Abstract
A limited number of papers have addressed the association between non-dipping-blood pressure (BP) obstructive sleep apnea (OSA), and no study has assessed BP-dipping during rapid eye movement (REM) and non-REM sleep in OSA patients. This study sought to noninvasively assess BP-dipping during REM and non-REM (NREM)-sleep using a beat-by-beat measurement method (pulse-transit-time (PTT)). Thirty consecutive OSA patients (men = 50%) who had not been treated for OSA before and who had > 20-min of REM-sleep were included. During sleep, BP was indirectly determined via PTT. Patients were divided into dippers and non-dippers based on the average systolic-BP during REM and NREM-sleep. The studied group had a a median age of 50 (42–58.5) years and a body mass index of 33.8 (27.6–37.5) kg/m2. The median AHI of the study group was 32.6 (20.1–58.1) events/h (range: 7–124), and 89% of them had moderate-to-severe OSA. The prevalence of non-dippers during REM-sleep was 93.3%, and during NREM-sleep was 80%. During NREM sleep, non-dippers had a higher waist circumference and waist-hip-ratio, higher severity of OSA, longer-time spent with oxygen saturation < 90%, and a higher mean duration of apnea during REM and NREM-sleep. Severe OSA (AHI ≥ 30) was defined as an independent predictor of non-dipping BP during NREM sleep (OR = 19.5, CI: [1.299–292.75], p-value = 0.03). This short report demonstrated that BP-dipping occurs during REM and NREM-sleep in patients with moderate-to-severe OSA. There was a trend of more severe OSA among the non-dippers during NREM-sleep, and severe OSA was independently correlated with BP non-dipping during NREM sleep.
Similar content being viewed by others
Introduction
Blood pressure (BP) follows a circadian rhythm, where it decreases at night during sleep1. If the reduction in BP is ≥ 10% of the wake-BP, this is called a dipping pattern, and if it is < 10%, the pattern is called a non-dipping pattern2. On the other hand, those with an increase in nocturnal-BP are called reverse dippers. The nocturnal dipping in BP is related to the physiological decrease in sympathetic activity and increased parasympathetic activity3.
Obstructive sleep apnea (OSA) is characterized by recurrent narrowing of the upper airway during sleep, leading to upper airway obstruction, arousal, hypercapnia, and intermittent hypoxemia. These pathological changes have a pathophysiological impact on the cardiovascular system and BP. Closure of the upper airway results in sudden swings in intrathoracic pressure leading to acute perturbations of BP4. Intermittent hypoxia in animal models caused a rise in BP that continued even after eliminating hypoxic exposure5. Similar findings have been reported in humans, too6. OSA causes persistent elevations of sympathetic tone, changes in baroreceptor function, and remodeling of the cardiovascular system7.
Previous studies have indicated a relationship between the severity of OSA and non-dipping BP in both community and clinic-based populations8,9,10. Moreover, non-dipping BP in OSA patients has been linked with cardiovascular diseases and systemic inflammation11,12.
When assessing BP during sleep, a validated noninvasive method that does not disturb sleep is desirable to continuously assess BP during different sleep stages to remove the effects of sleep disturbances on BP. A BP measurement method based on the association between BP and the pulse wave velocity (PWV) is a noninvasive practical method to measure BP. It has been shown that BP measured using the pulse transit time (PTT) is comparable with that assessed by reference methods13,14,15,16. As noninvasive measurements were not commonly applied for BP measurements during sleep, BP-dipping was not measured during different sleep stages in previous studies.
In general, a relatively limited number of papers have addressed the association between non-dipping-BP and OSA17; a recent meta-analysis included 14 studies only. Moreover, no study has assessed BP-dipping during REM and NREM sleep in OSA patients. Because a more considerable sympathetic surge accompanies REM sleep and more accentuated hemodynamic variations than in NREM sleep, the assessment of BP-dipping during REM sleep is essential. This needs to be correlated with cardiovascular complications in patients with OSA, particularly that recent data suggested that REM predominant OSA is independently related to incident non-dipping of BP8.
We hypothesized that in OSA patients (in REM and NREM sleep), BP declines (dips) less frequently during REM sleep than NREM sleep. Therefore, this study sought to noninvasively assess BP-dipping during REM and NREM-sleep using a beat-by-beat measurement method (PTT) in patients who had obstructive events in both REM and NREM sleep.
