Abstract
Although several prognostic factors in nivolumab therapy have been reported in recurrent or metastatic head and neck cancer (RM-HNC) patients, these factors remain controversial. Here, we conducted a multicenter retrospective cohort study to investigate the impact of clinico-hematological factors on survival in RM-HNC patients treated with nivolumab. We reviewed 126 RM-HNC patients from seven institutes. We evaluated the prognostic effects of clinico-hematological factors on survival. The median overall survival (OS) was 12.3 months, and the 1 year-OS rate was 51.2%. Patients without immune-related adverse events, lower relative eosinophil count, worse best overall response, higher performance status, and higher modified Glasgow Prognostic Score had worse survival. The score, generated by combining these factors, was associated with survival. Patients with score of 4–5 had worse survival than those with score of 2–3 and 0–1 [adjusted HR for PFS: score of 4–5, 7.77 (3.98–15.15); score of 2–3, 3.44 (1.95–6.06), compared to score of 0–1], [adjusted HR for OS: score of 4–5, 14.66 (4.28–50.22); score of 2–3, 7.63 (2.29–25.37), compared to score of 0–1]. Our novel prognostic score utilizing clinico-hematological factors might be useful to establish an individual treatment strategy in RM-HNC patients treated with nivolumab therapy.
Similar content being viewed by others
Introduction
Immune checkpoint inhibitors (ICIs) have become standard therapy for various types of cancer1,2,3,4. Nivolumab, a programmed cell death 1 (PD-1) inhibitor, was approved in 2017 to treat platinum-refractory recurrent or metastatic head and neck cancer (RM-HNC) in Japan, receiving the results of CheckMate 141 trial1. Although nivolumab is highly effective in some patients, more than half of these patients do not have clinical benefits.
To date, several studies have reported the association of patient-related factors, including the best overall response (BOR), the occurrence of immune-related adverse events (irAEs), programmed death-ligand 1 (PD-L1) expression, high mutational burden, hematological inflammatory, and nutritional markers, and clinical outcomes in RM-HNC patients treated with nivolumab therapy5,6,7,8,9,10,11,12,13. Although several studies reported the utility of the combination of these factors in ICIs therapy, we found only one study in head and neck caner13,14,15,16,17. Therefore, the optimal prognostic factors for nivolumab therapy in patients with RM-HNC are still controversial. Furthermore, in considering the cost-effectiveness of ICI therapy, it is essential to elucidate the optimal prognostic factors in RM-HNC patients treated with ICI therapy.
Here, we conducted a multicenter retrospective cohort study to investigate the impact of clinico-hematological factors on survival in RM-HNC patients treated with nivolumab therapy among the Japanese population.
Results
Patient characteristics
The median follow-up interval was 7.5 months (range 0.5–33 months) for all patients in this study. The patient characteristics are summarized in Table 1. The median age was 68 years (range 35–90 years), and men were predominant. The primary tumor sites were oral cavity cancer in 12, oropharyngeal cancer in 27, hypopharyngeal cancer in 20, laryngeal cancer in 21, sinonasal cancer in 13, nasopharyngeal cancer in 11, salivary gland cancer in 8, external ear canal cancer in 7, and unknown primary cancer in 7. Fifty-four patients had locoregional diseases, and 72 patients had distant metastases. Regarding Eastern Cooperative Oncology Group Performance Status (ECOG PS), most patients were less than 1.
IrAE profile
The irAE profiles are shown in Supplementary Table S1. Forty-one patients (32.5%) presented with 50 irAEs. The most common irAE category was endocrine irAEs (hypothyroidism, hypophysitis, etc.), followed by skin irAEs (rash, dermatitis, rash acneiform, etc.). There were 24 grade 3 or higher irAEs, and steroid therapy was administered to 25 patients.
