted from the arthropodial membrane at the 3rd swimming legs by using a disposable sterile syringe and stored in a 2 mL centrifuge tube. Then, the hepatopancreas and posterior gills (6th and 7th gills) were removed and frozen in liquid nitrogen, and all samples were maintained at − 80 °C for further analysis.
The haemolymph osmolality was measured with a BS-88 freezing point osmometer (Ashtec, USA) and expressed in mOsm/kg H2O. The activities of Na+/K+ ATPase (One activity unit was defined as the amount of ATP decomposed by ATPase in tissue to produce 1 μmol inorganic phosphorus per milligram of tissue per hour at 37℃) in the posterior gills and lysozyme (One activity unit was defined as the increase in absorbance by the decomposition of bacterial solution per mL sample per two minutes at 37℃ and 530 nm), alkaline phosphatase (One activity unit was defined as 1 mg tissue protein that interacts with the matrix at 37℃ for 15 min and produces 1 mg of phenol) and lipase (At 37℃, each gram of tissue protein reacted with the substrate for 1 min, and each micromole of substrate consumed was defined as one enzyme activity unit) in the hepatopancreas were detected with commercial assay kits (Jiancheng Bioengineering Institute, Nanjing, China), according to the manufacturer’s instructions46,47. Before the test, 0.1 g gill or hepatopancreas sample was mixed with 900 mL normal saline (0.9%). Then, the mixture was thoroughly homogenized and centrifuged at 3500 rpm for 10 min (4 ℃). The supernatant was collected for further analyses.
Phenoloxidase activity was determined using the method of Ashida (1971). l-DOPA (Jiebes Biological Co., Ltd., Shanghai, China) was prepared with 0.01 mol/L l-DOPA solution (l-DOPA:HCL-K3PO4 = 0.002:1). HCl-K3PO4 was prepared by mixing 50 mL NaH2PO4-2H2O (0.2 mol/L) and 22 mL HCl (0.1 mol/L) in a 200 mL volumetric flask for constant volume. Then, 10 μL of the tissue homogenate to be tested and 10 μL of l-DOPA solution were added successively to the aseptic enzyme plate in a constant temperature water bath (28℃) for 40 min. Next, 200 μL of pre-cooled potassium phosphate buffer solution (pH 6.0) was added, and light absorption was measured. One phenoloxidase activity unit (U) was defined as 0.001 increase in OD490 per minute.
Trypsin activity was determined as follows: 100 μL of the tissue homogenate to be tested was put in a 2 mL centrifuge tube, and 500 μL of 0.5% casein solution, 25 μL of 0.04 mol/L EDTA-Na2, 100 μL of 0.05 mol/L borax-sodium hydroxide buffer solution (pH 9.8) and 150 μL of double distilled water were added to obtain a total volume of 875 μL. The solution was mixed in a water bath at 37 ℃ for 15 min. Then, 250 μL trichloroacetic acid (30%) was added, and the mixture was centrifuged at 1400 rpm for 10 min (4 ℃). The supernatant was used to determine tyrosine content with the l-phenol method. The tyrosine content was determined using colorimetry at 660 nm. Enzyme activity (U/mg prot) was defined as the catalytic hydrolysis of casein to 1 μg tyrosine by trypsin per minute per mg protein at 37 ℃.
Amylase activity was determined as follows: 100 mg starch was added to 10 mL phosphate buffer (0.067 mol/L, pH 6.8) to prepare 1% starch solution. Next, 50 μL of the tissue homogenate to be tested and 50 μL of the starch solution were mixed and placed in a water bath at 25 ℃ for 3 min. Then, 200 μL of 3,5-dinitrosalicylic acid indicator was added and placed in a boiling water bath for 5 min. The indicator was cooled to 1 mL with running water, and maltose content was determined using colorimetry at 540 nm. Enzyme activity (U/mg prot) was defined as the catalytic hydrolysis of starch to 1 μg maltose by amylase per minute per mg protein.
Protein concentrations (mg/ml) of the enzyme solutions were determined using a protein quantification kit (Coomassie brilliant blue) produced by Jiancheng Institute of Biotechnology (Nanjing, China).