Materials and methods
Subjects
We recruited consecutive patients who had not used positive airway pressure therapy for OSA from the sleep disorders clinic. Inclusion criteria were ≥ 18-years-old, OSA diagnosis based on overnight polysomnography (PSG) (SOMNOmedics GmbH, Randersacker, Germany), and having REM-sleep (≥ 20-min) during monitoring between April and October 2018. Exclusion criteria included daytime hypercapnia, alternative treatments for OSA (e.g., surgical procedures), known neuromuscular, respiratory, or cardiovascular diseases, secondary causes of hypertension (other than OSA), or requirement for home oxygen therapy. All patients underwent arterial blood gas analysis.
Ethics approval was obtained from the Institutional Review Board in our institute, and all participants signed a written informed consent form.
Blood pressure measurement
The recruited patients were instructed to stop smoking on the evaluation day. None of the participants drink alcohol. During sleep, BP was indirectly determined via a pulse-transit-time (PTT), which is validated-method using the DOMINO-Software (DOMINO-2.2.0 provided with the SOMNOscreen-plus, Randersacker, Germany)18. We have described the details of the used method in a previous paper19. In brief, the noninvasive measurement of BP using the SOMNOscreen plus has been validated following the European Society of Hypertension protocol18. The system assesses BP continuously and noninvasively (beat-to-beat determination of PTT), calculated as the interval between the ECG R-waves and the detection of the corresponding pulse wave (revealed from the finger photoplethysmography signal) at the peripheral site.
The algorithm utilizes the relationship between BP and the pulse-wave-velocity (PWV), and good results have been reported for BP measurement using the PTT in OSA patients20. The computation of the systolic blood pressure (SBP) depends on a non-linear correlation between BP and PTT13. Calibration was done based on a PWV-BP relation following the manufacturer’s instructions18. The mean values of SBP during REM and NREM-sleep were used in the analysis.
Awake-BP was determined via measuring BP for one hour while awake in the sitting position using the same (PTT) method between 9 and 10 AM on the day of the study, and the mean awake-BP was also used in the analysis. The all-night PTT recording was viewed, and data segments containing movement artifacts were excluded from the analysis. Clean 30 s epochs were analyzed, and the mean SBP and DBP were calculated. BP determined automatically beat-to-beat with the DOMINO software based on a non-linear pulse wave velocity-SBP function in combination with an initial BP calibration.
The patients were divided into dippers if a reduction in the mean systolic-BP of ≥ 10% of the wake systolic-BP was documented during sleep; they were called non-dippers if the reduction was < 10%. Further, patients were divided into dippers and non-dippers based on the average systolic-BP during REM and NREM-sleep.
Polysomnography
All patients underwent a standard overnight attended type-I PSG (SOMNOscreen-plus, Randersacker, Germany). PSG scoring was done manually following the American Academy of Sleep Medicine scoring criteria by a certified sleep technologist who had no access to the clinical information21. Apnea was scored when there was a drop of flow signal of ≤ 90% for at least 10-s. Hypopnea was defined as a drop in flow signal by ≥ 30% for at least ≥ 10 s, associated with arousal or ≥ 3% oxyhemoglobin desaturation. The apnea–hypopnea index (AHI) was calculated during REM and NREM sleep. The severity of OSA was graded according to the AHI: 5– < 15, mild OSA; 15– < 30, moderate OSA; and ≥ 30, severe OSA22.
Statistical analysis
For categorical variables, data were expressed as numbers (percentages), and for continuous variables, mean and standard deviation were used and median with interquartile range (IQR) in the Tables. The Student t-test was used to compare continuous variables if data passed the normality test (Kolmogorov–Smirnov); upon failing normality testing, the Mann–Whitney U test was used. For dichotomous variables, the Chi-square test was used. However, when the expected frequencies were < 5, we used the Fisher's exact test.
Multiple logistic regression analysis (Forward: Wald method) was performed to assess the association between markers of OSA severity (AHI, desaturation, apnea duration, and arousals) and BP-dipping while adjusting for age, sex, BMI, and smoking status expressed as odds ratio [OR] and confidence interval [CI]. A p-value of ≤ 0.05 was considered significant. Data were analyzed using the SPSS-statistical (version-24; Chicago, IL, USA) was used for the analyses.