Clinical outcomes
For all the patients, the median overall survival (OS) was 12.3 months (95% CI 9.7–16.0), and 1 year-OS rate was 51.2% (95% CI 40.0–61.2), and the median progression-free survival (PFS) was 3.9 months (95% CI 2.8–5.4) and 1 year-PFS rate was 14.6% (95% CI 8.7–22.0) (Supplementary Fig. S1). BOR to nivolumab therapy was complete response (CR) in 5 patients (4.0%), partial response (PR) in 24 patients (19.0%), stable disease (SD) in 48 patients (38.1%), and progressive disease (PD) in 49 patients (38.9%). After PD in nivolumab therapy, chemotherapy was administered to 44 patients. Among these patients, paclitaxel and cetuximab therapy was the most common (47.7%) (Supplementary Table S2). BOR to chemotherapy after nivolumab was CR in 1 patient (2.3%), PR in 17 patients (38.6%), SD in 6 patients (13.6%), and PD in 19 patients (43.2%).
The association between clinico-hematological factors and clinical outcomes is shown in Table 2. In univariate analysis, OS was better for patients with any irAEs (HR 0.49; 95%CI, 0.27–0.87; p = 0.016), higher REC (HR 0.51, 95% CI 0.31–0.86, p = 0.011), and better BOR (HR 0.18, 95% CI 0.08–0.41, p < 0.001). Moreover, OS was worse in patients with higher ECOG PS (HR 3.14; 95%CI, 1.77–5.57; p < 0.001) and higher modified Glasgow Prognostic Score (mGPS) (HR 2.40, 95% CI 1.41–4.10, p = 0.001). Furthermore, PFS was better for patients with any irAEs (HR 0.55, 95% CI 0.36–0.84, p = 0.006), higher relative eosinophil count (REC) (HR 0.68, 95% CI 0.46–1.00, p = 0.049), and better BOR (HR 0.19, 95% CI 0.11–0.33, p < 0.001). Additionally, PFS was worse for patients with higher ECOG PS (HR 1.90; 95%CI, 1.18–3.06; p = 0.008) and higher mGPS (HR 2.02, 95% CI 1.34–3.03, p < 0.001).
In multivariate analysis, OS was better for patients with any irAEs (HR 0.47, 95% CI 0.26–0.86, p = 0.015), higher REC (HR 0.49, 95% CI 0.29–0.83, p = 0.008), and better BOR (HR 0.18, 95% CI 0.07–0.42, p < 0.001). Besides, OS was worse in patients with higher ECOG PS (HR 2.94; 95%CI, 1.55–5.55; p < 0.001) and higher mGPS (HR 2.51, 95% CI 1.45–4.35, p < 0.001). Furthermore, PFS was better for patients with any irAEs (HR 0.52, 95% CI 0.34–0.80, p = 0.003), higher REC (HR 0.68, 95% CI 0.46–1.00, p = 0.048), and better BOR (HR 0.17, 95% CI 0.10–0.30, p < 0.001). Additionally, PFS was worse for patients with higher ECOG PS (HR 2.17; 95% CI 1.30–3.63, p = 0.003) and higher mGPS (HR 2.37, 95% CI 1.55–3.63, p < 0.001).
The impact of prognostic score using the sum of numbers from clinico-hematological factors
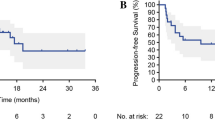
Finally, we investigated the impact of prognostic score using the sum of numbers from worse prognostic factors that were statistically significant in clinico-hematological factors, including without irAEs, lower REC, worse BOR, higher ECOG PS, and higher mGPS (Fig. 1 and Table 2). We found that higher scores were associated with worse OS and PFS, with a significant trend (p for trend < 0.001). Patients with score of 4–5 had worse survival than those with score of 2–3 and 0–1 [1 year-PFS: 0.0% vs. 10.1% vs. 38.2%, respectively; adjusted HR score of 4–5, 7.77 (95% CI 3.98–15.15); score of 2–3, 3.44 (95% CI 1.95–6.06), compared to score of 0–1], [1 year-OS: 26.3% vs. 43.1% vs. 87.7%, respectively; adjusted HR score of 4–5, 14.66 (95% CI 4.28–50.22); score of 2–3, 7.63 (95% CI 2.29–25.37), compared to score of 0–1].