Statistical analyses
All the data variables were expressed as mean ± standard error (SEM) values. Differences in the investigated variables (water salinity and number of crabs) with respect to different areas, osmolality and enzyme activities (Na+/K+-ATPase, lysozyme, alkaline phosphatase, phenoloxidase, trypsin, amylase and lipase) were analysed using one-way ANOVA, followed by Tukey’s tests. Independent-samples t-test was used to detect differences between the male and female crabs. The relationship between the number of crabs and water salinity was evaluated with linear regression analysis. Statistically significant differences were accepted at P < 0.05, and all statistical analyses were performed using the SPSS v19.0 software.
References
Chen, L. et al. Spatiotemporal dynamics of coastal wetlands and reclamation in the Yangtze estuary during past 50 years (1960s–2015). Chin. Geogr. Sci. 28(3), 386–399 (2018).
Lv, W. et al. Effect of freshwater inflow on self-restoration of macrobenthic diversity in seaward intertidal wetlands influenced by reclamation projects in the Yangtze estuary, China. Mar. Pollut. Bull. 138, 177–186 (2019).
Lv, W. et al. Loss and selfrestoration of macrobenthic diversity in reclamation habitats of estuarine islands in Yangtze Estuary, China. Mar. Pollut. Bull. 103, 128–136 (2016).
Matsuda, O. & Kokubu, H. Recent coastal environmental management based on new concept of Satoumi which promotes land-ocean interaction: A case study in Japan. Estuar. Coast. Shelf S 183, 179–186 (2016).
Wang, J. et al. Exotic Spartina alterniflora provides compatible habitats for native estuarine crab Sesarma dehaani in the Yangtze River estuary. Ecol. Eng. 34, 57–64 (2008).
Lee, S. Y. & Khim, J. S. Hard science is essential to restoring soft-sediment intertidal habitats in burgeoning East Asia. Chemosphere 168, 765–776 (1998).
Wang, L. The complete larval development of Sesarma dehaani. J. Shanghai Fisheries Univ. 10(3), 199–206 (2001).
Liu, Z. et al. Different effects of reclamation methods on macrobenthos community structure in the Yangtze Estuary, China. Mar. Pollut. Bull. 127, 429–436 (2018).
Henry, R. P., Lucu, C., Onken, H. & Weihrauch, D. Multiple functions of the crustacean gill: Osmotic/ionic regulation, acid-base balance, ammonia excretion, and bioaccumulation of toxic metals. Front. Physiol. 3, 431 (2012).
McNamara, J. C. & Faria, S. C. Evolution of osmoregulatory patterns and gill ion transport mechanisms in the decapod Crustacea: A review. J. Comp. Physiol. B. 182(8), 997–1014 (2012).
Thabet, R., Ayadi, H., Koken, M. & Leignel, V. Homeostatic responses of crustaceans to salinity changes. Hydrobiologia 799(1), 1–20 (2017).
Boonsanit, P. & Pairohakul, S. Effects of salinity on haemolymph osmolality, gill Na+/K+ ATPase and antioxidant enzyme activities in the male mud crab Scylla olivacea (Herbst, 1796). Mar. Biol. Res. 17(1), 86–97 (2021).
Wang, R. et al. Osmotic and ionic regulation and Na+/K+-ATPase, carbonic anhydrase activities in mature Chinese mitten crab, Eriocheir sinensis H. Milne Edwards, 1853 (Decapoda, Brachyura) exposed to different salinities. Crustaceana 85(12–13), 1431–1447 (2012).
Garçon, D. P. et al. Na+, K+-ATPase activity in the posterior gills of the blue crab, Callinectes ornatus (Decapoda, Brachyura): Modulation of ATP hydrolysis by the biogenic amines spermidine and spermine. J. Membr. Biol. 244, 9–20 (2011).
Jiang, S. & Xu, Q. Influence of salinity stress on the activity of gill Na+/K+-ATPase in swimming crab(Portunus trituberculatus). J. Fish. China 35(10), 1475–1480 (2011).