Informed consent
Written informed consent was obtained from all participants.
Research involving human subjects
The study protocol was approved by the institutional review board at King Saud University, and informed consent was obtained from all the participants prior to inclusion in this study. The used methods were carried out in accordance with the Declaration of Helsinki and the relevant guidelines and regulations.
Results
Thirty patients (males = 14) who had a median age of 50 (42–58.5) years and a BMI of 33.8 (27.6–37.5) kg/m2, met the inclusion criteria and were included. Nine patients have been previously diagnosed with hypertension. The median systolic and diastolic-BP of the study group were 128 (114–145) mmHg and 74 (68–85) mmHg, respectively.
Among the whole group, 89% of the sample had moderate-to-severe OSA. The median AHI of the study group was 32.6 (20.1–58.1) events/h (range: 7–124). The AHI during NREM-sleep (AHI-NREM) was 30.9 (17.2–56.7) events/h, and the AHI REM-sleep (AHI-REM) was 48.4 (29–74.10 events/h.
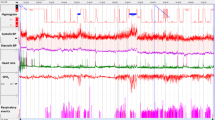
The prevalence of non-dippers during REM-sleep was 93.3%, and during NREM-sleep was 80%. Two patients had BP-dipping during REM and NREM-sleep, and four patients had BP-dipping only in NREM-sleep. Figure 1A shows an example of a patient who had BP-dipping in REM and NREM-sleep, and Fig. 1B shows an example of reverse dipping during REM-sleep.
(A) The histogram is showing more than 10% dipping in blood pressure during REM-sleep. The patient also has obstructive respiratory hypopneas during REM-sleep associated with desaturation. (B) The histogram is showing non-dipping during NREM-sleep and reverse dipping during REM-sleep. There are also obstructive respiratory events and significant desaturation during REM-sleep.
Table 1 presents demographics, comorbidities, arterial blood gases, and polysomnographic findings in dippers and non-dippers during REM and NREM sleep. The small sample size of dippers during REM sleep (n = 2) did not allow performing a comparison between dippers and non-dippers. However, during NREM sleep, non-dippers had a higher waist circumference and waist-hip-ratio, higher severity of OSA, longer-time spent with oxygen saturation < 90% (SpO2), and higher mean duration of apnea during REM and NREM-sleep.
Table 2 depicts the demographics, comorbidities, and polysomnographic findings among non-dippers in REM and NREM sleep. Non-dippers during NREM sleep had a higher prevalence of diabetes mellitus and had a lower percentage of stage N3.
A multiple logistic regression analysis after adjusting for age, sex, BMI, and smoking status identified severe OSA (AHI ≥ 30/h) as an independent correlate of non-dipping BP during NREM sleep (OR = 19.5, CI: [1.299—292.75], p-value = 0.03) (Table 3).
Discussion
This preliminary report demonstrated that BP-dipping is low among patients with moderate-to-severe OSA. However, the central message of this preliminary report is that BP-dipping may occur in both REM and NREM-sleep in patients with moderate-to-severe OSA; nevertheless, as we hypothesized, the prevalence of BP-dipping was more during NREM than REM-sleep in OSA patients. OSA severity was apparent among non-dippers during NREM sleep. The small sample size of non-dippers in REM sleep did not allow proper comparison and detection of potential differences between dippers and non-dippers. Non-dippers during NREM had a lower percentage of N3 and a higher prevalence of diabetes mellitus than non-dippers during REM sleep.
Nocturnal BP-dipping is an active process controlled by the central nervous system; it is an essential process for the regulation of daytime-BP. In healthy individuals, as sleep progresses from N1 to N3-sleep, BP slowly declines due to increased vagal tone and decreased sympathetic-activity, and BP reaches its nadir during stage-N323. On the other hand, REM-sleep is associated with intermittent increases in sympathetic-activity and BP23. However, findings in healthy individuals may not apply to patients with OSA. Therefore, it is essential to objectively monitor the decline in the average BP in OSA patients during REM and NREM-sleep. Changes in BP during sleep stages and in OSA patients are quick with significant swings; therefore, a method that measures these rapid BP changes in different sleep stages with accuracy is needed. A strong point of this study is the use of a valid beat-by-beat measurement of BP, which allowed us to detect the dipping in BP in Rem and NREM with accuracy. The PTT and PWV method is an accurate application of beat-to-beat BP recordings13,15,16,24. The beat-by-beat measurement of BP permits precise recording of the very short-term variability in BP that may accompany changes in sleep stages and obstructive respiratory events25.