Kaplan–Meier curves of progression-free survival (PFS) and overall survival (OS) in patients with recurrent or metastatic head and neck cancer treated with nivolumab, stratified by prognostic score. Prognostic score is the sum of numbers from worse prognostic factors in clinico-hematological factors including without immune-related adverse events (irAEs), lower relative eosinophil count (REC), worse best overall response (BOR), higher Eastern Cooperative Oncology Group Performance Status (ECOG PS), and higher modified Glasgow Prognostic Score (mGPS). (a) Patients with a prognostic score of 4–5 (N = 28) had significantly worse PFS than those with prognostic scores of 2–3 (N = 61) and 0–1 (N = 27) (1 year-PFS: 0.0% vs. 10.1% [95% CI 3.9–19.8] vs. 38.2% [95% CI 18.9–57.4], p < 0.001). (b), Patients with a prognostic score of 4–5 (N = 28) had significantly worse OS than those with prognostic scores of 2–3 (N = 61) and 0–1 (N = 27) (1 year-OS: 26.3% [95% CI 10.3–45.6] vs. 43.1% [95% CI 27.4–57.9] vs. 87.7% [95% CI 56.9–97.0], p < 0.001).
Discussion
This study found the prognostic effect of clinico-hematological factors, including the occurrence of irAEs, REC, BOR, ECOG PS, and mGPS in RM-HNC patients treated with nivolumab therapy. Furthermore, our analysis showed that the sum of numbers from worse prognostic factors in clinico-hematological factors might be the optimal prognostic score in the Japanese population.
Similar to previous studies reported5,18, we found that the occurrence of irAEs was significantly associated with better prognosis in RM-HNC patients treated with nivolumab therapy. Matsuo et al. reported that gastrointestinal irAEs were significantly associated with better PFS5. In other types of cancer, cutaneous, gastrointestinal, and endocrine irAEs are associated with better survival19,20. Although the endocrine irAEs category was most common in this study, we could not detect which type of irAE was strongly associated with clinical outcomes. However, since appropriate management of irAEs could lead to the clinical benefit of nivolumab therapy, early detection of irAEs and management in multidisciplinary teams should be advocated.
Additionally, we found that a better BOR to nivolumab therapy was associated with better survival. In the CheckMate 141 trial, the response rate in the nivolumab group was higher than that in the investigator's choice group, and tumor reduction was more durable with nivolumab1. Matsuki et al. also demonstrated that a better BOR was significantly associated with better survival21. It is obvious that response evaluation is important even when using immunotherapeutic agents.
Consistent with our results, Nishikawa et al. demonstrated that higher eosinophil counts and increases were associated with better survival13,22. Furthermore, eosinophil accumulation was associated with better survival in patients with melanoma treated with ICI23. They mentioned that the high number of peripheral blood eosinophils might reflect the high number of tumor-infiltrating eosinophils and the increase in tumor antigens due to tumor necrosis and collapse.
In this study, modified GPS and ECOG PS were associated with clinical outcomes in RM-HNC patients treated with nivolumab therapy. These factors are known to be prognostic factors in patients with ICI and other treatments24,25. Therefore, these biomarkers may be useful in various treatment modalities. The advantage of these biomarkers and eosinophil count, described above, is that they can be evaluated before nivolumab treatment. Since these two factors indicate the patient's general condition and/or inflammation, it might be better to use ICI without any symptoms associated with RM-HNC.