Mo, J. L., Devos, P. & Trausch, G. Active absorption of Cl– and Na+ in posterior gills of Chinese crab, Eriocheir sinensis: modulation by dopamine and cAMP. J. Crust. Biol. 23, 505–512 (2003).
Charmantier, G. Ontogeny of osmoregulation in crustaceans: A review. Invertebr. Reprod. Dev. 33(2–3), 177–190 (1998).
Vargas-Chacoff, L. et al. Effects on the metabolism, growth, digestive capacity and osmoregulation of juvenile of sub-Antarctic Notothenioid fish Eleginops maclovinus acclimated at different salinities. Fish Physiol. Biochem. 41, 1369–1381 (2015).
Wang, R. et al. The response of digestive enzyme activity in the mature Chinese mitten crab, Eriocheir sinensis (Decapoda: Brachyura), to gradual increase of salinity. Sci. Mar. 77(2), 323–329 (2013).
Li, E. et al. Comparison of digestive and antioxidant enzymes activities, haemolymph oxyhemocyanin contents and hepatopancreas histology of white shrimp, Litopenaeus vannamei, at various salinities. Aquaculture 274, 80–86 (2008).
Asaro, A., del Valle, J. C. & López Mañanes, A. A. Amylase, maltase and sucrase activities in hepatopancreas of the euryhaline crab Neohelice granulata (Decapoda: Brachyura: Varunidae): Partial characterization and response to low environmental salinity. Sci. Mar. 75, 517–524 (2011).
Sǒderhǎll, I. et al. Hemocyte production andmaturation in an invertebrate animal; proliferation and gene expression in hematopoietic stem cells of Pacifastacus leniusculus. Dev. Comp. Immunol. 97(8), 661–672 (2004).
Liu, S., Jiang, X., Mou, H., Wang, H. & Guan, H. Effects of immunopoiysaccharide on LSZ, ALP, ACP and POD activities of Penaeus chinensis serum. Oceanol. Limnol. Sin. 30(3), 278–283 (1999).
Ma, Z., Zhang, F. & Jing, A. Overview and graph theory of the immune system of crustacean. Aquacul. Sci. Technol. 11(8), 19–23 (2010).
Gu, Q. & He, L. Analysis of hemolymph osmotic pressure in crab (Eriocheir sinensis H. Milne Edwards) during oogenesis. Acta Zool. Sin. 36(2), 165–171 (1990).
Esser, L. J. & Cumberlidge, N. Evidence that salt water may not be a barrier to the dispersal of Asian freshwater crabs (Decapoda: Brachyura: Gecarcinucidae and potamidae). Raffles B. Zool. 59(2), 259–268 (2011).
Novo, M. S., Miranda, R. B. & Bianchini, A. Sexual and seasonal variations in osmoregulation and ionoregulation in the estuarine crab Chasmagnathus granulatus (Crustacea, Decapoda). J. Exp. Mar. Biol. Ecol. 323(2), 118–137 (2005).
Huong, D. T. T., Yang, W., Okuno, A. & Wilder, M. N. Changes in free amino acids in the hemolymph of giant freshwater prawn Macrobrachium rosenbergii exposed to varying salinities: Relationship to osmoregulatory ability. Comp. Biochem. Phys. A 128(2), 317–326 (2001).
Malmsten, M. & Larsson, A. Immobilization of trypsin on porous glycidyl methacrylate beads: Effects of polymer hydrophilization. Colloid. Surf. B 18, 277–284 (2000).
Hosoi, M. et al. Effect of salinity change on free amino acid content in Pacific oyster. Fish. Sci. 69(2), 395–400 (2003).
Wang, G. D., Xu, K. F., Tian, X. L., Dong, S. L. & Fang, Z. H. Changes in plasma osmolality, cortisol and amino acid levels of tongue sole (Cynoglossus semilaevis) at different salinities. J. Ocean Univ. China 14(5), 881–887 (2015).
Johnston, D. & Freeman, J. Dietary preference and digestive enzyme activities as indicators of trophic resource utilization by six species of crab. Biol. Bull. 208, 36–46 (2005).