Theoretically, sleep stages may impact nocturnal-BP changes in OSA patients through the severity of the obstructive events, duration, and accompanied arousals and desaturations in each stage. In the current study, stage-N3% was significantly lower in the non-dippers during NREM sleep. Experimental studies that assessed slow-wave-sleep deprivation on BP in healthy-volunteers suggested an impact of slow-wave-sleep on BP-dipping during sleep. Tasali et al. reported a decrease in vagal-tone and an increase in sympathetic-activity with slow-wave-sleep deprivation26. Another study randomly deprived 11 healthy subjects of slow-wave-sleep via acoustic stimulation for one-night, and the effects were compared with one-night of undisturbed sleep27. Suppression of slow-wave-sleep resulted in a significant decrease in BP-dipping; however, no significant changes were reported in the morning-BP and no changes in urine catecholamine levels27.
There was a clear trend towards a more severe OSA among non-dippers, and severe OSA was an independent correlate of non-dipping BP during NREM-sleep. This concurs with previous studies, which demonstrated that non-dipping correlated with the severity of respiratory events in OSA patients25. Additionally, apnea duration and time-spent with SpO2 < 90% were longer in the non-dippers in NREM-sleep. Previous studies demonstrated that increased duration of the obstructive respiratory event during sleep is associated with increased nocturnal BP28,29. Additionally, longer duration of apnea results in a higher level of hypercapnia and hypoxemia28, which work together to enhance sympathetic nerve activity and hence increase BP30.
This current short report has some strengths; it included patients with > 20-min of REM sleep to assess the changes in BP during REM-sleep, and the study used a validated beat-to-beat measurement method. Nevertheless, the study's limitations include being a preliminary report with a relatively small sample; hence, a larger study is needed to confirm the current findings and identify the predictors of BP-dipping during REM-sleep. Second, the present report did not include controls. Third, antihypertensive medications were not stopped in patients with hypertension. Finally, the difference between diurnal and nocturnal BP values is ideally assessed via measuring the 24-h BP recording to evaluate the changes in mean BP from day to night. However, as we used the PTT method built-in the PSG device, we could not do 24-h monitoring, as we were keen to use the same measuring method and device to avoid discrepancy between methods. To mitigate that, we measured BP using the same PTT method on the day of the study for one hour between 9 and 10 AM. In normal individuals, BP usually shows two peaks, one in the morning and the other in the late afternoon or early evening31. Studies have reported differences in peak BP timing, while a higher morning peak was reported mainly in Asian individuals, higher evening peak was reported in other studies, mainly in European individuals25,32. Moreover, 24-h changes in BP can be revealed by BP measurements taken at different times of the day, e.g., morning and evening, and do not need to be a continuous measurement25. Therefore, we think that measuring BP in the morning for 1 h was a reasonable method to compare with BP during sleep to detect BP-dipping.
Additionally, in this study, we used the same definition of BP dipping during REM and NREM sleep. Most previous studies averaged the BP during the whole night or the whole epoch of recording without distinguishing BP dipping in REM and NREM sleep. Nevertheless, a longitudinal analysis of the Wisconsin Sleep Cohort used the same definition for BP dipping in REM and NREM sleep8. Therefore, future research should determine whether the definition of BP dipping during REM and NREM sleep should be the same.
In the current literature and published guidelines, it is unclear whether dipping phenomena should be based on SBP and/or DBP, and there is debate among experts33,34. It is possible that the use of SBP is better as people get old because data collected in several studies demonstrated that DBP becomes lower in most patients when they get older33. Previous studies have used SBP, DBP, or MABP. According to experts in BP monitoring, this area is still ambiguous33,34,35. Nevertheless, the extent of percentage of nocturnal reduction is on average greater for DBP than for SBP36. It has also been suggested that the SBP night-to-day ratio may be similar in auscultatory and oscillometric recordings, whereas this could not be the case for the DBP night-to-day ratio37. Therefore, we opted to use SBP in this paper. Nonetheless, more future research is needed to elucidate this ambiguity.