Some studies have also reported the utility of the combination of each factor in several type of cancer treated with ICIs. In non–small cell lung cancer patients treated with anti-PD-1/PD-L1 antibodies, the ESPILoN score [smoking history, liver metastasis, lactate dehydrogenase (LDH), and neutrophil to lymphocyte ratio (NLR)], the Gustave Roussy Immune Score (GRIm-Score) (LDH, serum albumin (Alb), and NLR) and the RHM score (LDH, Alb, and the number of metastatic sites) was reported to be significantly correlated with prognosis14,15,16. The Emory risk scoring system including the monocyte-to-lymphocyte ratio (MLR), body mass index (BMI) and number of metastatic sites was significantly associated with survival in recurrent or metastatic renal cell carcinoma treated with anti-PD-1 antibodies17. In recurrent or metastatic head and neck squamous cell carcinoma treated with anti-PD-1 antibodies, the Eosinophil Prognostic Score (REC, the ratio of eosinophil increase, and ECOG PS) was reported to be significantly correlated with survival13. We demonstrated that the sum of numbers from worse prognostic factors could be the optimal prognostic score in RM-HNC patients treated with nivolumab. To the best of our knowledge, this is the first report to evaluate the impact of prognostic score associated with clinico-hematological factors, which are routinely available in clinical settings and included irAE and BOR, on survival in RM-HNC patients with nivolumab. However, the concern is that the poor nutritional condition and infection could affect these factors. Therefore, supportive therapy, including nutritional support, oral care, and smoking cessation, should be considered in patients with RM-HNC.
Regarding the primary tumor site, while we performed nivolumab therapy in patients not included in the CheckMate 141 trial, such as nasopharyngeal cancer, clinical outcomes were comparable to those included in the CheckMate 141 trial1,7. In patients excluded from the CheckMate 141 trial, the efficacy of nivolumab therapy has been reported with primary tumors at other sites, including the nasopharynx, while its efficacy is limited in the salivary gland cancer5,26,27,28. However, there is insufficient evidence for the optimal systematic therapy for HNC other than squamous cell carcinoma (SCC). A larger collaborative study to evaluate the efficacy of nivolumab therapy in patients with HNC other than SCC is required.
Regarding salvage chemotherapy following nivolumab therapy, we mainly performed paclitaxel and cetuximab therapy, and our clinical outcomes, including 41% ORR, were comparable to those of previous studies29,30,31. As mentioned above, since the efficacy of cetuximab and other chemotherapies following ICI has been reported, relatively early changes in treatment modalities might be acceptable.
Our study had several strengths. Since this is a multi-institutional cohort study, our sample size is one of the largest studies in patients with RM-HNC in a real-world setting. Second, detailed individual data were available for this study. Third, because physicians had no information on the association of clinic-hematological factors with survival, information bias appears unlikely. Fourth, we could perform the analysis adjusted for potential confounders, including detailed clinical information.
Moreover, several limitations should be mentioned. First, this study was conducted with a retrospective and multi-institutional design. Second, our information, especially hematological factors, reflected pre-treatment status only and not post-treatment factors, which might be associated with clinical outcomes. Third, we could not fully remove the potential effect of factors of infectious diseases, inflammation other than that derived from RM-HNC, and the use of glucocorticoid hormones. To overcome these limitations, future large scale, multicenter and prospective studies are warranted.
In conclusion, we found that the occurrence of irAEs, higher REC, better BOR, lower ECOG PS, and lower mGPS were the better prognostic factors for survival in patients with RM-HNC treated with nivolumab. Furthermore, the sum of the numbers of worse prognostic factors might be the optimal prognostic score. Using this novel prognostic score, more effective treatment strategies, including nivolumab therapy, could be established for patients with RM-HNC.