Zhang, Y. & Tong, C. Stomach content characteristics and feeding preference of Chiromantes dehaani in the salt marsh of Yangtze estuary. Chinese J. Ecol. 37(7), 2059–2066 (2018).
Ye, Y. et al. Comparative study on some traits of male and female Eriocheir sinensis raised in pond. Contemp. Aquacult. 38(4), 7–8 (2000).
Han, S. & Guan, W. Growth and maturity of Chiromantes dehaani in Dazhi River Estuary. Trans. Oceanol. Limnol. 15(1), 51–65 (2012).
Li, W., Guan, Y. & Yu, Z. Effects of salinity variation on outbreak of white spot syndrome and immunocompetence in Penaeus japonicas. Mar. Environ. Sci. 21(4), 6–9 (2002).
Pan, L. & Jiang, L. The effect of sudden changes in salinity and pH on immune activity of two species of shrimps. J. Ocean Univ. Qingdao 32(6), 903–910 (2002).
Gamperl, A. K., Vijayan, M. M. & Boutilier, R. G. Experimental control of stress hormone levels in fishes: Techniques and applications. Rev. Fish Biol. Fish. 4(2), 215–255 (1994).
Weerd, J. H. V. & Komen, J. The effects of chronic stress on growth in fish: A critical appraisal. Comp. Biochem. Phys. A 120(1), 107–112 (1998).
Barton, B. A., Schreck, C. B. & Barton, L. D. Effects of chronic cortisol administration and daily acute stress on growth, physiological conditions, and stress responses in juvenile rainbow trout. Dis. Aquat. Organ. 2(3), 173–185 (1987).
Zhao, Q., Qin, F., Li, C. & Jin, S. Preliminary study on the activities of enzymes in haemolymph of three species of marine crabs. J. Ningbo Univ. 22(1), 33–38 (2009).
Lv, W. et al. Macrobenthic diversity in protected, disturbed, and newly formed intertidal wetlands of a subtropical estuary in China. Mar. Pollut. Bull. 89, 259–266 (2014).
Ma, Z., Jing, K., Tang, S. & Chen, J. Shorebirds in the eastern intertidal areas of Chongming island during the 2001 northern migration. Stilt 41, 6–10 (2002).
Sui, L., Wille, M., Cheng, Y., Wu, X. & Sorgeloos, P. Larviculture techniques of Chinese mitten crab Eriocheir sinensis. Aquaculture 315(1–2), 16–19 (2011).
Luo, M. et al. Community characteristics of macrobenthos in waters around the nature reserve of the Chinese sturgeon Acipenser sinensis and the adjacent waters in Yangtze River estuary. J. Appl. Ichthyol. 27, 425–432 (2011).
Yang, Z., Zhu, L., Zhao, X. & Cheng, Y. Effects of salinity stress on osmotic pressure, free amino acids, and immune-associated parameters of the juvenile Chinese mitten crab, Eriocheir sinensis. Aquaculture 549, 737776 (2022).
Tian, L., Tan, P., Yang, L., Zhu, W. & Xu, D. Effects of salinity on the growth, plasma ion concentrations, osmoregulation, non-specific immunity, and intestinal microbiota of the yellow drum (Nibea albiflora). Aquaculture 528, 735470 (2020).
Acknowledgements
This study was supported by the Shanghai Municipal Science and Technology Commission, China [grant number 19ZR1436900] and the China Agriculture Research System of MOF and MARA [grant number CARS-46]. We also thank ELIXIGEN CO. for improving the language of our manuscript.
Author information
Authors and Affiliations
Contributions
W.L. wrote the manuscript. Q.Y. collected the data. W.H. analyzed the data. L.S. contributed to the plot management. W.Z. performed the experiment. Y.Z. designed the experiment.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Lv, W., Yuan, Q., Huang, W. et al. Effects of reduced salinity caused by reclamation on population and physiological characteristics of the sesarmid crab Chiromantes dehaani. Sci Rep 12, 1591 (2022). https://doi.org/10.1038/s41598-022-05639-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-022-05639-4
This article is cited by
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.