In summary, this preliminary report demonstrated that BP-dipping occurs during both REM and NREM-sleep in a small percentage of patients with OSA. There was a trend of more severe OSA among the non-dippers during NREM-sleep.
Data availability
Data are available upon request but need an institutional approval.
References
Douma, L. G. & Gumz, M. L. Circadian clock-mediated regulation of blood pressure. Free Radic. Biol. Med. 119, 108–114. https://doi.org/10.1016/j.freeradbiomed.2017.11.024 (2018).
ESH/ESC Task Force for the Management of Arterial Hypertension. 2013 Practice guidelines for the management of arterial hypertension of the European Society of Hypertension (ESH) and the European Society of Cardiology (ESC): ESH/ESC task force for the management of arterial hypertension. J. Hypertens. 31, 1925–1938. https://doi.org/10.1097/HJH.0b013e328364ca4c (2013).
Sherwood, A., Steffen, P. R., Blumenthal, J. A., Kuhn, C. & Hinderliter, A. L. Nighttime blood pressure dipping: the role of the sympathetic nervous system. Am. J. Hypertens. 15, 111–118. https://doi.org/10.1016/s0895-7061(01)02251-8 (2002).
Salles, G. F. et al. Prognostic effect of the nocturnal blood pressure fall in hypertensive patients: the ambulatory blood pressure collaboration in patients with hypertension (ABC-H) meta-analysis. Hypertension 67, 693–700. https://doi.org/10.1161/HYPERTENSIONAHA.115.06981 (2016).
Brooks, D., Horner, R. L., Kozar, L. F., Render-Teixeira, C. L. & Phillipson, E. A. Obstructive sleep apnea as a cause of systemic hypertension. Evidence from a canine model. J. Clin. Invest. 99, 106–109. https://doi.org/10.1172/JCI119120 (1997).
Fletcher, E. C., Miller, J., Schaaf, J. W. & Fletcher, J. G. Urinary catecholamines before and after tracheostomy in patients with obstructive sleep apnea and hypertension. Sleep 10, 35–44. https://doi.org/10.1093/sleep/10.1.35 (1987).
Kohli, P., Balachandran, J. S. & Malhotra, A. Obstructive sleep apnea and the risk for cardiovascular disease. Curr. Atheroscler. Rep. 13, 138–146. https://doi.org/10.1007/s11883-011-0161-8 (2011).
Mokhlesi, B. et al. Obstructive sleep apnoea during REM sleep and incident non-dipping of nocturnal blood pressure: a longitudinal analysis of the Wisconsin sleep cohort. Thorax 70, 1062–1069. https://doi.org/10.1136/thoraxjnl-2015-207231 (2015).
Seif, F. et al. Obstructive sleep apnea and diurnal nondipping hemodynamic indices in patients at increased cardiovascular risk. J. Hypertens. 32, 267–275. https://doi.org/10.1097/HJH.0000000000000011 (2014).
Kwon, Y. et al. Blood pressure monitoring in sleep: time to wake up. Blood Press. Monit. 25, 61–68. https://doi.org/10.1097/MBP.0000000000000426 (2020).
Ulasli, S. S. et al. Effects of nondipping pattern on systemic inflammation in obstructive sleep apnea. Sleep Breath 19, 1185–1190. https://doi.org/10.1007/s11325-015-1135-9 (2015).
Sasaki, N. et al. Impact of non-dipping on cardiovascular outcomes in patients with obstructive sleep apnea syndrome. Clin. Exp. Hypertens. 37, 449–453. https://doi.org/10.3109/10641963.2015.1057833 (2015).
Gesche, H., Grosskurth, D., Kuchler, G. & Patzak, A. Continuous blood pressure measurement by using the pulse transit time: comparison to a cuff-based method. Eur. J. Appl. Physiol. 112, 309–315. https://doi.org/10.1007/s00421-011-1983-3 (2012).