Materials and methods
Patients
We conducted a retrospective cohort study to investigate the efficacy and safety of nivolumab therapy in RM-HNC patients in seven institutes, including Nagoya City University Hospital, Konan Kosei Hospital, Toyohashi Municipal Hospital, Kainan Hospital, Toyota Kosei Hospital, Anjo Kosei Hospital, and Ichinomiya Municipal Hospital. Among these institutes, 126 RM-HNC patients were treated with nivolumab between April 2017 and November 2019. The total of 10 patients who lacked information on clinico-hematological factors were excluded. Finally, 116 patients were enrolled in the prognostic score analysis. The Institutional Review Board (IRB) of Nagoya City University Graduate School of Medical Sciences approved our protocols (approval number: 60-21-0001). Concerning consent to participate, patients could reject participation by opting out, to an announcement on Nagoya City University Hospital’s website. This study was conducted in accordance with the principles of the Declaration of Helsinki.
Treatment and follow-up
All patients had been treated with platinum-based therapy before nivolumab therapy. Nivolumab was administered at a dose of 3 mg/kg every 2 weeks. The response to nivolumab therapy was evaluated according to Response Evaluation Criteria in Solid Tumor (RECIST) criteria version 1.132, using computed tomography (CT) or magnetic resonance imaging (MRI) every 8–12 weeks. Patients in whom nivolumab administration was terminated due to clinically obvious disease progression were diagnosed with PD, even when image evaluation was not performed. Chemotherapy with cytotoxic agents was administered to patients diagnosed with PD. Follow-up was continued until death or the cut-off date (May 6, 2020).
Prognostic effect of clinical and hematological factors
This study evaluated the prognostic effect of clinical factors, including age, sex, primary tumor site, ECOG PS, site of recurrence, and platinum sensitivity. A platinum-refractory tumor was defined as a tumor that progressed within 6 months after the last platinum-based chemotherapy or a residual tumor after platinum-based chemoradiotherapy. A platinum-sensitive tumor was defined as a tumor that progressed from 6 months or longer after the last platinum-based chemotherapy. Moreover, we also evaluated the prognostic effect of hematological factors: REC, NLR, PLR, and mGPS. Hematological factors were calculated by eosinophil count, neutrophil count, lymphocyte count, platelet count, C-reactive protein (CRP), and Alb in peripheral blood just before the start of nivolumab therapy. Regarding mGPS, patients with both elevated CRP level (> 1.0 mg/dL) and decreased Alb (< 3.5 g/dL) were assigned a score of 2; those with elevated CRP level (> 1.0 mg/dL) and non-decreased Alb (≥ 3.5 g/dL) were assigned a score of 1, and those with a non-elevated CRP level (≤ 1.0 mg/dL) were assigned a score of 0 according to a previous study33.
Statistical analysis
We investigated overall effectiveness, including BOR, PFS, and OS. BOR was defined as the best response from the initiation of nivolumab administration to PD. PFS was defined as the time from the first nivolumab administration to the date of PD or clinically unequivocal progression. OS was defined as the time from the first nivolumab administration to the date of death or the last visit. The irAEs were evaluated according to the protocol described in a previous study34. Toxicity was assessed using the Common Terminology Criteria for Adverse Events (CTCAE) version 5.035.
Additionally, we evaluated the frequency of irAEs and the association between clinico-hematological factors and clinical outcomes in nivolumab therapy. The association of clinico-hematological factors with PFS or OS was assessed using the Kaplan–Meier product-limit method and univariate and multivariate Cox proportional hazards models. In the multivariate analysis, the forced entry method was performed. The measure of association in this study was hazard ratios (HRs) with 95% confidence intervals (CIs). Statistical significance was set at P < 0.05. Statistical analyses were performed using GraphPad Prism (version 9.00; GraphPad Software, San Diego, CA, USA) and EZR version 1.40 (Saitama Medical Center, Jichi Medical University, Saitama, Japan), which is a graphical user interface for R (R version 3.6.1, The R Foundation for Statistical Computing, Vienna, Austria)36. More precisely, it is a modified version of R commander designed to add statistical functions frequently used in biostatistics.
Ethical approval
The study protocol was approved by the Institutional Review Board (IRB) of Nagoya City University Graduate School of Medical Sciences (# 60-21-0001). Concerning consent to participate, patients could reject participation by opting out, to an announcement on Nagoya City University Hospital’s website. Therefore, written informed consent was waived, which was approved by the IRB of Nagoya City University Graduate School of Medical Sciences. This study was conducted in accordance with the principles of the Declaration of Helsinki.
Data availability
The datasets generated in this study are available from the corresponding author on request.
References
Ferris, R. L. et al. Nivolumab for recurrent squamous-cell carcinoma of the head and neck. New Engl. J. Med. 375, 1856–1867. https://doi.org/10.1056/NEJMoa1602252 (2016).
Wolchok, J. D. et al. Overall survival with combined nivolumab and ipilimumab in advanced melanoma. New Engl. J. Med. 377, 1345–1356. https://doi.org/10.1056/NEJMoa1709684 (2017).
Overman, M. J. et al. Durable clinical benefit with nivolumab plus ipilimumab in DNA mismatch repair-deficient/microsatellite instability-high metastatic colorectal cancer. J. Clin. Oncol. 36, 773–779. https://doi.org/10.1200/jco.2017.76.9901 (2018).
Garon, E. B. et al. Pembrolizumab for the treatment of non-small-cell lung cancer. New Engl. J. Med. 372, 2018–2028. https://doi.org/10.1056/NEJMoa1501824 (2015).
Matsuo, M. et al. Relationship between immune-related adverse events and the long-term outcomes in recurrent/metastatic head and neck squamous cell carcinoma treated with nivolumab. Oral Oncol. 101, 104525. https://doi.org/10.1016/j.oraloncology.2019.104525 (2020).
Hanai, N. et al. Effectiveness and safety of nivolumab in patients with head and neck cancer in Japanese real-world clinical practice: A multicenter retrospective clinical study. Int. J. Clin. Oncol. 26, 494–506. https://doi.org/10.1007/s10147-020-01829-0 (2021).
Ferris, R. L. et al. Nivolumab vs investigator’s choice in recurrent or metastatic squamous cell carcinoma of the head and neck: 2-year long-term survival update of CheckMate 141 with analyses by tumor PD-L1 expression. Oral Oncol. 81, 45–51. https://doi.org/10.1016/j.oraloncology.2018.04.008 (2018).
Yarchoan, M., Hopkins, A. & Jaffee, E. M. Tumor mutational burden and response rate to PD-1 inhibition. New Engl. J. Med. 377, 2500–2501. https://doi.org/10.1056/NEJMc1713444 (2017).
Hanna, G. J. et al. Frameshift events predict anti-PD-1/L1 response in head and neck cancer. JCI Insight https://doi.org/10.1172/jci.insight.98811 (2018).
Yu, Y. et al. Pretreatment neutrophil to lymphocyte ratio in determining the prognosis of head and neck cancer: A meta-analysis. BMC Cancer 18, 383. https://doi.org/10.1186/s12885-018-4230-z (2018).
Proctor, M. J. et al. An inflammation-based prognostic score (mGPS) predicts cancer survival independent of tumour site: A Glasgow Inflammation Outcome Study. Br. J. Cancer 104, 726–734. https://doi.org/10.1038/sj.bjc.6606087 (2011).
Matsuki, T. et al. Hematological predictive markers for recurrent or metastatic squamous cell carcinomas of the head and neck treated with nivolumab: A multicenter study of 88 patients. Cancer Med. 9, 5015–5024. https://doi.org/10.1002/cam4.3124 (2020).
Nishikawa, D. et al. Eosinophil prognostic scores for patients with head and neck squamous cell carcinoma treated with nivolumab. Cancer Sci. 112, 339–346. https://doi.org/10.1111/cas.14706 (2021).
Bigot, F. et al. Prospective validation of a prognostic score for patients in immunotherapy phase I trials: The Gustave Roussy Immune Score (GRIm-Score). Eur. J. Cancer 84, 212–218. https://doi.org/10.1016/j.ejca.2017.07.027 (2017).