Bartsch, S. et al. Validation of continuous blood pressure measurements by pulse transit time: a comparison with invasive measurements in a cardiac intensive care unit. Dtsch. Med. Wochenschr. 135, 2406–2412. https://doi.org/10.1055/s-0030-1269408 (2010).
Schmalgemeier, H. et al. Pulse transit time: validation of blood pressure measurement under positive airway pressure ventilation. Sleep Breath 16, 1105–1112. https://doi.org/10.1007/s11325-011-0609-7 (2012).
Patzak, A., Mendoza, Y., Gesche, H. & Konermann, M. Continuous blood pressure measurement using the pulse transit time: comparison to intra-arterial measurement. Blood Press. 24, 217–221. https://doi.org/10.3109/08037051.2015.1030901 (2015).
Cuspidi, C. et al. Blood pressure non-dipping and obstructive sleep apnea syndrome: a meta-analysis. J. Clin. Med. https://doi.org/10.3390/jcm8091367 (2019).
Bilo, G. et al. Validation of the Somnotouch-NIBP noninvasive continuous blood pressure monitor according to the European Society of Hypertension International Protocol revision 2010. Blood Press. Monit. 20, 291–294. https://doi.org/10.1097/MBP.0000000000000124 (2015).
Almeneessier, A. S. et al. Comparison between blood pressure during obstructive respiratory events in REM and NREM sleep using pulse transit time. Sci. Rep. 10, 3342. https://doi.org/10.1038/s41598-020-60281-2 (2020).
Pitson, D. J. & Stradling, J. R. Value of beat-to-beat blood pressure changes, detected by pulse transit time, in the management of the obstructive sleep apnoea/hypopnoea syndrome. Eur. Respir. J. 12, 685–692 (1998).
Berry, R. B. et al. AASM scoring manual updates for 2017 (version 2.4). J. Clin. Sleep Med. 13, 665–666. https://doi.org/10.5664/jcsm.6576 (2017).
Quan, S. F., Gillin, J. C., Littner, M. R. & Shepard, J. W. Sleep-related breathing disorders in adults: recommendations for syndrome definition and measurement techniques in clinical research. The report of an American Academy of Sleep Medicine Task Force. Sleep Breath 22, 667–689 (1999).
Javaheri, S. & Redline, S. Sleep, slow-wave sleep, and blood pressure. Curr. Hypertens. Rep. 14, 442–448. https://doi.org/10.1007/s11906-012-0289-0 (2012).
Garcia, M. T. G. et al. Can pulse transit time be useful for detecting hypertension in patients in a sleep unit?. Arch. Bronconeumol. 50, 278–284. https://doi.org/10.1016/j.arbres.2013.12.001 (2014).
Marrone, O. & Bonsignore, M. R. Blood-pressure variability in patients with obstructive sleep apnea: current perspectives. Nat. Sci. Sleep 10, 229–242. https://doi.org/10.2147/NSS.S148543 (2018).
Tasali, E., Leproult, R., Ehrmann, D. A. & Van Cauter, E. Slow-wave sleep and the risk of type 2 diabetes in humans. Proc. Natl. Acad. Sci. U. S. A. 105, 1044–1049. https://doi.org/10.1073/pnas.0706446105 (2008).
Sayk, F. et al. Effects of selective slow-wave sleep deprivation on nocturnal blood pressure dipping and daytime blood pressure regulation. Am. J. Physiol. Regul. Integr. Comp. Physiol. 298, R191-197. https://doi.org/10.1152/ajpregu.00368.2009 (2010).
Alex, R. et al. Effect of apnea duration on apnea induced variations in cerebral blood flow velocity and arterial blood pressure. Conf. Proc. IEEE Eng. Med. Biol. Soc. 270–273, 2014. https://doi.org/10.1109/EMBC.2014.6943581 (2014).
Wu, H., Zhan, X., Zhao, M. & Wei, Y. Mean apnea-hypopnea duration (but not apnea-hypopnea index) is associated with worse hypertension in patients with obstructive sleep apnea. Med. Baltim. 95, e5493. https://doi.org/10.1097/MD.0000000000005493 (2016).
Somers, V. K., Mark, A. L. & Abboud, F. M. Sympathetic activation by hypoxia and hypercapnia–implications for sleep apnea. Clin. Exp. Hypertens. A 10(Suppl 1), 413–422 (1988).