Arkenau, H. T. et al. Clinical outcome and prognostic factors for patients treated within the context of a phase I study: The Royal Marsden Hospital experience. Br. J. Cancer 98, 1029–1033. https://doi.org/10.1038/sj.bjc.6604218 (2008).
Prelaj, A. et al. EPSILoN: A prognostic score for immunotherapy in advanced non-small-cell lung cancer: A validation cohort. Cancers (Basel) https://doi.org/10.3390/cancers11121954 (2019).
Martini, D. J. et al. Novel risk scoring system for patients with metastatic renal cell carcinoma treated with immune checkpoint inhibitors. Oncologist 25, e484–e491. https://doi.org/10.1634/theoncologist.2019-0578 (2020).
Okamoto, I. et al. Efficacy and safety of nivolumab in 100 patients with recurrent or metastatic head and neck cancer—A retrospective multicentre study. Acta Otolaryngol. 139, 918–925. https://doi.org/10.1080/00016489.2019.1648867 (2019).
Freeman-Keller, M. et al. Nivolumab in resected and unresectable metastatic melanoma: Characteristics of immune-related adverse events and association with outcomes. Clin. Cancer Res. 22, 886–894. https://doi.org/10.1158/1078-0432.Ccr-15-1136 (2016).
Ricciuti, B. et al. Impact of immune-related adverse events on survival in patients with advanced non-small cell lung cancer treated with nivolumab: Long-term outcomes from a multi-institutional analysis. J. Cancer Res. Clin. Oncol 145, 479–485. https://doi.org/10.1007/s00432-018-2805-3 (2019).
Matsuki, T. et al. Real-world, long-term outcomes of nivolumab therapy for recurrent or metastatic squamous cell carcinoma of the head and neck and impact of the magnitude of best overall response: A retrospective multicenter study of 88 patients. Cancers (Basel) https://doi.org/10.3390/cancers12113427 (2020).
Nishikawa, D. et al. Prognostic markers in head and neck cancer patients treated with nivolumab. Cancers (Basel) https://doi.org/10.3390/cancers10120466 (2018).
Simon, S. C. S. et al. Eosinophil accumulation predicts response to melanoma treatment with immune checkpoint inhibitors. Oncoimmunology 9, 1727116. https://doi.org/10.1080/2162402x.2020.1727116 (2020).
Argiris, A., Li, Y. & Forastiere, A. Prognostic factors and long-term survivorship in patients with recurrent or metastatic carcinoma of the head and neck. Cancer 101, 2222–2229. https://doi.org/10.1002/cncr.20640 (2004).
Chang, P. H. et al. Impact of the pretreatment Glasgow prognostic score on treatment tolerance, toxicities, and survival in patients with advanced head and neck cancer undergoing concurrent chemoradiotherapy. Head Neck 39, 1990–1996. https://doi.org/10.1002/hed.24853 (2017).
Niwa, K. et al. Multicentre, retrospective study of the efficacy and safety of nivolumab for recurrent and metastatic salivary gland carcinoma. Sci. Rep. 10, 16988. https://doi.org/10.1038/s41598-020-73965-6 (2020).
Ma, B. B. Y. et al. Antitumor activity of nivolumab in recurrent and metastatic nasopharyngeal carcinoma: an international, multicenter study of the mayo clinic phase 2 consortium (NCI-9742). J. Clin. Oncol. 36, 1412–1418. https://doi.org/10.1200/jco.2017.77.0388 (2018).
Kokkali, S. et al. Nivolumab in patients with rare head and neck carcinomas: A single center’s experience. Oral Oncol 101, 104359. https://doi.org/10.1016/j.oraloncology.2019.07.002 (2020).
Saleh, K. et al. Response to salvage chemotherapy after progression on immune checkpoint inhibitors in patients with recurrent and/or metastatic squamous cell carcinoma of the head and neck. Eur. J. Cancer 121, 123–129. https://doi.org/10.1016/j.ejca.2019.08.026 (2019).
Pestana, R. C. et al. Response rates and survival to systemic therapy after immune checkpoint inhibitor failure in recurrent/metastatic head and neck squamous cell carcinoma. Oral Oncol. 101, 104523. https://doi.org/10.1016/j.oraloncology.2019.104523 (2020).
Cabezas-Camarero, S. et al. Safety and efficacy of cetuximab-based salvage chemotherapy after checkpoint inhibitors in head and neck cancer. Oncologist https://doi.org/10.1002/onco.13754 (2021).
Eisenhauer, E. A. et al. New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1). Eur. J. Cancer 45, 228–247. https://doi.org/10.1016/j.ejca.2008.10.026 (2009).
McMillan, D. C., Crozier, J. E., Canna, K., Angerson, W. J. & McArdle, C. S. Evaluation of an inflammation-based prognostic score (GPS) in patients undergoing resection for colon and rectal cancer. Int. J. Colorectal Dis. 22, 881–886. https://doi.org/10.1007/s00384-006-0259-6 (2007).
Michot, J. M. et al. Immune-related adverse events with immune checkpoint blockade: A comprehensive review. Eur. J. Cancer 54, 139–148. https://doi.org/10.1016/j.ejca.2015.11.016 (2016).
Common Terminology Criteria for Adverse Events (CTCAE) Version 5.0. https://ctep.cancer.gov/protocolDevelopment/electronic_applications/docs/CTCAE_v5_Quick_Reference_8.5x11.pdf#search=%22common%20terminology%20criteria%22.
Kanda, Y. Investigation of the freely available easy-to-use software “EZR” for medical statistics. Bone Marrow Transpl. 48, 452–458. https://doi.org/10.1038/bmt.2012.244 (2013).
Acknowledgements
The authors thank all the patients, their families, and caregivers. The authors thank all the clinicians for their involvement and contribution to the study. This work was supported by JSPS Grant-in-Aid for Young Scientists (B) to D. Kawakita (No. 17K18006) and T. Matoba (No. 19K18779), and by a JSPS Grant-in-Aid for Scientific Research (C) to S. Iwasaki (No. 18K09370) and D. Kawakita (No. 20K10508), and by Grants from the Japan Agency for Medical Research and Development to S. Iwasaki (No. 20hk0102050h0403).
Author information
Authors and Affiliations
Contributions
Study concept and design: K.M., T.M., D.K., and S.I.; acquisition of data: K.M., T.M., D.K., G.T., K.O., A.M., K.N., S.I., W.H., A.M., S.O., T.O., I.H., N.T., S.M., H.T., S.I., S.M., K.M., S.E., and S.I.; analysis and interpretation of data: K.M., T.M., and D.K.; statistical analysis: K.M., T.M., and D.K.; drafting of the manuscript: K.M., T.M., D.K., and S.I.; manuscript review: all authors.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Minohara, K., Matoba, T., Kawakita, D. et al. Novel Prognostic Score for recurrent or metastatic head and neck cancer patients treated with Nivolumab. Sci Rep 11, 16992 (2021). https://doi.org/10.1038/s41598-021-96538-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-021-96538-7
This article is cited by
-
Novel Scoring Formula to Predict Survival in Patients of Primary Tongue Cancer Belonging to Tobacco Chewing Population
Indian Journal of Surgical Oncology (2023)
-
A systematic review and meta-analysis of prognostic indicators in patients with head and neck malignancy treated with immune checkpoint inhibitors
Journal of Cancer Research and Clinical Oncology (2023)
-
Site of distant metastasis affects the prognosis with recurrent/metastatic head and neck squamous cell carcinoma patients treated with Nivolumab
International Journal of Clinical Oncology (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.