Smolensky, M. H., Hermida, R. C. & Portaluppi, F. Circadian mechanisms of 24-hour blood pressure regulation and patterning. Sleep Med. Rev. 33, 4–16. https://doi.org/10.1016/j.smrv.2016.02.003 (2017).
Aparicio, L. S. et al. Determinants of the morning-evening home blood pressure difference in treated hypertensives: the HIBA-home study. Int. J. Hypertens. 2014, 569259. https://doi.org/10.1155/2014/569259 (2014).
O’Brien, E., Parati, G. & Stergiou, G. Response to: nocturnal blood pressure dipping: systolic, diastolic or both?. J. Hypertens. 32, 700–701. https://doi.org/10.1097/HJH.0000000000000104 (2014).
Schillaci, G., Battista, F. & Pucci, G. Nocturnal blood pressure dipping: systolic, diastolic or both?. J. Hypertens. 32, 699–700. https://doi.org/10.1097/HJH.0000000000000103 (2014).
Parati, G. et al. European Society of Hypertension practice guidelines for ambulatory blood pressure monitoring. J. Hypertens. 32, 1359–1366. https://doi.org/10.1097/HJH.0000000000000221 (2014).
Schillaci, G. et al. Predictors of diurnal blood pressure changes in 2042 subjects with essential hypertension. J. Hypertens. 14, 1167–1173. https://doi.org/10.1097/00004872-199610000-00003 (1996).
Staessen, J. A. et al. Nocturnal blood pressure fall on ambulatory monitoring in a large international database. The “Ad Hoc’’ Working Group”. Hypertension 29, 30–39. https://doi.org/10.1161/01.hyp.29.1.30 (1997).
Acknowledgements
In this paper, we remember the coauthor Awad H. Olaish who deceased before the publication of the paper; we all miss you.
Funding
This work was supported by the Strategic Technologies Program of the National Plan for Sciences and Technology and Innovation in the Kingdom of Saudi Arabia (MED511-02–08).
Author information
Authors and Affiliations
Contributions
A.S.B.: Conception and design of the work; data acquisition, analysis, and interpretation; securing funds; writing the manuscript; and final approval of the version to be submitted, agreed both to be personally accountable for the author's own contributions and to ensure that questions related to the accuracy or integrity of any part of the work, even ones in which the author was not personally involved. M.A.: Contributions to the conception and design of the work; writing the manuscript; and final approval of the version to be submitted, agreed both to be personally accountable for the author's own contributions and to ensure that questions related to the accuracy or integrity of any part of the work, even ones in which the author was not personally involved. S.A.: Contributions to the conception and design of the work; writing the manuscript; and final approval of the version to be submitted, agreed both to be personally accountable for the author's own contributions and to ensure that questions related to the accuracy or integrity of any part of the work, even ones in which the author was not personally involved. A.H.O.: Data cleaning, interpretation of the data, statistical analysis; writing of the manuscript and final approval of the version to be submitted, agreed both to be personally accountable for the author's own contributions and to ensure that questions related to the accuracy or integrity of any part of the work, even ones in which the author was not personally involved. M.H.A.: Data cleaning; writing of the manuscript and final approval of the version to be submitted, agreed both to be personally accountable for the author's own contributions and to ensure that questions related to the accuracy or integrity of any part of the work, even ones in which the author was not personally involved. A.S.: Data cleaning; writing of the manuscript and final approval of the version to be submitted, agreed both to be personally accountable for the author's own contributions and to ensure that questions related to the accuracy or integrity of any part of the work, even ones in which the author was not personally involved.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
BaHammam, A.S., Alshahrani, M., Aleissi, S.A. et al. Blood pressure dipping during REM and non-REM sleep in patients with moderate to severe obstructive sleep apnea. Sci Rep 11, 7990 (2021). https://doi.org/10.1038/s41598-021-87200-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-021-87200-3
This article is cited by
-
Role of Sleep and Sleep Disorders in Cardiometabolic Risk: a Review and Update
Current Sleep Medicine Reports (2024)
-
Neurocognitive, mood changes, and sleepiness in patients with REM-predominant obstructive sleep apnea
Sleep and Breathing (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.